Investigation on the Thermal–Mechanical Properties of YbRESiO5 (RE = Yb, Eu, Gd, Ho, Tm, Lu, Y, Sc): First-Principles Calculations and Thermal Performance Experiments
Abstract
:1. Introduction
2. Computational Models and Experimental Procedures
2.1. Computational Models
2.2. Material Preparation and Microstructure Characterization
2.3. Thermal Properties
3. Result and Discussion
3.1. First-Principles Calculations
3.1.1. Crystal Lattice Distortion Calculation
3.1.2. Water Vapor Corrosion Resistance
3.1.3. Elastic Constants
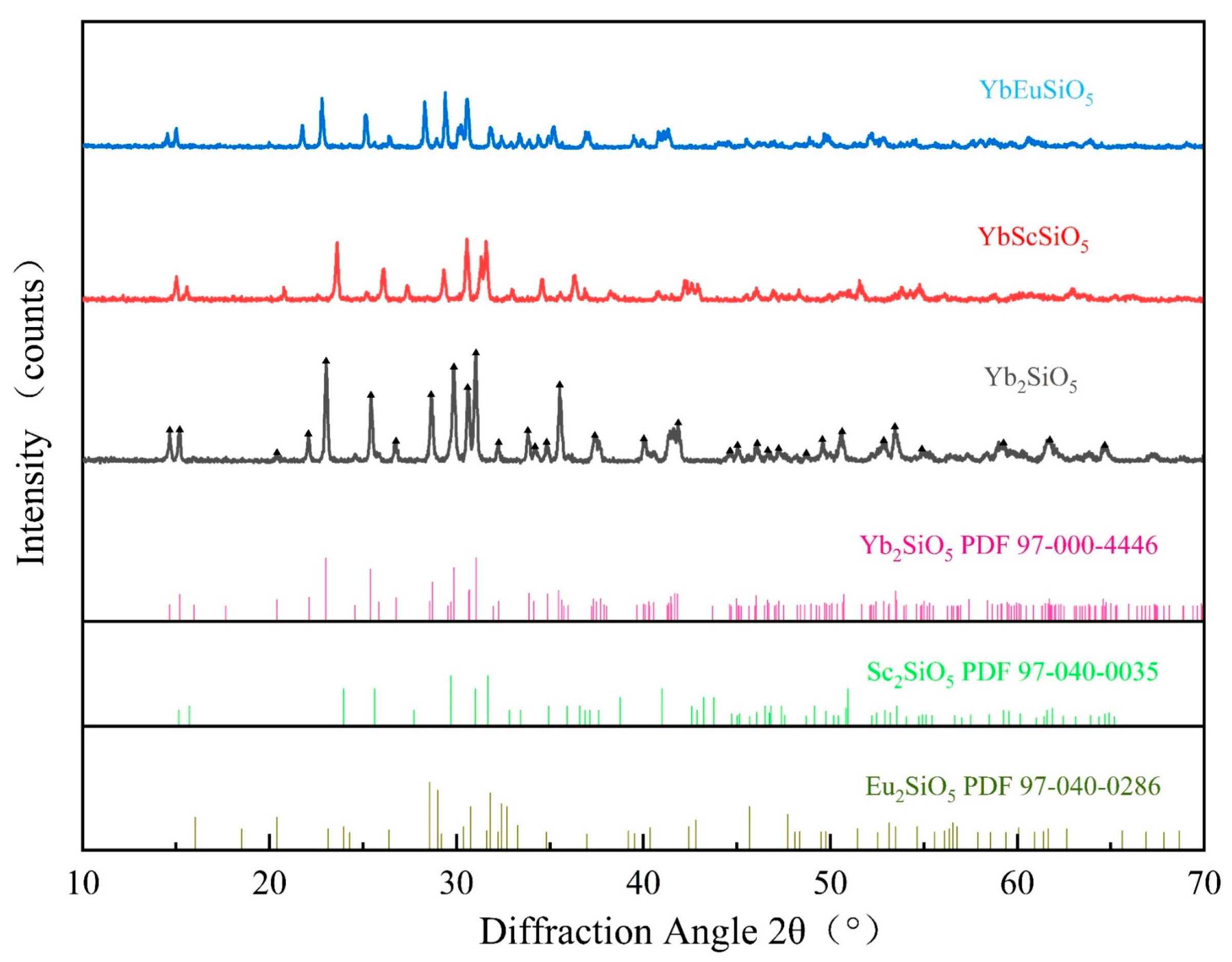
3.2. Phases and Microstructure of Sintered Bulk Materials
3.3. Thermal Performance Test
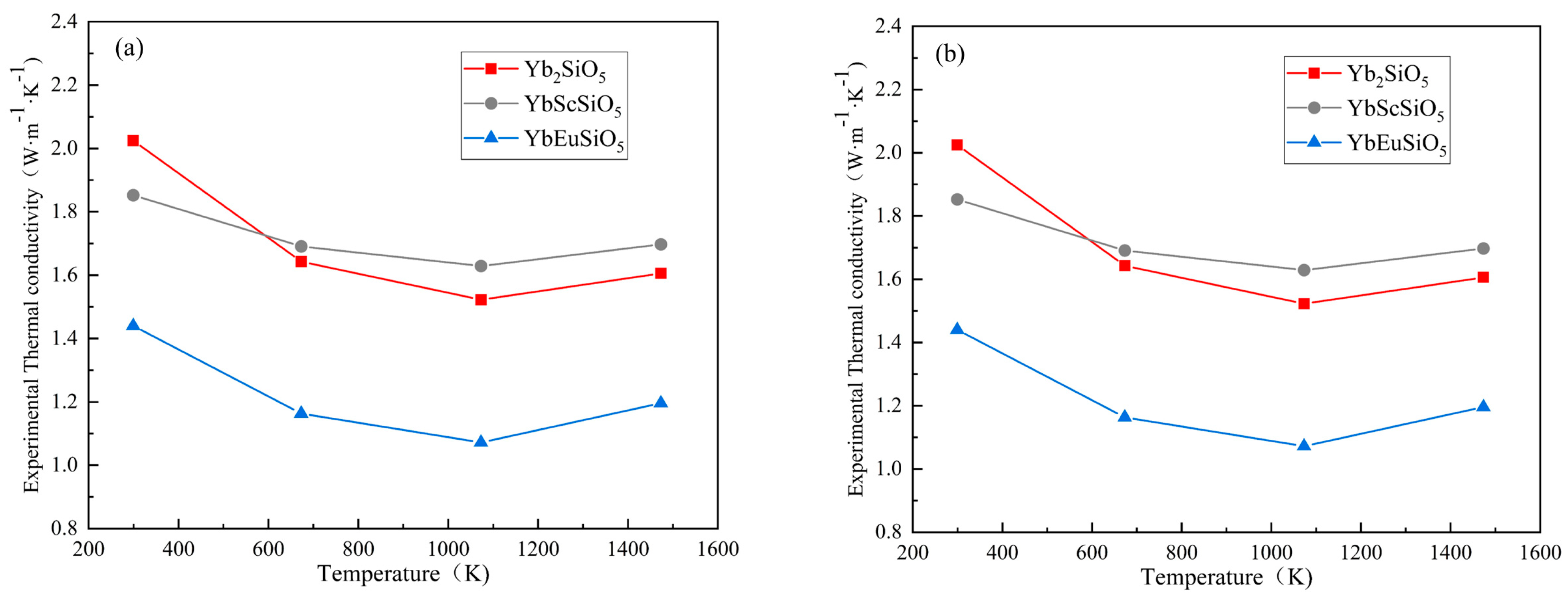
3.3.1. Thermal Conductivity
