Extraction of Anthocyanin Dye from Staghorn Sumac Fruit in Various Solvents and Use for Pigment Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Staghorn Sumac and Chemicals for Extraction
2.1.2. Pigment Printing Pastes
2.1.3. Printing Substrates
2.2. Methods
2.2.1. Dye Extraction
2.2.2. Preparation of Printing Inks
2.2.3. Printing
2.2.4. Color Measurements
2.2.5. Fastness Tests
3. Results and Discussion
3.1. Extracts, Printing Inks, and Prints
3.2. Fastness of Prints
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Zhu, F. Chemical composition and biological activity of staghorn sumac (Rhus typhina). Food Chem. 2017, 237, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Kossah, R.; Nsabimana, C.; Zhao, J.X.; Chen, H.Q.; Tian, F.W.; Zhang, H.; Chen, W. Comparative study on the chemical composition of Syrian sumac (Rhus coriaria L.) and Chinese sumac (Rhus typhina L.) fruits. Pak. J. Nutr. 2009, 8, 1570–1574. [Google Scholar] [CrossRef]
- Wu, T.; McCallum, J.L.; Wang, S.; Liu, R.; Zhu, H.; Tsao, R. Evaluation of antioxidant activities and chemical characterisation of staghorn sumac fruit (Rhus hirta L.). Food Chem. 2013, 138, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.W.; Wu, T.; Tsao, R.; McCallum, J.L. Isolation and structural characterization of unusual pyranoanthocyanins and related anthocyanins from Staghorn sumac (Rhus typhina L.) via UPLC–ESI-MS, 1H, 13C, and 2D NMR spectroscopy. Phytochemistry 2013, 94, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Wang, H.; Wang, D.; Fang, F.; Wang, F.; Wu, T. Ultrasonic Extraction of Antioxidants from Chinese Sumac (Rhus typhina L.) Fruit Using Response Surface Methodology and Their Characterization. Molecules 2014, 19, 9019–9032. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, H.; Liu, R.; Mine, Y.; McCallum, J.; Kirby, C.; Tsao, R. Antioxidant and anti-inflammatory activities of pyranoanthocyanins and other polyphenols from staghorn sumac (Rhus hirta L.) in Caco-2 cell models. J. Funct. Foods 2016, 20, 139–147. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Quality attributes of bread fortified with staghorn sumac extract. J. Texture Stud. 2018, 49, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Iskra, J.; Pavlič, M.; Žigon, J.; Merela, M. Wood dyes from invasive alien plants. Les/Wood 2020, 69, 37–48. [Google Scholar] [CrossRef]
- Horvat, M.; Iskra, J. Invazivna tujerodna rastlina octovec-naravni pH indikator. Invasive alien plant staghorn sumac-a natural pH indicator. Kem. Šoli Družbi 2020, 1, 1–5. [Google Scholar]
- Matoh, L.; Krejan, E.; Horvat, M.; Iskra, J. Uporaba barvil invazivnih tujerodnih rastlin pri izdelavi sončnih celic. Use of dyes from invasive alien plants in the manufacture of solar cells. Kem. Šoli Družbi 2020, 1, 1–4. [Google Scholar]
- Petrovčič, E. Vpliv Načina Ekstrakcije Barvila iz Plodov Octovca in Predobdelave Bombažne Tkanine na Njeno Obarvljivost. Influence of Extraction Process of Dye from Staghorn Sumac Fruit and Pretreatment of Cotton Fabric on Its Dyeability. Diploma Thesis, University of Ljubljana, Ljubljana, Slovenia, 2020. Available online: https://repozitorij.uni-lj.si/IzpisGradiva.php?lang=slv&id=119495 (accessed on 20 May 2024).
