Mechanism of Ag-SiO2-TiO2 Nanocomposite Coating Formation on NiTi Substrate for Enhanced Functionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial Materials
2.2. Ag-SiO2-TiO2 Coatings Production
2.3. Method of Testing
3. Results and Discussion
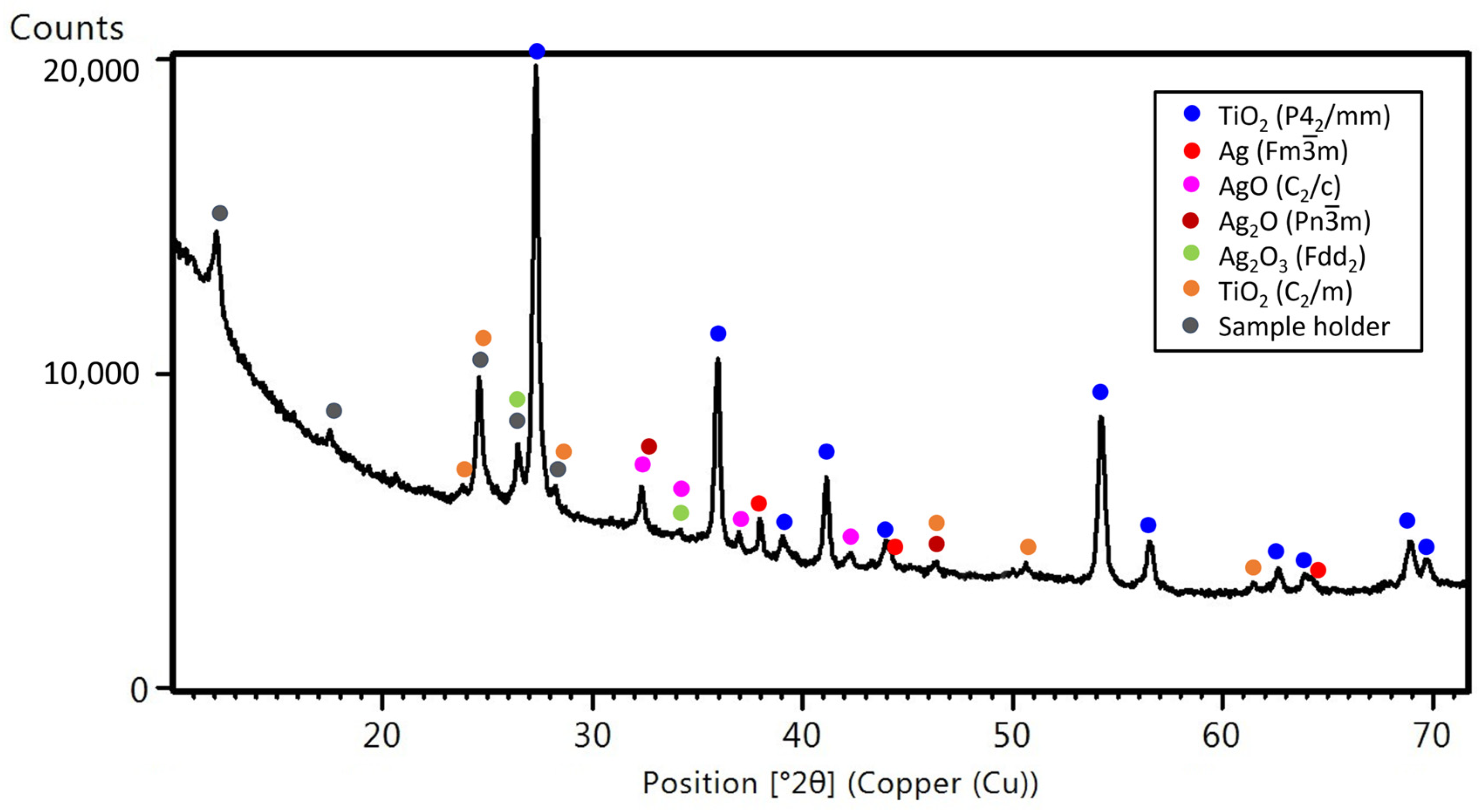
3.1. The Influence of Deposition Conditions on the Microstructure and Structure of the Coatings
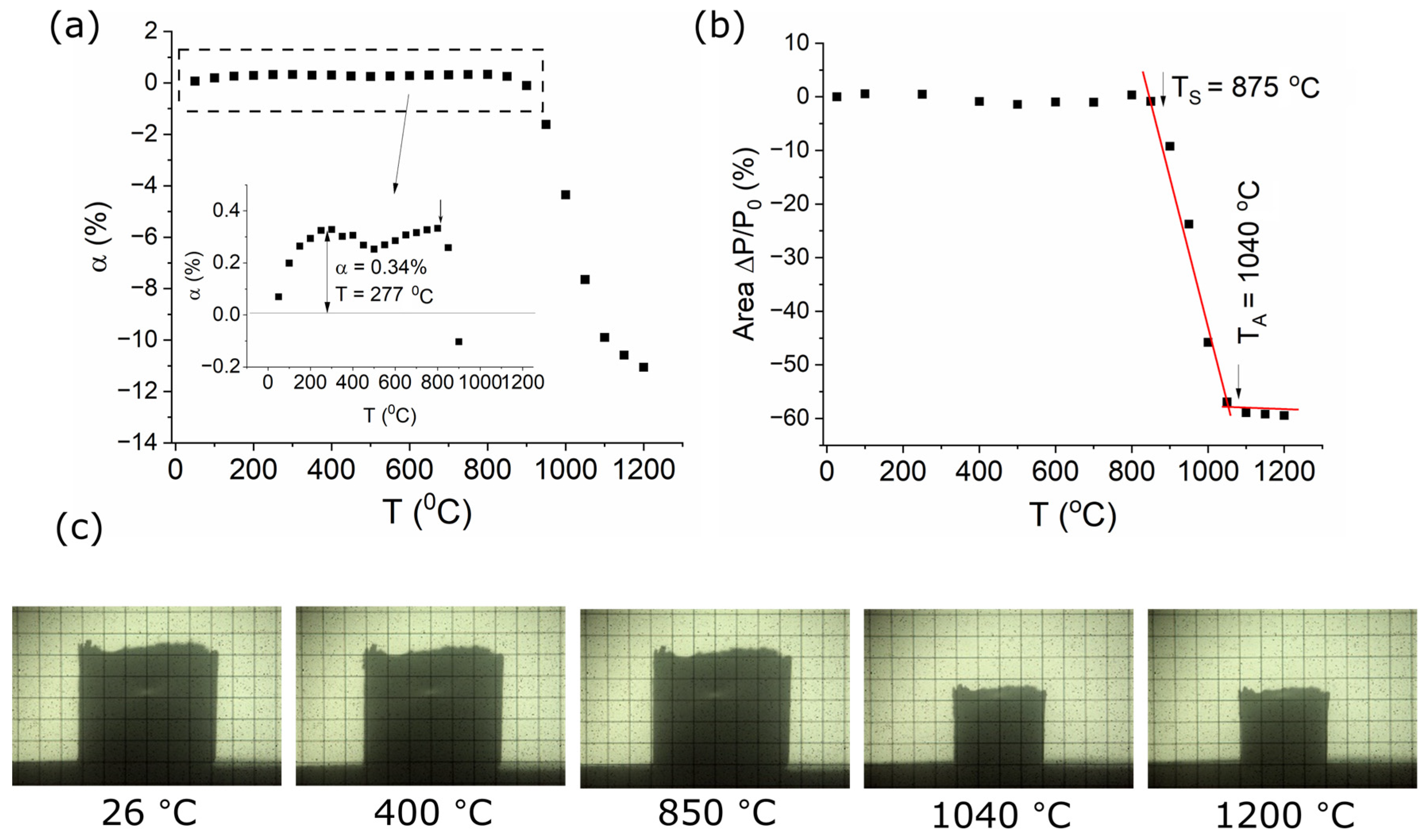
3.2. Thermal Behavior of the Ag-SiO2-TiO2 Nanocomposite
3.3. The Influence of Heating Conditions on the Morphology and Structure of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murthy, S.K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Haq Khan, Z.U.; Khan, T.M.; Khan, A.; Shah, N.S.; Muhammad, N.; Tahir, K.; Iqbal, J.; Rahim, A.; Khasim, S.; Ahmad, I.; et al. Brief Review: Applications of Nanocomposite in Electrochemical Sensor and Drugs Delivery. Front. Chem. 2023, 11, 1152217. [Google Scholar] [CrossRef]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bhatia, R.; Webster, T.J. Atomic Layer Deposition of Nano-TiO2 Thin Films with Enhanced Biocompatibility and Antimicrobial Activity for Orthopedic Implants. Int. J. Nanomed. 2017, 12, 8711–8723. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Stróż, A.; Osak, P.; Maszybrocka, J.; Gerle, A.; Dudek, K.; Balin, K.; Łukowiec, D.; Gawlikowski, M.; Bogunia, S. Production, Characterization and Application of Oxide Nanotubes on Ti–6Al–7Nb Alloy as a Potential Drug Carrier. Materials 2021, 14, 6142. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mazinani, A.; Barati, M.R.; Losic, D. Engineered Titanium Implants for Localized Drug Delivery: Recent Advances and Perspectives of Titania Nanotubes Arrays. Expert. Opin. Drug Deliv. 2018, 15, 1021–1037. [Google Scholar] [CrossRef]
- Shaban, M.; Hasanzadeh, M. Biomedical Applications of Dendritic Fibrous Nanosilica (DFNS): Recent Progress and Challenges. RSC Adv. 2020, 10, 37116–37133. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of New Antibacterial Composite Coating for Titanium Based on Highly Ordered Nanoporous Silica and Silver Nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 146–153. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles-A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chen, J.; Nugen, S.R.; Goddard, J.M. Hybrid Antifouling and Antimicrobial Coatings Prepared by Electroless Co-Deposition of Fluoropolymer and Cationic Silica Nanoparticles on Stainless Steel: Efficacy against Listeria Monocytogenes. ACS Appl. Mater. Interfaces 2016, 8, 15926–15936. [Google Scholar] [CrossRef] [PubMed]