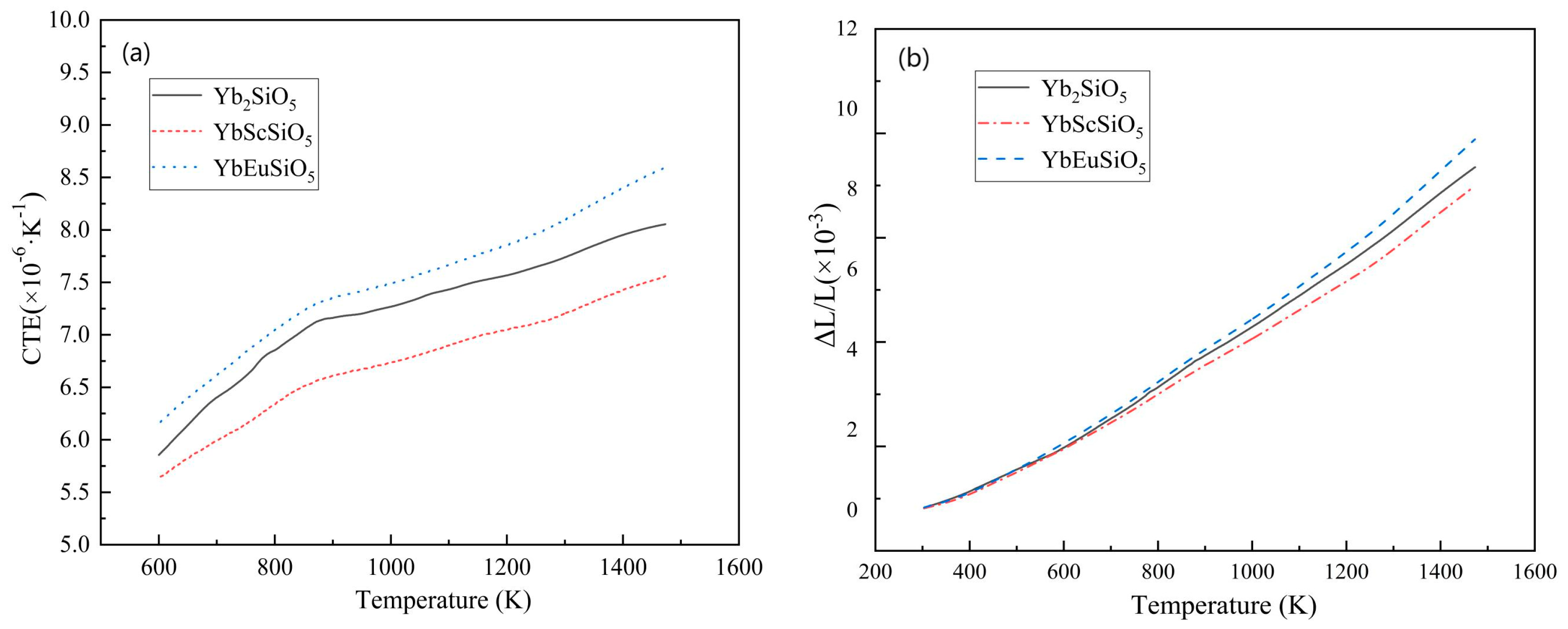
3.3.2. CTE
4. Conclusions
- (1)
- According to first-principles calculations, the crystal structure and elastic properties of Yb2SiO5 are affected differently when equimolar amounts of rare earth elements RE (RE = Eu, Gd, Ho, Tm, Lu, Y, Sc) are added. In particular, doping the material with Eu, which has the largest atomic radius, and Sc, which has the smallest, seems to have opposite effects. While YbEuSiO5 has the opposite behavior, YbScSiO5 has the biggest lattice distortion, average sound velocity, Debye temperature, and Young’s modulus. It also has the least average RE-O bond length and unit cell volume. The material’s Young’s modulus is increased when Yb2SiO5 is doped with Sc, mostly as a result of an increase in cation field strength and a decrease in average cation radius.
- (2)
- The Si-O bond lengths and bond energies of YbRESiO5 (RE = Yb, Eu, Gd, Ho, Tm, Lu, Y, Sc) were calculated using first-principles methods. The seven aforementioned elements can be divided into two groups based on the outcomes of the simulation: In comparison to Yb2SiO5, one group (Gd, Eu, Y, and Lu) decreases the bond energy and lengthens the Si-O bond when replacing Yb, which lowers the material’s resistance to oxygen and water corrosion. When doped, the other group (Sc, Ho, and Tm) shortens the Si-O bond and lengthens the bond energy, improving the material’s resistance to oxygen and water corrosion.
