Facile Preparation of Smart Sponge Based on a Zeolitic Imidazolate Framework for the Efficient Separation of Oily Wastewater
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of ZIF-8/APTES/MS
2.3. Adsorption Experiments for Oil and Organic Solvent
2.4. Recyclability and Reusability Tests
2.5. Oil/Water Separation
2.6. Emulsion Separation
2.7. Characterization
3. Results and Discussion
3.1. Characterization of ZIF-8/APTES/MS Composites
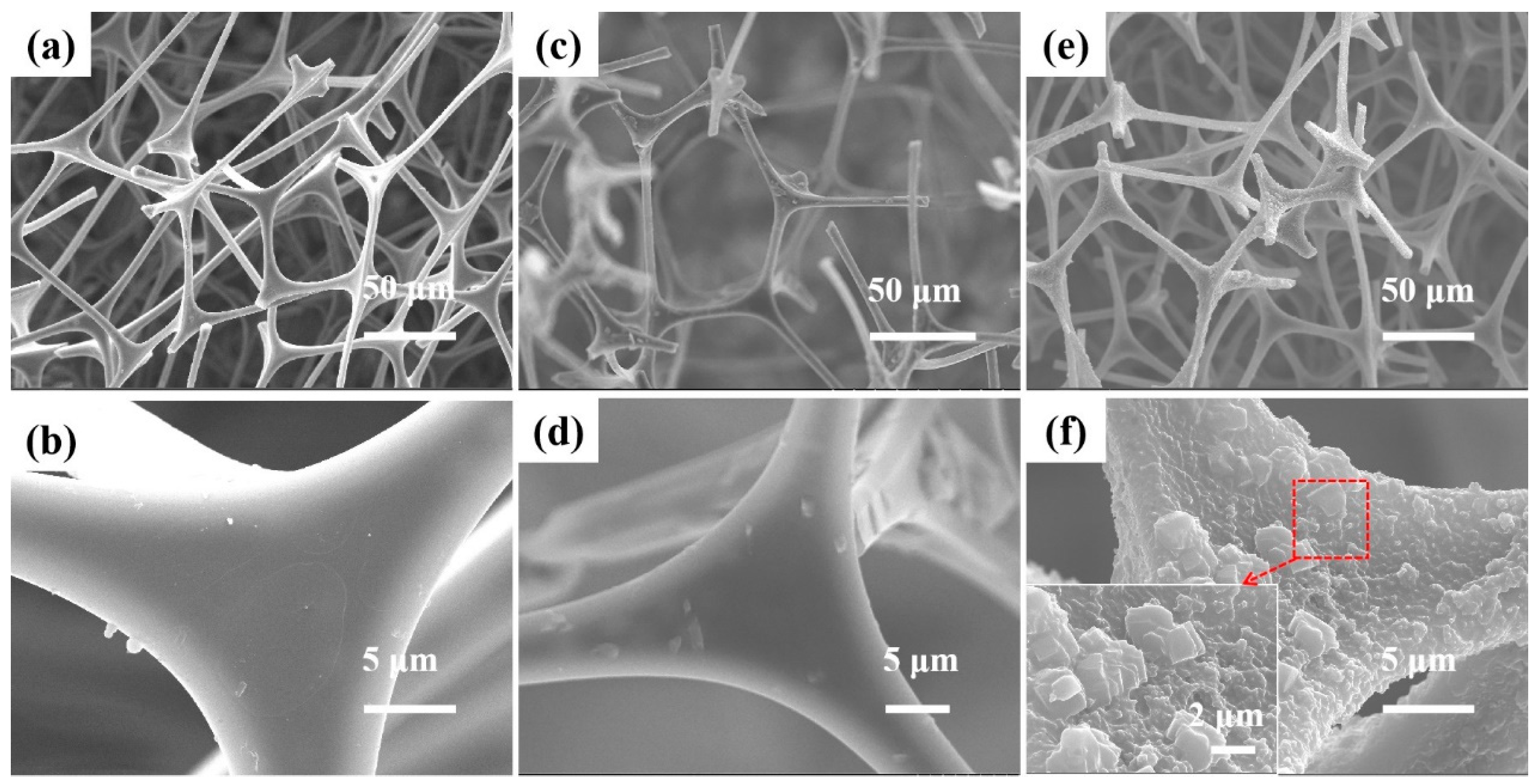
3.1.1. SEM Analysis
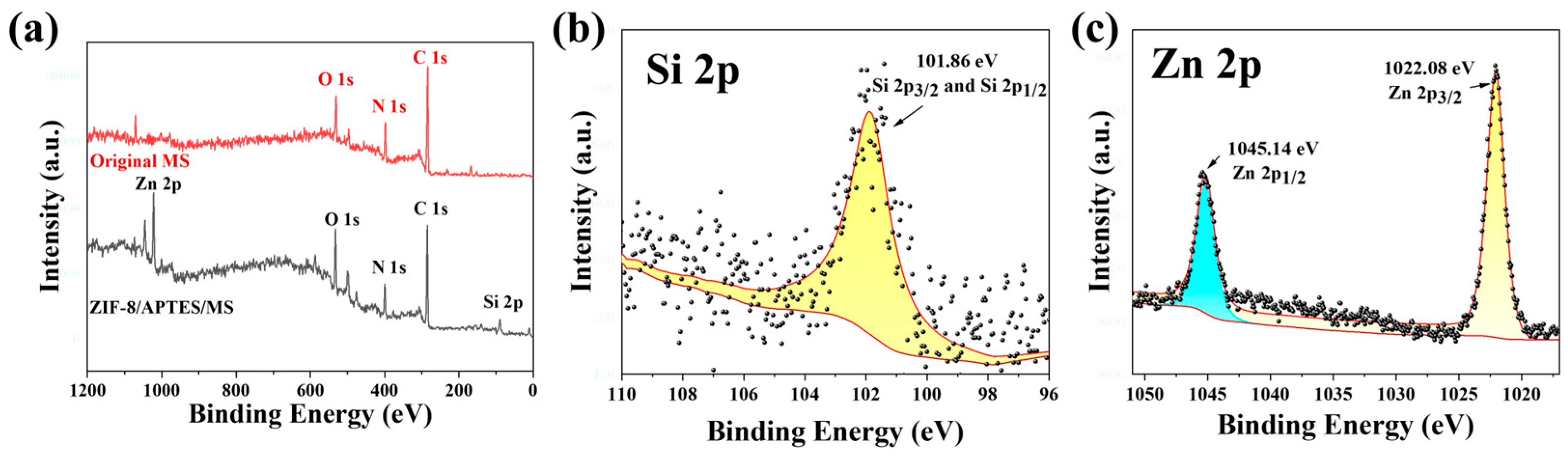
3.1.2. XPS Analysis
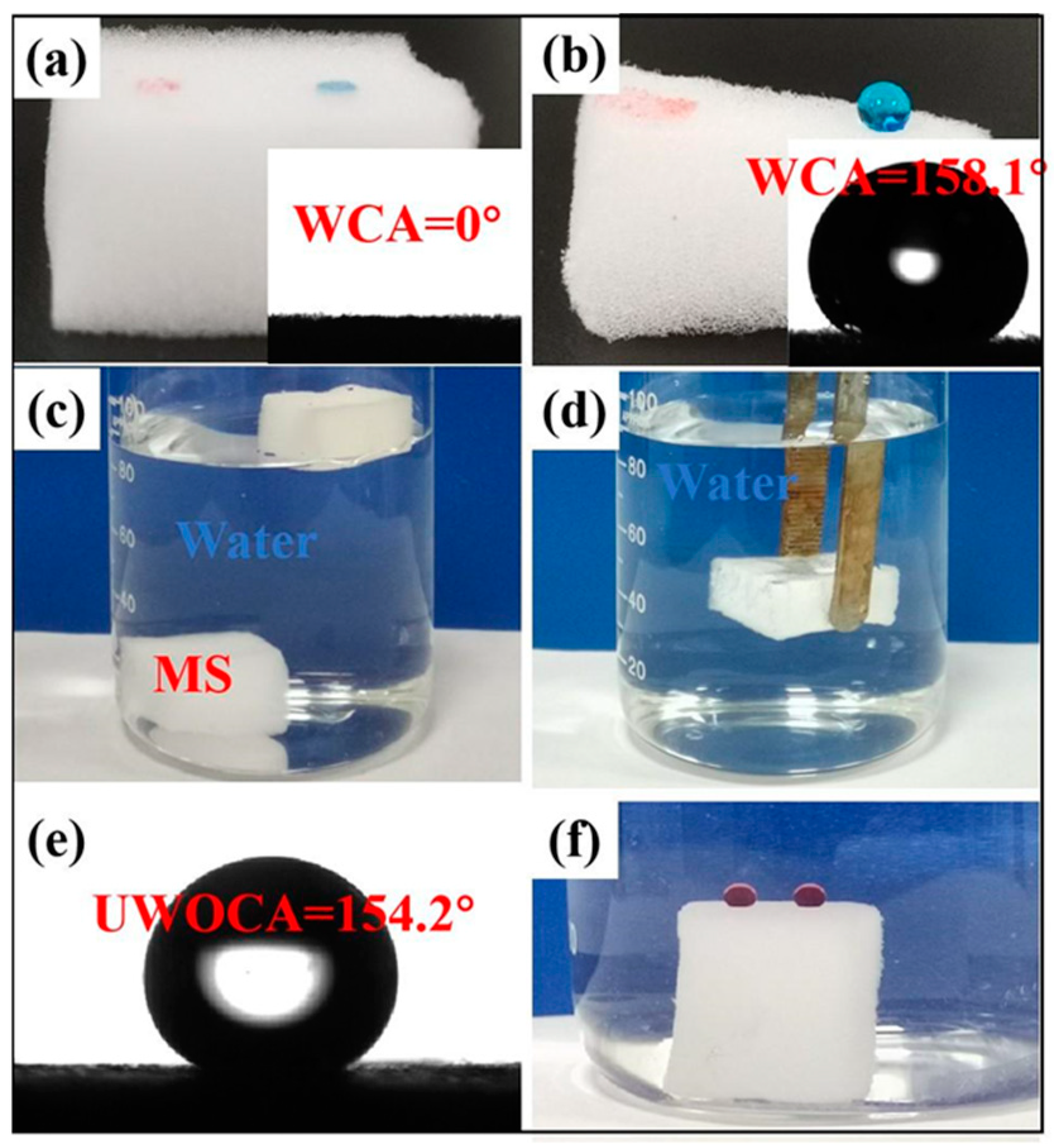
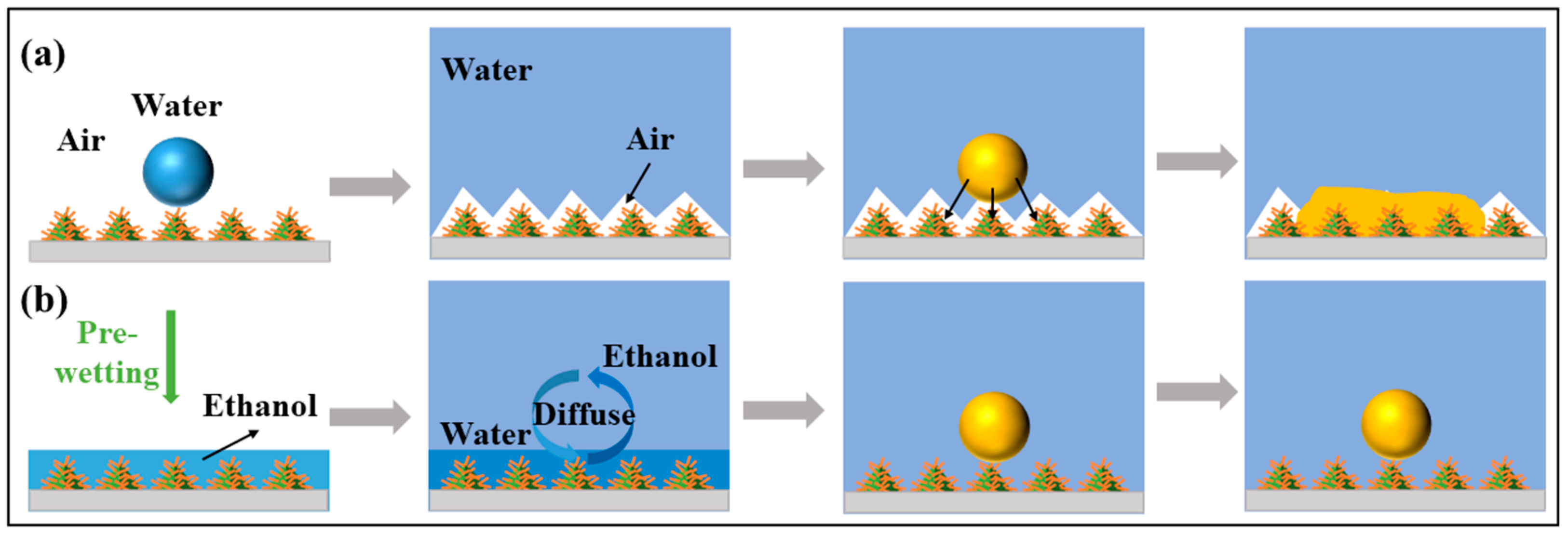
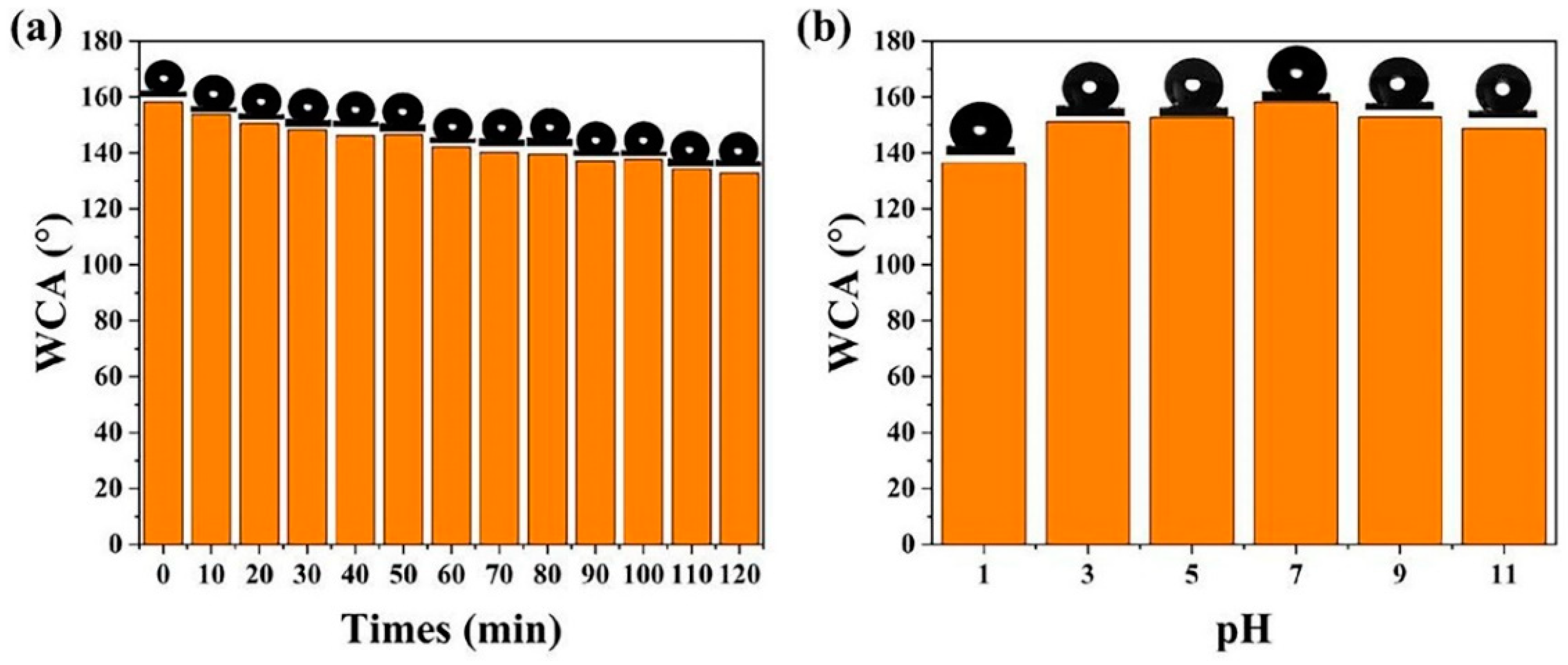
3.2. Surface Wettability Analysis and Antifouling Properties
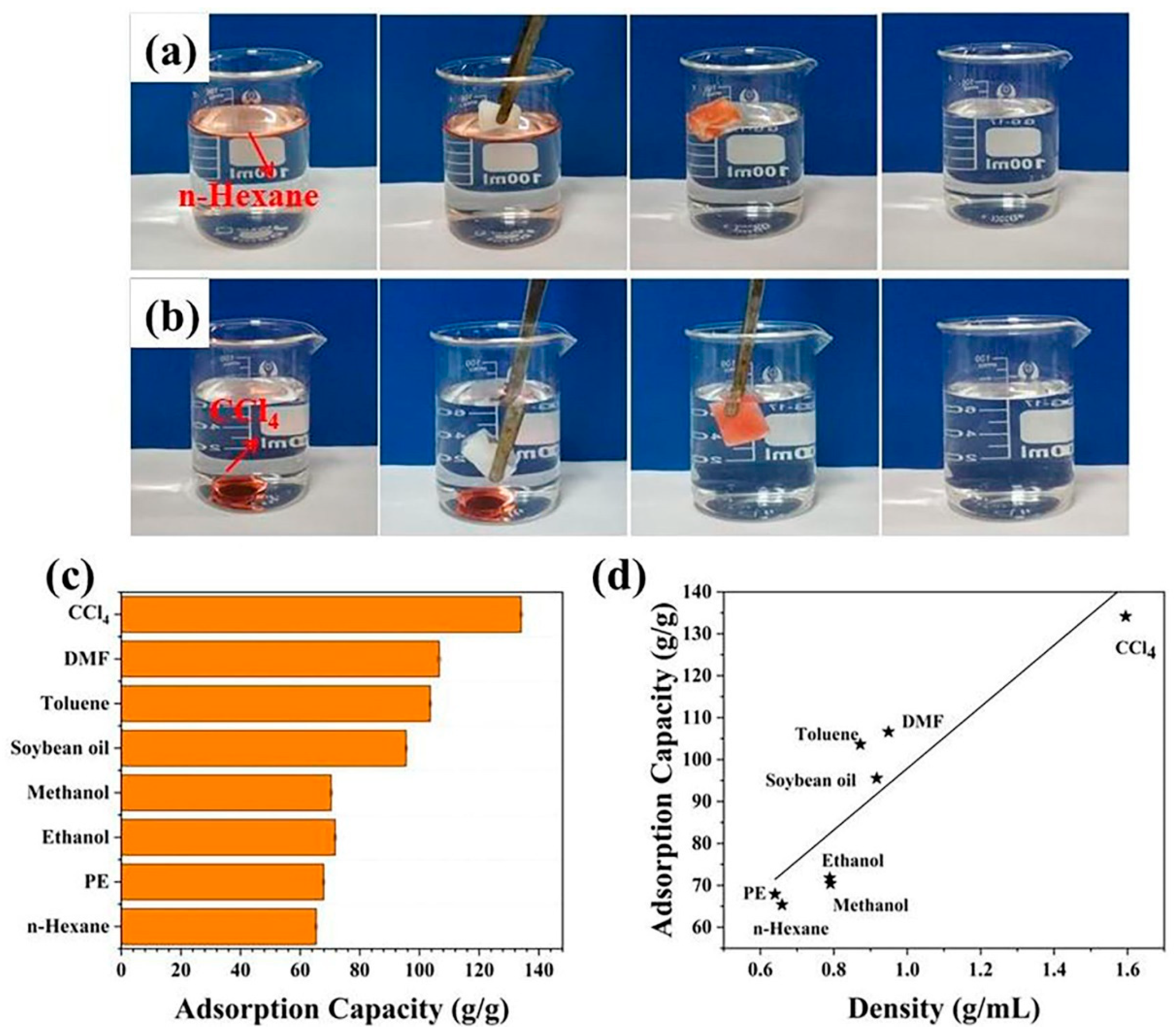
3.3. Adsorption of Oil and Organic Solvents and Oil/Water Separation Properties
3.4. Emulsion Separation
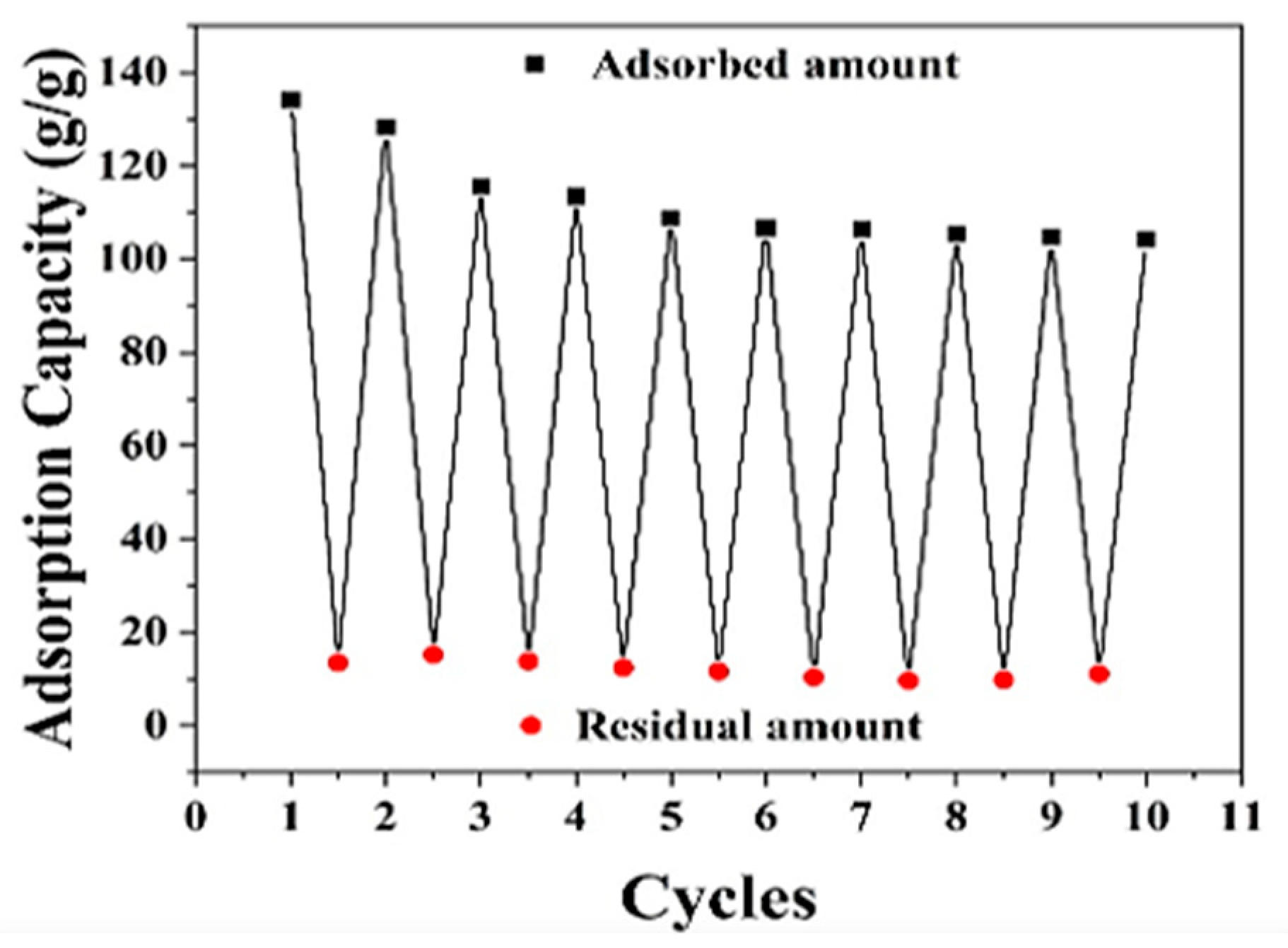
3.5. Durability and Chemical Stability
3.6. Flame Retardancy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Guan, M.; Yang, X.; Li, H.; Zhao, Y.; Chen, Y. A Trifecta membrane modified by multifunctional superhydrophilic coating for oil/water separation and simultaneous absorption of dyes and heavy metal. Sep. Purifi. Technol. 2024, 333, 125904. [Google Scholar] [CrossRef]