- Zarys Technologii Materiałów Ogniotrwałych/Franciszek Nadachowski.|Biblioteka Główna. Available online: https://bg.pcz.pl/apiszb/book/3137/Zarys-technologii-materialow-ogniotrwalych-Franciszek-Nadachowski (accessed on 13 July 2024).
- Dudek, K.; Dulski, M.; Łosiewicz, B. Functionalization of the NiTi Shape Memory Alloy Surface by HAp/SiO2/Ag Hybrid Coatings Formed on SiO2-TiO2 Glass Interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and Their Biomedical Applications—A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Polepalli, L.; Chowdhury, S.; Carr, M.A.; Janorkar, A.V.; Marquart, M.E.; Griggs, J.A.; Bumgardner, J.D.; Roach, M.D. Silver-Doped Titanium Oxide Layers for Improved Photocatalytic Activity and Antibacterial Properties of Titanium Implants. J. Funct. Biomater. 2024, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Dulski, M.; Dudek, K.; Chalon, D.; Kubacki, J.; Sulowicz, S.; Piotrowska-Seget, Z.; Mrozek-Wilczkiewicz, A.; Gawecki, R.; Nowak, A. Toward the Development of an Innovative Implant: NiTi Alloy Functionalized by Multifunctional β-TCP + Ag/SiO2 Coatings. ACS Appl. Bio Mater. 2019, 2, 987–998. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Petrini, L.; Migliavacca, F. Biomedical Applications of Shape Memory Alloys. J. Metall. 2011, 2011, 501483. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; der Biest, O.V.; Clasen, R.; Uchikoshi, T. Electrophoretic Deposition: Fundamentals and Applications III; Trans Tech Publications Ltd.: Bäch, Switzerland, 2009; ISBN 978-3-03813-267-7. [Google Scholar]
- Sarkar, P.; De, D.; Uchikochi, T.; Besra, L. Electrophoretic Deposition (EPD): Fundamentals and Novel Applications in Fabrication of Advanced Ceramic Microstructures. In Electrophoretic Deposition of Nanomaterials; Dickerson, J.H., Boccaccini, A.R., Eds.; Springer: New York, NY, USA, 2012; pp. 181–215. ISBN 978-1-4419-9730-2. [Google Scholar]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic Deposition of Biomaterials. J. R. Soc. Interface 2010, 7 (Suppl. 5), S581–S613. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Gerle, A.; Rawicka, P. Functionalization of the NiTi Shape Memory Alloy Surface through Innovative Hydroxyapatite/Ag-TiO2 Hybrid Coatings. Materials 2024, 17, 604. [Google Scholar] [CrossRef]