- Baaka, N. Sumac (Rhus tripartita): A Natural Dye Used for Simultaneous Coloration and Functional Finishing on Textiles. J. Nat. Fibers 2022, 19, 7265–7274. [Google Scholar] [CrossRef]
- Verbič, A.; Brenčič, K.; Dolenec, M.; Primc, G.; Recek, N.; Šala, M.; Gorjanc, M. Designing UV-protective and hydrophilic or hydrophobic cotton fabrics through in-situ ZnO synthesis using biodegradable waste extracts. Appl. Surf. Sci. 2022, 599, 153931. [Google Scholar] [CrossRef]
- Brenčič, K.; Ogrizek, L.; Gorjanc, M. Priprava pigmentov iz invazivnih tujerodnih rastlin in izdelava okolju prijaznih tiskarskih past za tisk tekstilij. Preparation of pigments from invasive alien plant species and development of environmentally friendly printing pastes for textile application. Tekstilec 2022, 65, 16–22. [Google Scholar]
- Klančnik, M. Screen Printing with Natural Dye Extract from Japanese Knotweed Rhizome. Fiber Polym. 2021, 22, 2498–2506. [Google Scholar] [CrossRef]
- Klančnik, M. Printing with Natural Dye Extracted from Impatiens glandulifera Royle. Coatings 2021, 11, 445. [Google Scholar] [CrossRef]
- ASTM D 5264-98; Standard Practice for Abrasion Resistance of Printed Materials by the Sutherland Rub Tester. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 105-X12; Textiles—Tests for Colour Fastness—Part X12: Colour Fastness to Rubbing. ISO: Geneva, Switzerland, 2016.
- ISO 105-X11; Textiles—Tests for Colour Fastness—Part X11: Colour Fastness to Hot Pressing. ISO: Geneva, Switzerland, 1994.
- ISO 105-C06; Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering. ISO: Geneva, Switzerland, 2012.
- ISO 105-A02; Textiles—Tests for Colour Fastness—Part A02: Grey Scale for Assessing Change in Colour. ISO: Geneva, Switzerland, 1993.
- ISO 105-A03; Textiles—Tests for Colour Fastness—Part A03: Grey Scale for Assessing Staining. ISO: Geneva, Switzerland, 2019.
- ISO 105-B02; Textiles—Tests for Colour Fastness—Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test. ISO: Geneva, Switzerland, 2014.
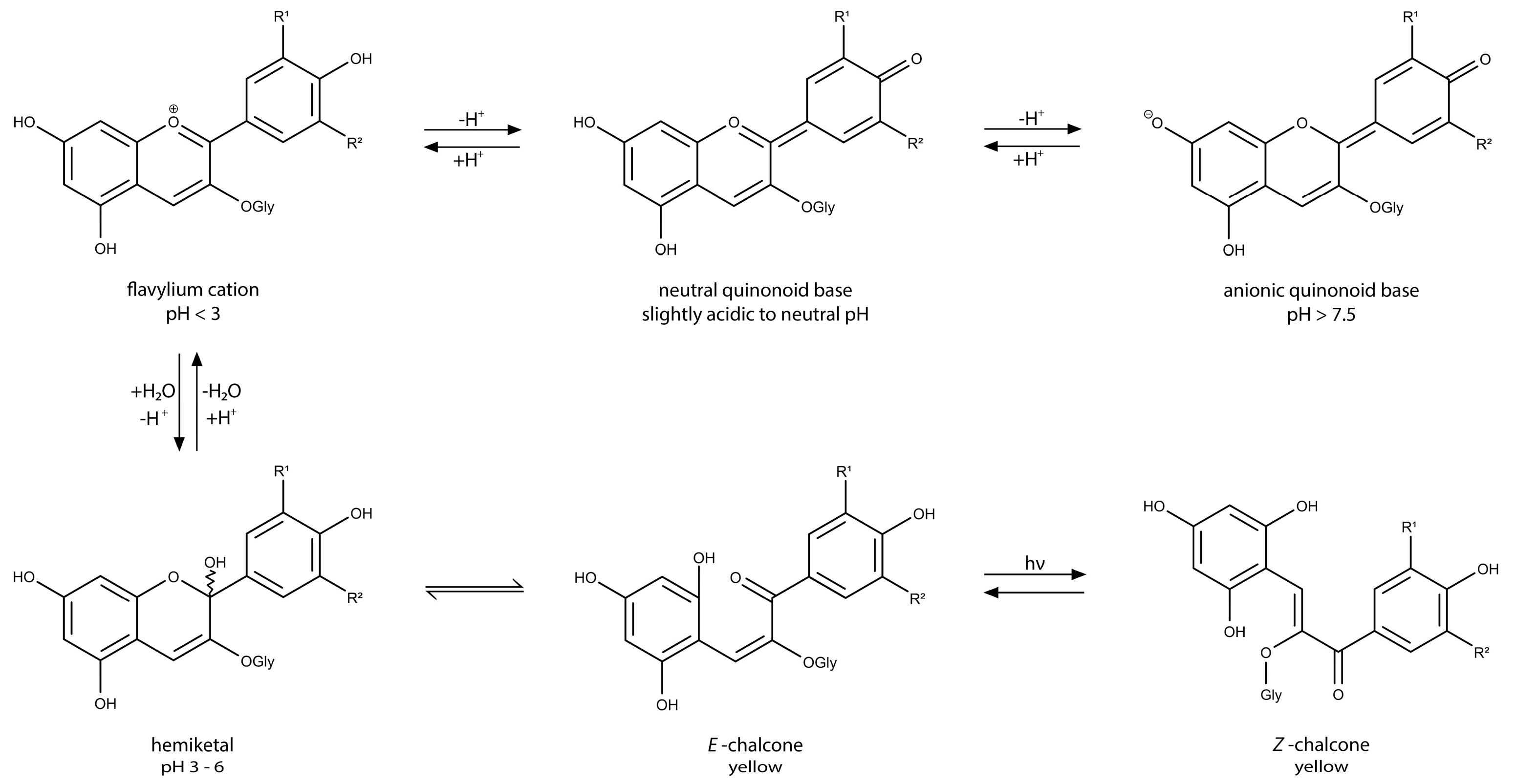
- Constantin, O.E.; Istrati, D.I. Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review. Horticulturae 2022, 8, 1084. [Google Scholar] [CrossRef]