- Zhu, N.X.; Wei, Z.W.; Chen, C.X.; Wang, D.; Cao, C.C.; Qiu, Q.F.; Jiang, J.J.; Wang, H.P.; Su, C.Y. Self-generation of surface roughness by low-surface-energy alkyl chains for highly stable superhydrophobic/superoleophilic MOFs with multiple functionalities. Angew. Chem. Int. Ed. 2019, 58, 17033–17040. [Google Scholar] [CrossRef]
- Zhang, N.; Qi, Y.F.; Zhang, Y.N.; Luo, J.L.; Cui, P.; Jiang, W. A Review on the Oil/Water Mixture Separation Material. Ind. Eng. Chem. Res. 2020, 59, 14546–14568. [Google Scholar] [CrossRef]
- Shome, A.; Moses, J.C.; Rather, A.M.; Mandal, B.B.; Manna, U. Unconventional and Facile Fabrication of Chemically Reactive Silk Fi-broin Sponges for Environmental Remediation. ACS Appl. Mater. Interfaces 2021, 13, 24258–24271. [Google Scholar] [CrossRef] [PubMed]
- Shome, A.; Maji, K.; Rather, A.M.; Yashwanth, A.; Patel, D.K.; Manna, U. A Scalable Chemical Approach for the Synthesis of a Highly Tolerant and Efficient Oil Absorbent. Chem. Asian J. 2019, 14, 4732–4740. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Guan, M.; Li, H.; Guo, J.; Shi, H.; Li, S. Triple-defense design of multifunctional superhydrophilic surface with outstanding underwater superoleophobicity, anti-oil-fouling properties, and high transparency. J. Membr. Sci. 2024, 689, 122161. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Zhu, L.; Liu, H.; Yu, S.; Xue, J.; Zhu, X.; Xue, Q. Reusable membrane with multifunctional skin layer for effective removal of insoluble emulsified oils and soluble dyes. J. Hazard. Mater. 2021, 415, 125677–125686. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, Y.; Wang, T.; Zhang, J.; Cui, Z.; Guo, J.; Li, S.; Chen, Y. Janus membranes with asymmetric superwettability for high-performance and long-term on-demand oil/water emulsion separation. ACS Appl. Mater. Interfaces 2024, 16, 15558–15568. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.; Luo, Y.; Wu, R.; Fang, R.; Umar, A.; Zhang, Z.; Zhao, Z.; Yao, J.; Zhao, S. Superhydrophobic MOF based materials and their applications for oil-water separation. J. Clean. Prod. 2023, 420, 138347. [Google Scholar] [CrossRef]
- Narayan Thorat, B.; Kumar Sonwani, R. Current technologies and future perspectives for the treatment of complex petroleum refinery wastewater: A review. Bioresour. Technol. 2022, 355, 127263. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.D.; Yu, F.Y.; Zhang, H. In-situ construction of MOFs-based superhydrophobic/superoleophilic coating on filter paper with self-cleaning and antibacterial activity for efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126976. [Google Scholar] [CrossRef]
- Du, J.C.; Zhou, C.L.; Yang, Z.J.; Cheng, J.; Shen, Y.; Zeng, X.; Tan, L.; Dong, L. Conversion of solid Cu2(OH)2CO3 into HKUST-1 metal-organic frameworks: Toward an under-liquid superamphiphobic surface. Surf. Coat. Technol. 2019, 363, 282–290. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Mu, S.; Jiang, C.; Song, M.; Fang, Q.; Xue, M.; Qiu, S.; Chen, B. UiO-66-Coated mesh membrane with underwater superoleophobicity for high-efficiency oil-water separation. ACS Appl. Mater. Interfaces 2018, 10, 17301–17308. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Sun, Y.; Guo, Z. Designing novel superwetting surfaces for high-efficiency oil–water separation: Design principles, opportunities, trends and challenges. J. Mater. Chem. A 2020, 8, 16831–16853. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and underwater superoleophobic membranes—A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166–101205. [Google Scholar] [CrossRef]
- Dong, X.X.; Cui, M.; Huang, R.L.; Su, R.X.; Qi, W.; He, Z.M. Polydopamine-assisted surface coating of MIL-53 and dodecanethiol on a melamine sponge for oil–water separation. Langmuir 2020, 36, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Tamsilian, Y.; Ansari-Asl, Z.; Maghsoudian, A.; Abadshapoori, A.K.; Agirre, A.; Tomovska, R. Superhydrophobic ZIF8/PDMS-coated polyurethane nanocomposite sponge: Synthesis, characterization and evaluation of organic pollutants continuous separation. J. Taiwan Inst. Chem. Eng. 2021, 125, 204–214. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, N.; Zhou, S.; XLv, i.; Yang, C.; Chen, W.; Hu, Y.; Jiang, W. Facile preparation of ZIF-67 coated melamine sponge for efficient oil/water separation. Ind. Eng. Chem. Res. 2019, 58, 17380–17388. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Hou, S.Y.; Song, H.L.; Qin, G.W.; Li, P.; Zhang, K.; Li, T.; Han, L.; Liu, W.; Ji, S. A green and facile one-step hydration method based on ZIF-8-PDA to prepare melamine composite sponges with excellent hydrophobicity for oil-water separation. J. Hazard. Mater. 2023, 451, 131064–131083. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhang, Y.P.; Du, H.L.; Wan, L.; Zuo, C.-G.; Qu, L.-B. “Two birds with one stone” strategy for oil/water separation based on Ni foams assembled using metal–organic frameworks. New J. Chem. 2023, 47, 14289–14296. [Google Scholar] [CrossRef]
- Xiang, W.; Guo, Z. Nonflammable, robust and recyclable hydrophobic zeolitic imidazolate frameworks/sponge with high oil absorption capacity for efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129570. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, X.; Feng, S.Y. Nonflammable and magnetic sponge decorated with polydimethylsiloxane brush for multitasking and highly efficient oil-water separation. Adv. Funct. Mater. 2019, 29, 1902488–1902500. [Google Scholar] [CrossRef]
- Li, X.F.; Yan, B.Y.; Huang, W.Q.; Bian, H.; Wang, X.Y.; Zhu, J.; Dong, S.; Wang, Y.; Chen, W. Room-temperature synthesis of hydrophobic/oleophilic ZIF-90-CF3/melamine foam composite for the efficient removal of organic compounds from wastewater. Chem. Eng. J. 2022, 428, 132501–132514. [Google Scholar] [CrossRef]
