Bibliometric Study of Electrochemical Advanced Oxidation Processes (EAOPs) for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
Data Collection and Analysis
3. Results and Discussion
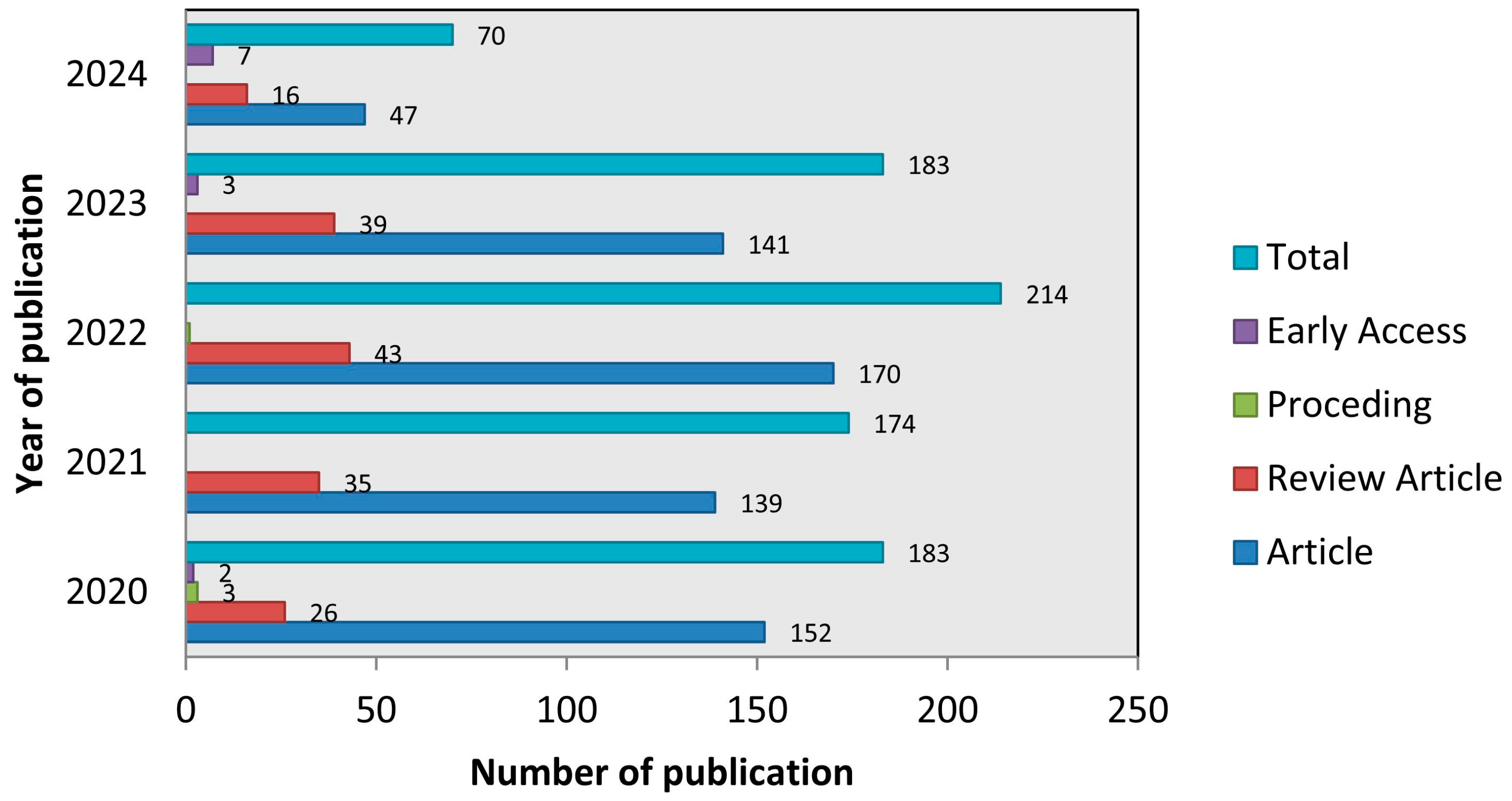