- Joshi, D.R. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant (Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, G.W.; Jurs, P.C. Prediction of Surface Tension, Viscosity, and Thermal Conductivity for Common Organic Solvents Using Quantitative Structure−Property Relationships. J. Chem. Inf. Comput. Sci. 2001, 41, 408–418. [Google Scholar] [CrossRef] [PubMed]
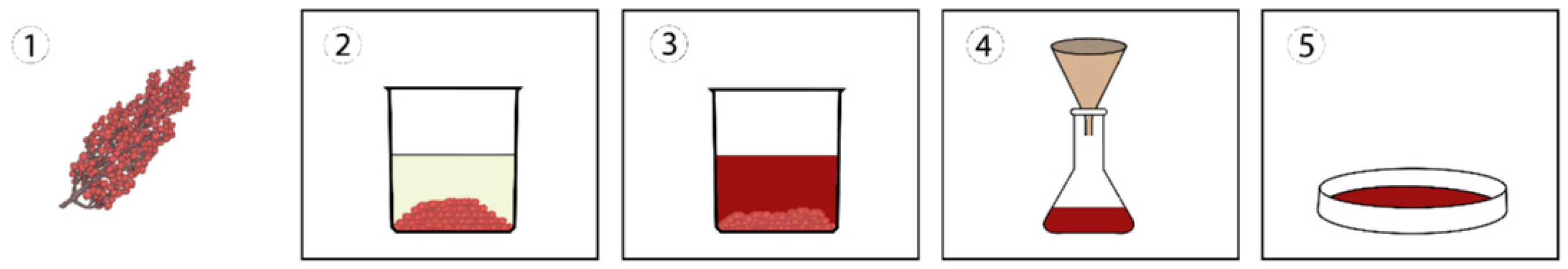
| Color of Staghorn Sumac Fruit | Dye Extract | Color of Solution after Filtering | Color of Extract after Solvent Evaporation | Concentration of Printing Ink | Color of Printing Ink | Color of Print on | |
|---|---|---|---|---|---|---|---|
| Paper | Cotton Fabric | ||||||
| in distilled water |  |  | 1.5 g dye extract /50 g printing paste |  |  |  | |
| in distilled water |  |  | 1.5 g dye extract /50 g Elastil T. printing paste |  |  |  | |
| in methanol |  |  | 1.5 g dye extract /50 g printing paste |  |  |  | |
| in ethanol |  |  | 1.5 g dye extract /50 g printing paste |  |  |  | |
 | in propanol |  |  | 1.5 g dye extract /50 g printing paste |  |  |  |
| in acetonitrile |  |  | 1.48 g dye extract /50 g printing paste |  |  |  | |
| in acetone |  |  | 1.5 g dye extract /50 g printing paste |  |  |  | |
| in dichloro- methane |  |  | 0.67 g dye extract /50 g printing paste |  |  |  | |
| in water with soda in copper sulfate |  |  | 3 g precipitated pigment /50 g printing paste |  | - | - | |
| Dye Extract | L* | a* | b* | C*ab | hab [°] |
|---|---|---|---|---|---|
| Paper | |||||
| distilled water | 91.63 | 0.59 | −3.55 | 3.60 | 88.42 |
| distilled water/Elastil T. paste | 90.57 | −0.04 | −1.13 | 1.13 | 87.97 |
| methanol | 88.06 | 1.38 | 4.88 | 5.07 | 74.21 |
| ethanol | 91.59 | 1.51 | −4.18 | 4.44 | 70.14 |
| propanol | 92.11 | 0.40 | −3.27 | 3.29 | 78.67 |
| acetonitrile | 93.11 | 1.42 | −7.09 | 7.23 | 78.67 |
| acetone | 90.05 | −0.21 | 2.92 | 2.93 | 85.89 |
| dichloromethane | 92.33 | 0.87 | −5.18 | 5.25 | 80.47 |
| Cotton Fabric | |||||
| distilled water | 87.43 | −1.07 | 9.42 | 9.48 | 89.26 |
| distilled water/Elastil T. paste | 88.14 | −1.82 | 8.77 | 8.96 | 78.28 |
| methanol | 85.03 | 0.98 | 14.42 | 14.45 | 79.46 |