- (3)
- According to thermal conductivity measurements, below 600 K, YbScSiO5 has somewhat less thermal diffusivity and thermal conductivity than Yb2SiO5. They are marginally greater than Yb2SiO5 beyond 600 K, but the overall difference is negligible. When Eu is added, YbEuSiO5 exhibits much reduced thermal conductivity and diffusivity in comparison to Yb2SiO5. In particular, for YbEuSiO5, the minimum thermal conductivity is 1.072 W·m−1·K−1 between 400 °C and 1200 °C, while for Yb2SiO5, it is 1.52 W·m−1·K−1. This indicates that when YbEuSiO5 is compared to Yb2SiO5, its minimum thermal conductivity decreases by 29.4%. The primary cause of YbEuSiO5’s notable reduction in thermal conductivity is the addition of Eu, which results in the introduction of oxygen vacancies.
- (4)
- Coefficient of thermal expansion (CTE) testing results show that YbScSiO5 has a lower CTE than Yb2SiO5, but adding Eu raises the CTE of YbEuSiO5. In particular, at 1473 K, YbScSiO5 exhibits the lowest CTE (7.56 × 10−6·K−1) compared to Yb2SiO5 (7% lower) at the same temperature. YbEuSiO5, on the other hand, has the highest CTE at 8.6 × 10−6·K−1, which is 7.5% more than Yb2SiO5. According to an analysis of the experimental data, there may be a positive correlation between the base material and the CTE of multi-component silicates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, W.; Tan, Y.; Zhu, C.; Teng, Z.; Jia, P.; Zhang, H. Synthesis, microstructures, and corrosion behaviors of multi-components rare-earth silicates. Ceram. Int. 2021, 47, 32641–32647. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.-Z.; Liu, J.; Zhou, Y. High entropy (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 with strong anisotropy in thermal expansion. J. Mater. Sci. Technol. 2020, 36, 134–139. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, K.; Wang, W.; Yang, T.; Fang, H.; Qiang, L.; Zhang, X.; Zhang, C. Investigation of Thermal Shock Behavior of Multilayer Thermal Barrier Coatings with Superior Erosion Resistance Prepared by Atmospheric Plasma Spraying. Coatings 2022, 12, 804. [Google Scholar] [CrossRef]
- More, K.L.; Tortorelli, P.F.; Ferber, M.K.; Keiser, J.R. Observations of Accelerated Silicon Carbide Recession by Oxidation at High Water-Vapor Pressures. J. Am. Ceram. Soc. 2008, 83, 211–213. [Google Scholar] [CrossRef]
- Opila, E.J. Oxidation and Volatilization of Silica Formers in Water Vapor. J. Am. Ceram. Soc. 2004, 86, 1238–1248. [Google Scholar] [CrossRef]
- Opila, E.J.; Smialek, J.L.; Robinson, R.C.; Fox, D.S.; Jacobson, N.S. SiC Recession Caused by SiO2 Scale Volatility under Combustion Conditions: II, Thermodynamics and Gaseous-Diffusion Model. J. Am. Ceram. Soc. 2004, 82, 1826–1834. [Google Scholar] [CrossRef]
- Tian, Z.; Zheng, L.; Wang, J.; Wan, P.; Li, J.; Wang, J. Theoretical and experimental determination of the major thermo-mechanical properties of RE2SiO5 (RE = Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y) for environmental and thermal barrier coating applications. J. Eur. Ceram. Soc. 2016, 36, 189–202. [Google Scholar] [CrossRef]