- Dulski, M.; Gawecki, R.; Sułowicz, S.; Cichomski, M.; Kazek-Kęsik, A.; Wala, M.; Leśniak-Ziółkowska, K.; Simka, W.; Mrozek-Wilczkiewicz, A.; Gawęda, M.; et al. Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Rawicka, P. Optimization of the Electrophoretic Deposition Parameters and Mechanism of Formation of Ag-TiO2 Nanocoatings on a NiTi Shape Memory Alloy: Part I. Coatings 2024, 14, 44. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Gerle, A.; Rawicka, P. Synthesis and Characterization of Silica-Titanium Oxide Nano-Coating on NiTi Alloy. Coatings 2024, 14, 391. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Wong, J. A Review of Infrared Spectroscopic Studies of Vapor-Deposited Dielectric Glass Films on Silicon. J. Electron. Mater. 1976, 5, 113–160. [Google Scholar] [CrossRef]
- Pai, P.G.; Chao, S.S.; Takagi, Y.; Lucovsky, G. Infrared Spectroscopic Study of SiOx Films Produced by Plasma Enhanced Chemical Vapor Deposition. J. Vac. Sci. Technol. A 1986, 4, 689–694. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 Nanostructures: A Review of Efficient Synthesis, Growth Mechanism, Probing Capabilities, and Applications in Bio-Safety and Health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef]
- Kadam, A.N.; Dhabbe, R.S.; Kokate, M.R.; Gaikwad, Y.B.; Garadkar, K.M. Preparation of N Doped TiO2 via Microwave-Assisted Method and Its Photocatalytic Activity for Degradation of Malathion. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 133, 669–676. [Google Scholar] [CrossRef]
- Kil, H.-S.; Jung, Y.-J.; Moon, J.-I.; Song, J.-H.; Lim, D.-Y.; Cho, S.-B. Glycothermal Synthesis and Photocatalytic Properties of Highly Crystallized Anatase TiO2 Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 6193–6200. [Google Scholar] [CrossRef]