- Cao, M.J.; Feng, Y.; Chen, Q.; Zhang, P.C.; Guo, S.; Yao, J. Flexible Co-ZIF-L@melamine sponge with underwater superoleophobicity for water/oil separation. Mater. Chem. Phys. 2020, 241, 122385–122390. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhao, X.T.; Jia, N.; Cheng, L.; Liu, L.; Gao, C. Superwetting oil/water separation membrane constructed from in situ assembled metal-phenolic networks and metal-organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 10000–10008. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.H.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Nanofibrous metal-organic framework composite membrane for selective efficient oil/water emulsion separation. J. Membr. Sci. 2017, 543, 10–17. [Google Scholar] [CrossRef]
- Huang, A.S.; Bux, H.; Steinbach, F.; Caro, J. Molecular-sieve membrane with hydrogen permselectivity: ZIF-22 in LTA topology prepared with 3-aminopropyltriethoxysilane as covalent linker. Angew. Chem. Int. Ed. 2010, 49, 4958–4961. [Google Scholar] [CrossRef]
- Wang, J.Q.; Xu, J.K.; Chen, G.J.; Lian, Z.X.; Yu, H.D. Reversible wettability between underwater superoleophobicity and superhydrophobicity of stainless steel mesh for efficient oil–water separation. ACS Omega 2020, 6, 77–84. [Google Scholar] [CrossRef]
- Li, Q.Q.; Deng, W.J.; Li, C.H.; Sun, Q.; Huang, F.; Zhao, Y.; Li, S. High-flux oil/water separation with interfacial capillary effect in switchable superwetting Cu(OH)2@ZIF-8 nanowire membranes. ACS Appl. Mater. Interfaces 2018, 10, 40265–40273. [Google Scholar] [CrossRef]