- Fan, W.; Zou, B.; Fan, C.; Wang, Y.; Cai, X.; Tao, S.; Xu, J.; Wang, C.; Cao, X.; Zhu, L. Fabrication and oxidation resistant behavior of plasma sprayed Si/Yb2Si2O7/LaMgAl11O19 coating for Cf/SiC composites at 1673K. Ceram. Int. 2017, 43, 392–398. [Google Scholar] [CrossRef]
- Rohbeck, N.; Morrell, P.; Xiao, P. Degradation of ytterbium disilicate environmental barrier coatings in high temperature steam atmosphere. J. Eur. Ceram. Soc. 2019, 39, 3153–3163. [Google Scholar] [CrossRef]
- Raj, R. Fundamental research in structural ceramics for service near 2000 C. J. Am. Ceram. Soc. 1993, 76, 2147–2174. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Price, J.R.; Van Roode, M.; Stala, C. Ceramic Oxide-Coated Silicon Carbide for High Temperature Corrosive Environments. Key Eng. Mater. 1992, 72–74, 71–84. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y.-H.; Ding, Z.-Y.; Yang, H.-L.; Liu, Z.-G.; Ouyang, J.-H.; Wang, Y.-M.; Wang, Y.-J. Tunable corrosion resistance of rare-earth monosilicate to molten calcia-magnesia-aluminosilicate glass by RE-doping strategy. Corros. Sci. 2022, 202, 110319. [Google Scholar] [CrossRef]
- Lee, K.N.; Miller, R.A. Oxidation Behavior of MuIlite-Coated SiC and SiC/SiC Composites under Thermal Cycling between Room Temperature and 1200–1400 °C. J. Am. Ceram. Soc. 2005, 79, 620–626. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Kruger, S.E.; Moreau, C.; Lima, R.S. Elastic Modulus Evolution and Behavior of Si/Mullite/BSAS-Based Environmental Barrier Coatings Exposed to High Temperature in Water Vapor Environment. J. Therm. Spray Technol. 2011, 20, 92–99. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Lévesque, D.; Moreau, C.; Lima, R.S. Performance of thermally sprayed Si/mullite/BSAS environmental barrier coatings exposed to thermal cycling in water vapor environment. Surf. Coat. Technol. 2013, 216, 215–223. [Google Scholar] [CrossRef]
- Lee, K.N.; Fox, D.S.; Bansal, N.P. Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. J. Eur. Ceram. Soc. 2005, 25, 1705–1715. [Google Scholar] [CrossRef]
- Nasiri, N.A.; Patra, N.; Horlait, D.; Jayaseelan, D.D.; Lee, W.E. Thermal Properties of Rare-Earth Monosilicates for EBC on Si-Based Ceramic Composites. J. Am. Ceram. Soc. 2016, 99, 589–596. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Xu, F.; Li, K. Rare earth silicate environmental barrier coatings: Present status and prospective. Ceram. Int. 2017, 43, 5847–5855. [Google Scholar] [CrossRef]
- Felsche, J. The Crystal Chemistry of the Rare-Earth Silicates; Springer: Berlin/Heidelberg, Germany, 1973; pp. 99–197. [Google Scholar]
- Ridley, M.J.; Tomko, K.Q.; Tomko, J.A.; Hoglund, E.R.; Howe, J.M.; Hopkins, P.E.; Opila, E.J. Tailoring thermal and chemical properties of a multi-component environmental barrier coating candidate (Sc0.2Nd0.2Er0.2Yb0.2Lu0.2)2Si2O7. Materialia 2022, 26, 101557. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Y.; Zhong, X.; Shi, M.; Mao, F.; Zhang, L.; Li, Q.; Zheng, X. Water vapor corrosion behaviors of plasma sprayed RE2SiO5 (RE = Gd, Y, Er) coatings. Corros. Sci. 2020, 167, 108529. [Google Scholar] [CrossRef]