- Ricchiardi, G.; Damin, A.; Bordiga, S.; Lamberti, C.; Spanò, G.; Rivetti, F.; Zecchina, A. Vibrational Structure of Titanium Silicate Catalysts. A Spectroscopic and Theoretical Study. J. Am. Chem. Soc. 2001, 123, 11409–11419. [Google Scholar] [CrossRef] [PubMed]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite Characterization. 1. Structure, Adsorptive Capacity, and IR Spectroscopy of the Framework and Hydroxyl Modes. J. Phys. Chem. 1992, 96, 4985–4990. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite Characterization. 2. IR Spectroscopy of the Interaction of Carbon Monoxide with Internal and External Hydroxyl Groups. J. Phys. Chem. 1992, 96, 4991–4997. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Scarano, D.; Petrini, G.; Leofanti, G.; Padovan, M.; Areàn, C.O. Low-Temperature Fourier-Transform Infrared Investigation of the Interaction of CO with Nanosized ZSM5 and Silicalite. J. Chem. Soc. Faraday Trans. 1992, 88, 2959–2969. [Google Scholar] [CrossRef]
- Bordiga, S.; Ugliengo, P.; Damin, A.A.; Lamberti, C.; Spoto, G.; Zecchina, A.; Spanò, G.; Buzzoni, R.; Dalloro, L.; Rivetti, F. Hydroxyls Nests in Defective Silicalites and Strained Structures Derived upon Dehydroxylation: Vibrational Properties and Theoretical Modelling. Top. Catal. 2001, 15, 43–52. [Google Scholar] [CrossRef]
- Larouche, S.; Szymanowski, H.; Klemberg-Sapieha, J.E.; Martinu, L.; Gujrathi, S.C. Microstructure of Plasma-Deposited SiO2/TiO2 Optical Films. J. Vac. Sci. Technol. A 2004, 22, 1200–1207. [Google Scholar] [CrossRef]
- de Man, A.J.M.; Sauer, J. Coordination, Structure, and Vibrational Spectra of Titanium in Silicates and Zeolites in Comparison with Related Molecules. An Ab Initio Study. J. Phys. Chem. 1996, 100, 5025–5034. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman Spectra of Titanium Dioxide. J. Solid. State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Ocaña, M.; García-Ramos, J.V.; Serna Pereda, C.J. Low-Temperature Nucleation of Rutile Observed by Raman Spectroscopy during Crystallization of TiO2. J. Am. Ceram. Soc. 1992, 75, 2010–2012. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Li Bassi, A.; Bottani, C.E.; Ducati, C. Raman Spectroscopy Characterization of TiO2 Rutile Nanocrystals. Phys. Rev. B 2007, 75, 045416. [Google Scholar] [CrossRef]
- Zhang, Y.; Harris, C.X.; Wallenmeyer, P.; Murowchick, J.; Chen, X. Asymmetric Lattice Vibrational Characteristics of Rutile TiO2 as Revealed by Laser Power Dependent Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Parker, J.C.; Siegel, R.W. Calibration of the Raman Spectrum to the Oxygen Stoichiometry of Nanophase TiO2. Appl. Phys. Lett. 1990, 57, 943–945. [Google Scholar] [CrossRef]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Naumov, V.; Fedorenko, L.; Kshnyakin, V.; Baran, J. Laser-Excited Excitonic Luminescence of Nanocrystalline TiO2 Powder. Ukr. J. Phys. 2014, 59, 246. [Google Scholar] [CrossRef]