- Yang, B.H.; Shi, M.B.; Huang, R.L.; Qi, W.; Su, R.; He, Z. One-pot synthesis of fluorine functionalized Zr-MOFs and their in situ growth on sponge for oil absorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126322–126332. [Google Scholar] [CrossRef]
- Azam, T.; Pervaiz, E.; Farrukh, S.; Noor, T. Biomimetic highly hydrophobic stearic acid functionalized MOF sponge for efficient oil/water separation. Mater. Res. Express 2021, 8, 015019–015034. [Google Scholar] [CrossRef]
- Azam, T.; Pervaiz, E.; Javed, S.; Amina, S.J.; Khalid, M.S. Tuning the hydrophobicity of MOF sponge for efficient oil/water separation. Chem. Phys. Impact 2020, 1, 100001–100010. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, L.; Nagamine, S.; Ohshima, M. Study oil/water separation property of PE foam and its Improvement by in situ synthesis of zeolitic–imidazolate framework (ZIF-8). Polym. Eng. Sci. 2019, 59, 1354–1361. [Google Scholar] [CrossRef]
- Zhang, L.R.; Xie, J.L.; Luo, X.; Gong, X.B.; Zhu, M. Enhanced hydrophobicity of shell-ligand-exchanged ZIF-8/melamine foam for excellent oil-water separation. Chem. Eng. Sci. 2023, 273, 118663. [Google Scholar] [CrossRef]






| Solvent | Polarity | Surface Tension (mN/m) | UWOCA |
|---|---|---|---|
| Methanol | 6.6 | 23.6 | 151.7° |
| Ethanol | 4.3 | 22.39 | 154.2° |
| Acetonitrile | 6.2 | 22.75 | 153.1° |
| DMF | 7.87 | 25.7 | 150.6° |