| ethanol | 88.15 | 0.46 | 10.43 | 10.44 | 87.47 |
| propanol | 90.02 | −2.06 | 11.39 | 11.57 | 79.75 |
| acetonitrile | 90.33 | −0.71 | 5.28 | 5.33 | 82.34 |
| acetone | 86.65 | −1.43 | 15.95 | 16.01 | 84.88 |
| dichloromethane | 90.03 | −1.39 | 7.47 | 7.60 | 79.46 |
| Dye Extract | Rubbing | Light | |
|---|---|---|---|
| Print Surface | Color Transfer to White Paper | Color Change of the Print | |
| distilled water | unchanged | no | 6 |
| distilled water/Elastil T. paste | unchanged | no | 3 |
| methanol | unchanged | no | 6 |
| ethanol | unchanged | no | 6 |
| propanol | unchanged | no | 6 |
| acetonitrile | unchanged | no | 6 |
| acetone | unchanged | no | 6 |
| dichloromethane | unchanged | no | 6 |
| Dye Extract | Rubbing | Hot Pressing | Washing | Light | ||||
|---|---|---|---|---|---|---|---|---|
| Staining of Dry Cloth | Staining of Wet Cloth | Staining of Wet Cloth | Staining of Dry Cloth | Color Change of the Print | Staining of Cloth | Color Change of the Print | Color Change of the Print | |
| distilled water | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3–4 |
| distilled water/Elastil T. paste | 5 | 5 | 5 | 5 | 5 | 5 | 1–2 | 3 |
| methanol | 5 | 5 | 5 | 5 | 5 | 5 | 1–2 | 6 |
| ethanol | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 6–7 |
| propanol | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 8 |
| acetonitrile | 5 | 5 | 5 | 5 | 5 | 5 | 1–2 | 6 |
| acetone | 5 | 5 | 5 | 5 | 5 | 5 | 1–2 | 6 |
| dichloromethane | 5 | 5 | 5 | 5 | 5 | 5 | 4–5 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klančnik, M.; Koradin, E. Extraction of Anthocyanin Dye from Staghorn Sumac Fruit in Various Solvents and Use for Pigment Printing. Coatings 2024, 14, 1025. https://doi.org/10.3390/coatings14081025
Klančnik M, Koradin E. Extraction of Anthocyanin Dye from Staghorn Sumac Fruit in Various Solvents and Use for Pigment Printing. Coatings. 2024; 14(8):1025. https://doi.org/10.3390/coatings14081025
Chicago/Turabian StyleKlančnik, Maja, and Elena Koradin. 2024. "Extraction of Anthocyanin Dye from Staghorn Sumac Fruit in Various Solvents and Use for Pigment Printing" Coatings 14, no. 8: 1025. https://doi.org/10.3390/coatings14081025