- Qian, B.; Wang, Y.; Zu, J.; Xu, K.; Shang, Q.; Bai, Y. A review on multicomponent rare earth silicate environmental barrier coatings. J. Mater. Res. Technol. 2024, 29, 1231–1243. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Liu, R.; Jiang, D. Study on water vapor corrosion resistance of rare earth monosilicates RE2SiO5 (RE = Lu, Yb, Tm, Er, Ho, Dy, Y, and Sc) from first-principles calculations. Heliyon 2018, 4, e00857. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Feng, Z.; Zhou, Y. Mechanical and thermal properties of Yb2SiO5: First-principles calculations and chemical bond theory investigations. J. Mater. Res. 2014, 29, 1609–1619. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, Z.; Zheng, L.; Ming, K.; Ren, X.; Wang, J.; Li, B. (Ho0.25Lu0.25Yb0.25Eu0.25)2SiO5 high-entropy ceramic with low thermal conductivity, tunable thermal expansion coefficient, and excellent resistance to CMAS corrosion. J. Adv. Ceram. 2022, 11, 1279–1293. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Milman, V.; Warren, M.C. Elasticity of hexagonal BeO. J. Phys. Condens. Matter 2000, 13, 241–251. [Google Scholar] [CrossRef]
- Li, K.; Wang, W.; Yang, S.; Liu, Y.; Yang, T. First-principles calculations and thermal-mechanical experimental studies on middle-entropy rare-earth disilicates. Ceram. Int. 2024, 50, 22290–22305. [Google Scholar] [CrossRef]
- Leitner, J.; Chuchvalec, P.; Sedmidubský, D.; Strejc, A.; Abrman, P. Estimation of heat capacities of solid mixed oxides. Thermochim. Acta 2002, 395, 27–46. [Google Scholar] [CrossRef]
- Bruce, D.; Paradise, P.; Saxena, A.; Temes, S.; Clark, R.; Noe, C.; Benedict, M.; Broderick, T.; Bhate, D. A critical assessment of the Archimedes density method for thin-wall specimens in laser powder bed fusion: Measurement capability, process sensitivity and property correlation. J. Manuf. Process. 2022, 79, 185–192. [Google Scholar] [CrossRef]
- Leitner, J.; Voňka, P.; Sedmidubský, D.; Svoboda, P. Application of Neumann–Kopp rule for the estimation of heat capacity of mixed oxides. Thermochim. Acta 2010, 497, 7–13. [Google Scholar] [CrossRef]
- Charvat, F.R.; Kingery, W.D. Thermal Conductivity: XIII, Effect of Microstructure on Conductivity of Single-Phase Ceramics. J. Am. Ceram. Soc. 2006, 40, 306–315. [Google Scholar] [CrossRef]
- Hao, S.; Oleksak, R.P.; Doğan, Ö.N.; Gao, M.C. Computational Design of High-Entropy Rare Earth Disilicates as Next-Generation Thermal/Environmental Barrier Coatings. Acta Mater. 2023, 258, 119225. [Google Scholar] [CrossRef]
- Menke, Y.; Peltier-Baron, V.; Hampshire, S. Effect of rare-eath cations on properties of sialon glasses. J. Non Cryst. Solids 2000, 276, 145–150. [Google Scholar] [CrossRef]