3.1. Publications Trends
3.2. The Collaboration Network Analysis
3.2.1. Co-Authorship Countries
3.2.2. Co-Authorship Organizations
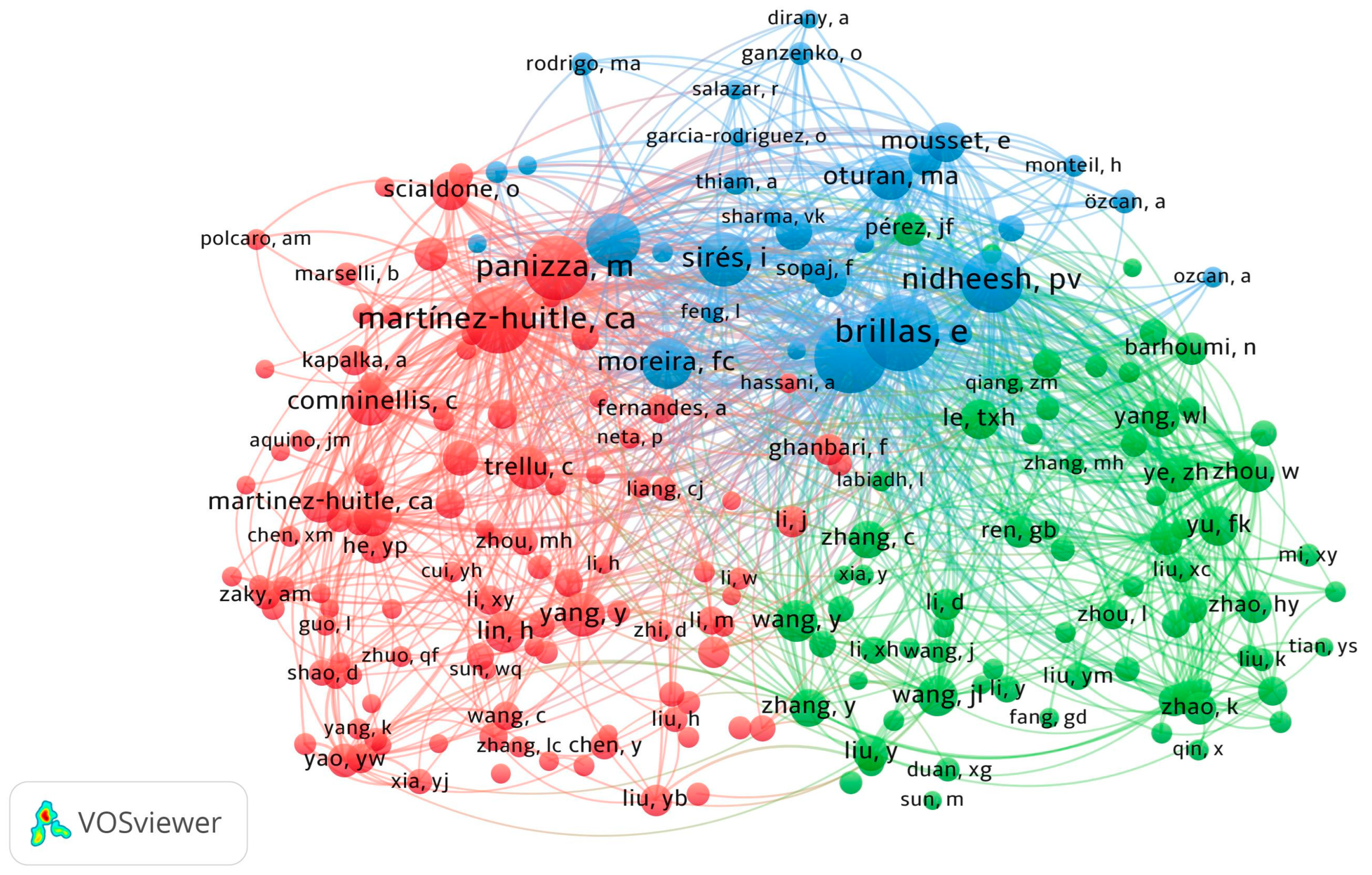
3.2.3. Co-Authorship Authors