- Wahlbeck, P.G.; Gilles, P.W. Reinvestigation of the Phase Diagram for the System Titanium–Oxygen. J. Am. Ceram. Soc. 1966, 49, 180–183. [Google Scholar] [CrossRef]
- Zahornyi, M.M.; Tyschenko, N.I.; Lobunet, T.F.; Kolomys, O.F.; Strelchuk, V.V.; Naumenko, K.S.; Biliavska, L.; Zahorodnia, S.; Kharchuk, M.; Skoryk, S.; et al. The Effect of Ag Content on the Structural, Optical, and Cytotoxicity Properties of TiO2 Nanopowders Grown from TiO(OH)2 Precursor by the Chemical Deposition Method. J. Nano-Electron. Phys. 2021, 19, 06009. [Google Scholar] [CrossRef]
- Pettinger, B.; Bao, X.; Wilcock, I.C.; Muhler, M.; Ertl, G. Surface-Enhanced Raman Scattering from Surface and Subsurface Oxygen Species at Microscopically Well-Defined Ag Surfaces. Phys. Rev. Lett. 1994, 72, 1561–1564. [Google Scholar] [CrossRef]
- Bao, X.; Muhler, M.; Pettinger, B.; Schlögl, R.; Ertl, G. On the Nature of the Active State of Silver during Catalytic Oxidation of Methanol. Catal. Lett. 1993, 22, 215–225. [Google Scholar] [CrossRef]
- Millar, G.J.; Metson, J.B.; Bowmaker, G.A.; Cooney, R.P. In Situ Raman Studies of the Selective Oxidation of Methanol to Formaldehyde and Ethene to Ethylene Oxide on a Polycrystalline Silver Catalyst. J. Chem. Soc. Faraday Trans. 1995, 91, 4149–4159. [Google Scholar] [CrossRef]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium Oral Implants: Surface Characteristics, Interface Biology and Clinical Outcome. J. R. Soc. Interface 2010, 7 (Suppl. 5), S515–S527. [Google Scholar] [CrossRef]
- Gaintantzopoulou, M.; Zinelis, S.; Silikas, N.; Eliades, G. Micro-Raman Spectroscopic Analysis of TiO2 Phases on the Root Surfaces of Commercial Dental Implants. Dent. Mater. 2014, 30, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.-T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the Surface Oxides on Turned and Electrochemically Oxidized Pure Titanium Implants up to Dielectric Breakdown: The Oxide Thickness, Micropore Configurations, Surface Roughness, Crystal Structure and Chemical Composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Lausmaa, J.; Kasemo, B.; Mattsson, H. Surface Spectroscopic Characterization of Titanium Implant Materials. Appl. Surf. Sci. 1990, 44, 133–146. [Google Scholar] [CrossRef]