- Giri, A.; Braun, J.L.; Rost, C.M.; Hopkins, P.E. On the minimum limit to thermal conductivity of multi-atom component crystalline solid solutions based on impurity mass scattering. Scr. Mater. 2017, 138, 134–138. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Ridley, M.; Gaskins, J.; Hopkins, P.; Opila, E. Tailoring thermal properties of multi-component rare earth monosilicates. Acta Mater. 2020, 195, 698–707. [Google Scholar] [CrossRef]






| Parameter | Yb2SiO5 Theory [20] | Yb2SiO5 Calculation | Deviation (%) |
|---|---|---|---|
| 14.2800 | 14.1961 | 0.5021 | |
| 10.2800 | 10.0852 | 1.9052 | |
| 6.6530 | 6.5614 | 1.3013 | |
| γ(°) | 122.200 | 122.2250 | 0.0220 |
| Parameter | γ(°) | ||||
|---|---|---|---|---|---|
| YbYSiO5 | 14.2749 | 10.1817 | 6.5710 | 122.0710 | 809.2980 |
| YbLuSiO5 | 14.3203 | 10.1851 | 6.5834 | 122.2090 | 812.4510 |
| YbHoSiO5 | 14.1741 | 10.1046 | 6.5834 | 122.3620 | 794.8210 |
| YbGdSiO5 | 14.2360 | 10.1655 | 6.5936 | 122.2250 | 806.6770 |
| YbScSiO5 | 13.9188 | 9.94632 | 6.4012 | 122.1350 | 750.4240 |
| YbTmSiO5 | 14.1493 | 10.1100 | 6.6152 | 122.2680 | 800.1510 |
| Yb2SiO5 | 14.1916 | 10.0858 | 6.5616 | 122.2250 | 794.7620 |
| YbEuSiO5 | 14.2760 | 10.1632 | 6.6205 | 122.2340 | 813.6240 |
| Compound | Bond | Polyhedra | Bond Length (Å) | Average Bond Length (Å) | Degree of Distortion (‰) |
|---|---|---|---|---|---|
| Yb2SiO5 | RE1-O | [REO7] | 2.17365, 2.26578, 2.27687, 2.28556, 2.29827, 2.3003, 2.50653 | 2.30098 | 1.63300 |
| RE2-O | [REO6] | 2.17305, 2.20835, 2.21083, 2.22665, 2.22665, 2.24791 | 2.21750 | 0.12334 | |
| Si-O | [SiO4] | 1.5915, 1.60382, 1.60696, 1.6228 | 1.60627 | 0.04824 | |
| YbTmSiO5 | RE1-O | [REO7] | 2.1845, 2.23035, 2.24948, 2.28916, 2.31377, 2.35425, 2.4962 | 2.30253 | 1.68259 |
| RE2-O | [REO6] | 2.15346, 2.20741, 2.21105, 2.22142, 2.22615, 2.26116 | 2.21344 | 0.20894 | |
| Si-O | [SiO4] | 1.59125, 1.60026, 1.60967, 1.62292 | 1.60602 | 0.05333 | |
| YbEuSiO5 | RE1-O | [REO7] | 2.22305, 2.26695, 2.31138, 2.31634, 2.32237, 2.36489, 2.50513 | 2.33001 | 1.26024 |
| RE2-O | [REO6] | 2.15427, 2.22478, 2.23663, 2.25526, 2.26148, 2.29384 | 2.23771 | 0.37126 | |
| Si-O | [SiO4] | 1.59478, 1.60092, 1.61483, 1.61983 | 1.60759 | 0.03971 | |
| YbGdSiO5 | RE1-O | [REO7] | 2.2152, 2.26505, 2.29988, 2.31368, 2.31368, 2.35175, 2.49526 | 2.32157 | 1.27335 |
| RE2-O | [REO6] | 2.19257, 2.21548, 2.23843, 2.23147, 2.25323, 2.26769 | 2.23314 | 0.11984 | |
| Si-O | [SiO4] | 1.59321, 1.60148, 1.6151, 1.62029 | 1.60752 | 0.04468 | |
| YbHoSiO5 | RE1-O | [REO7] | 2.20092, 2.25368, 2.28601, 2.30648, 2.30662, 2.34039, 2.58406 | 2.32545 | 2.37837 |
| RE2-O | [REO6] | 2.20766, 2.22172, 2.22778, 2.23061, 2.27294, 2.14123 | 2.21699 | 0.31473 | |
| Si-O | [SiO4] | 1.59119, 1.5994, 1.61279, 1.62194 | 1.60633 | 0.05451 | |
| YbLuSiO5 | RE1-O | [REO7] | 2.17016, 2.28852, 2.28869, 2.30331, 2.30836, 2.31393, 2.52894 | 2.31456 | 1.82196 |
| RE2-O | [REO6] | 2.21116, 2.23925, 2.24528, 2.25512, 2.25873, 2.26928 | 2.24643 | 0.07019 | |
| Si-O | [SiO4] | 1.59393, 1.60716, 1.60992, 1.61873 | 1.60744 | 0.03059 | |
| YbScSiO5 | RE1-O | [REO7] | 2.15248, 2.21908, 2.23995, 2.2501, 2.28767, 2.29817, 2.59642 | 2.29198 | 3.31675 |
| RE2-O | [REO6] | 2.11166, 2.02535, 2.08059, 2.11853, 2.13039, 2.17394 | 2.10674 | 0.47114 | |