| Adsorbents | WCA | Adsorption Capacity (g/g) | Ref. |
|---|---|---|---|
| ZIF-8/PDMS/PU | 156° | 42–58 | [18] |
| ZIF-8/APTES/MS | 130° | 76–164 | [20] |
| ZIF-8-PDA@MS | 162° | 85.45–168.95 | [22] |
| ZIF-8/SA/PU | 143.2° | 30.28–115.35 | [32] |
| ZIF-8/PU | 129.2° | 28–79 | [33] |
| ZIF-8/PE | - | 17.6–55.8 | [34] |
| ZIF-90/MS | 154° | 24–63 | [34] |
| ZIF-8/APTES/MS | 158.1° | 65.4–134.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, X.; Yuan, P.; Chen, H.; Li, S. Facile Preparation of Smart Sponge Based on a Zeolitic Imidazolate Framework for the Efficient Separation of Oily Wastewater. Coatings 2024, 14, 1058. https://doi.org/10.3390/coatings14081058
Zhang Y, Chen X, Yuan P, Chen H, Li S. Facile Preparation of Smart Sponge Based on a Zeolitic Imidazolate Framework for the Efficient Separation of Oily Wastewater. Coatings. 2024; 14(8):1058. https://doi.org/10.3390/coatings14081058
Chicago/Turabian StyleZhang, Yuping, Xinxin Chen, Pei Yuan, Haie Chen, and Songwei Li. 2024. "Facile Preparation of Smart Sponge Based on a Zeolitic Imidazolate Framework for the Efficient Separation of Oily Wastewater" Coatings 14, no. 8: 1058. https://doi.org/10.3390/coatings14081058
APA StyleZhang, Y., Chen, X., Yuan, P., Chen, H., & Li, S. (2024). Facile Preparation of Smart Sponge Based on a Zeolitic Imidazolate Framework for the Efficient Separation of Oily Wastewater. Coatings, 14(8), 1058. https://doi.org/10.3390/coatings14081058