- Chligui, M.; Guimbretiere, G.; Canizares, A.; Matzen, G.; Vaills, Y.; Simon, P. New Features in the Raman Spectrum of Silica: Key-Points in the Improvement on Structure Knowledge 2010. Available online: https://hal.science/hal-00520823/ (accessed on 24 September 2010).
- Mali, S.S.; Betty, C.A.; Bhosale, P.N.; Patil, P.S. Hydrothermal Synthesis of Rutile TiO2 with Hierarchical Microspheres and Their Characterization. CrystEngComm 2011, 13, 6349–6351. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Liu, J.; Chen, Q.; Zhu, X.; Liang, C. Carbon-Encapsulated Metal/Metal Carbide/Metal Oxide Core–Shell Nanostructures Generated by Laser Ablation of Metals in Organic Solvents. ACS Appl. Nano Mater. 2019, 2, 28–39. [Google Scholar] [CrossRef]
- Deng, Y.; Sheng, G.; Xu, C. Evaluation of the Microstructure and Mechanical Properties of Diffusion Bonded Joints of Titanium to Stainless Steel with a Pure Silver Interlayer. Mater. Des. (1980–2015) 2013, 46, 84–87. [Google Scholar] [CrossRef]






| TiO2 | a0 [Å] | c0 [Å] | Ti-O (x4) | Ti-O (x2) | Ti-O (Average) |
|---|---|---|---|---|---|
| Standard (NIST SRM 674b) | 4.5940 | 2.9589 | 1.9498 | 1.9783 | 1.9593 |
| After EPD | 4.5950(2) | 2.9583(2) | 1.9554 | 1.9703 | 1.9604 |
| After heat treatment | 4.5933(4) | 2.9589(4) | 1.9697 | 1.9476 | 1.9623 |
| Theoretical [29] | 1.9590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Rawicka, P. Mechanism of Ag-SiO2-TiO2 Nanocomposite Coating Formation on NiTi Substrate for Enhanced Functionalization. Coatings 2024, 14, 1055. https://doi.org/10.3390/coatings14081055
Dudek K, Dulski M, Podwórny J, Kujawa M, Rawicka P. Mechanism of Ag-SiO2-TiO2 Nanocomposite Coating Formation on NiTi Substrate for Enhanced Functionalization. Coatings. 2024; 14(8):1055. https://doi.org/10.3390/coatings14081055
Chicago/Turabian StyleDudek, Karolina, Mateusz Dulski, Jacek Podwórny, Magdalena Kujawa, and Patrycja Rawicka. 2024. "Mechanism of Ag-SiO2-TiO2 Nanocomposite Coating Formation on NiTi Substrate for Enhanced Functionalization" Coatings 14, no. 8: 1055. https://doi.org/10.3390/coatings14081055