| Si-O | [SiO4] | 1.59075, 1.5956, 1.60682, 1.61654 | 1.60243 | 0.05863 | |
| YbYSiO5 | RE1-O | [REO7] | 2.17206, 2.28342, 2.29012, 2.29846, 2.30223, 2.30753, 2.54975 | 2.31480 | 2.07000 |
| RE2-O | [REO6] | 2.19854, 2.23202, 2.23482, 2.23886, 2.24511, 2.25553 | 2.23415 | 0.06170 | |
| Si-O | [SiO4] | 1.5952, 1.60773, 1.60961, 1.61904 | 1.60620 | 0.02906 |
| Compound | Bond Average (Å) | Population of Bond | Density (Å−1) |
|---|---|---|---|
| Yb2SiO5 | 1.60627 | 0.61750 | 0.3844 |
| YbTmSiO5 | 1.60603 | 0.61850 | 0.3851 |
| YbEuSiO5 | 1.60759 | 0.61000 | 0.3794 |
| YbGdSiO5 | 1.60752 | 0.61250 | 0.3810 |
| YbHoSiO5 | 1.60620 | 0.6200 | 0.3860 |
| YbLuSiO5 | 1.60744 | 0.60500 | 0.3764 |
| YbScSiO5 | 1.60243 | 0.63500 | 0.3963 |
| YbYSiO5 | 1.60630 | 0.61720 | 0.3811 |
| Compound | (m/s) | (K) | B (Gpa) | G (Gpa) | E (Gpa) | |
|---|---|---|---|---|---|---|
| Yb2SiO5 | 3116.0476 | 400.2373 | 116.9618 | 59.8568 | 153.4019 | 0.2696 |
| YbTmSiO5 | 3115.4777 | 395.7621 | 105.4786 | 57.3296 | 145.6084 | 0.2699 |
| YbEuSiO5 | 3027.7198 | 392.4537 | 102.4466 | 57.1057 | 134.4732 | 0.2677 |
| YbGdSiO5 | 2875.6370 | 367.7621 | 92.0294 | 49.4669 | 125.8217 | 0.2721 |
| YbLuSiO5 | 3136.1570 | 403.6193 | 106.2719 | 60.1105 | 151.7248 | 0.2621 |
| YbHoSiO5 | 3047.4524 | 394.2189 | 97.2663 | 55.4342 | 139.7532 | 0.2605 |
| YbScSiO5 | 3669.7119 | 481.4030 | 126.2605 | 63.0056 | 162.0600 | 0.2861 |
| YbYSiO5 | 3282.3078 | 419.4569 | 95.0679 | 53.6648 | 145.4985 | 0.2625 |
| Compound | Average Ionic Radius | CFS |
|---|---|---|
| Yb2SiO5 | 0.86 | 4.056 |
| YbTmSiO5 | 0.865 | 4.009 |
| YbEuSiO5 | 0.905 | 3.662 |
| YbGdSiO5 | 0.9 | 3.370 |
| YbHoSiO5 | 0.88 | 3.387 |
| YbLuSiO5 | 0.855 | 4.104 |
| YbScSiO5 | 0.835 | 4.303 |
| YbYSiO5 | 0.875 | 3.918 |
| Compound | (Re2SiO5) (g·cm−3) | (Re2SiO5) (wt.%) | (SiO2) (wt.%) | (g·cm−3) | d (%) | (%) |
|---|---|---|---|---|---|---|
| Yb2SiO5 | 7.28 | 86.77 | 13.23 | 7.12 | 97.80 | 2.20 |
| YbEuSiO5 | 5.76 | 81.57 | 18.43 | 5.49 | 95.31 | 4.69 |
| YbScSiO5 | 6.69 | 86.13 | 13.87 | 6.40 | 95.66 | 4.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Wang, T.; Li, K.; Wang, W.; Liu, Y.; Yang, T. Investigation on the Thermal–Mechanical Properties of YbRESiO5 (RE = Yb, Eu, Gd, Ho, Tm, Lu, Y, Sc): First-Principles Calculations and Thermal Performance Experiments. Coatings 2024, 14, 1035. https://doi.org/10.3390/coatings14081035
Yang S, Wang T, Li K, Wang W, Liu Y, Yang T. Investigation on the Thermal–Mechanical Properties of YbRESiO5 (RE = Yb, Eu, Gd, Ho, Tm, Lu, Y, Sc): First-Principles Calculations and Thermal Performance Experiments. Coatings. 2024; 14(8):1035. https://doi.org/10.3390/coatings14081035
Chicago/Turabian StyleYang, Shilong, Tianying Wang, Kaibin Li, Weize Wang, Yangguang Liu, and Ting Yang. 2024. "Investigation on the Thermal–Mechanical Properties of YbRESiO5 (RE = Yb, Eu, Gd, Ho, Tm, Lu, Y, Sc): First-Principles Calculations and Thermal Performance Experiments" Coatings 14, no. 8: 1035. https://doi.org/10.3390/coatings14081035