3.3. Most Cited Articles
3.4. Co-Citation Network Analysis
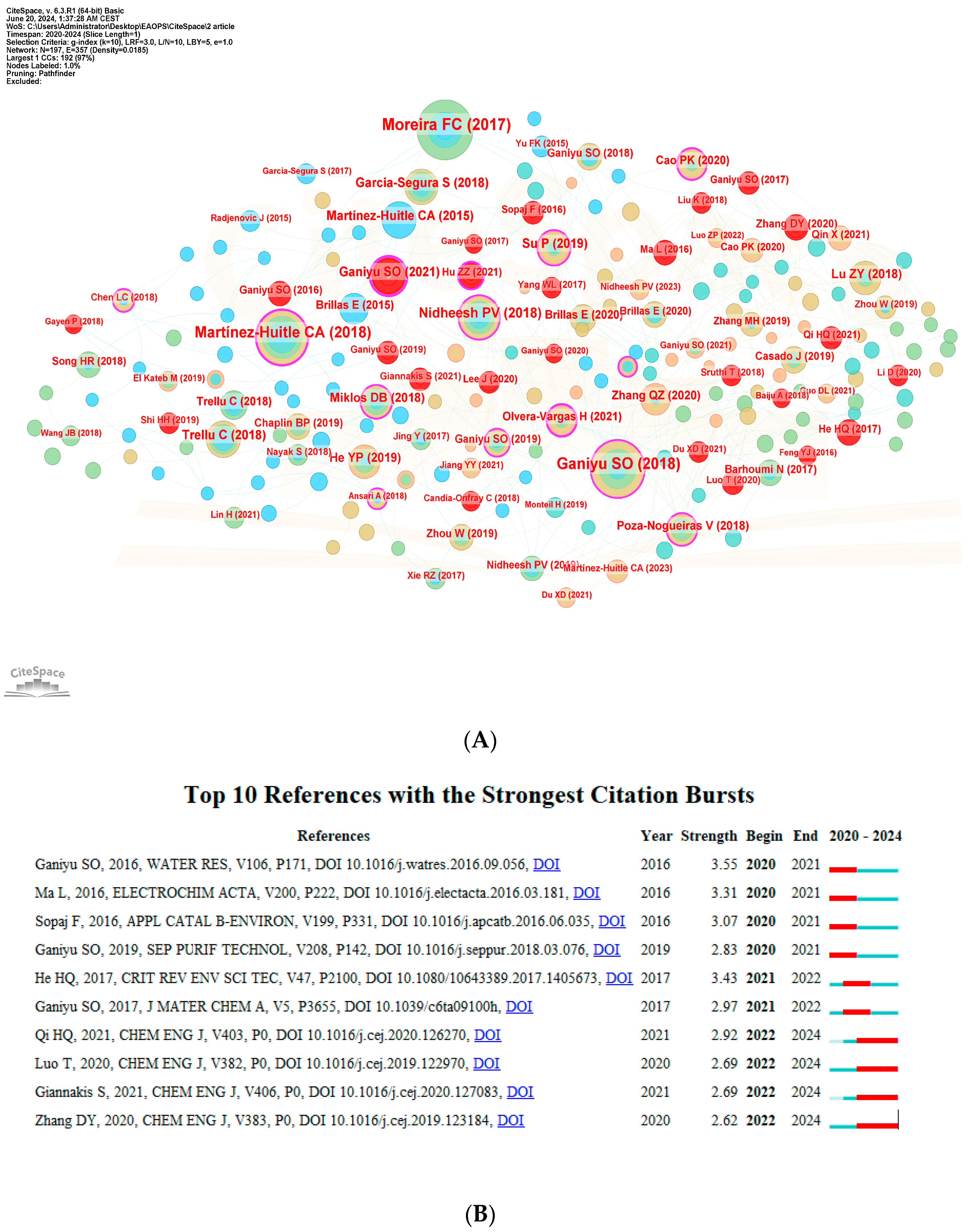
3.4.1. Document Co-Citation Network
3.4.2. Author Co-Citation Network
3.4.3. Journal Co-Citation Analysis
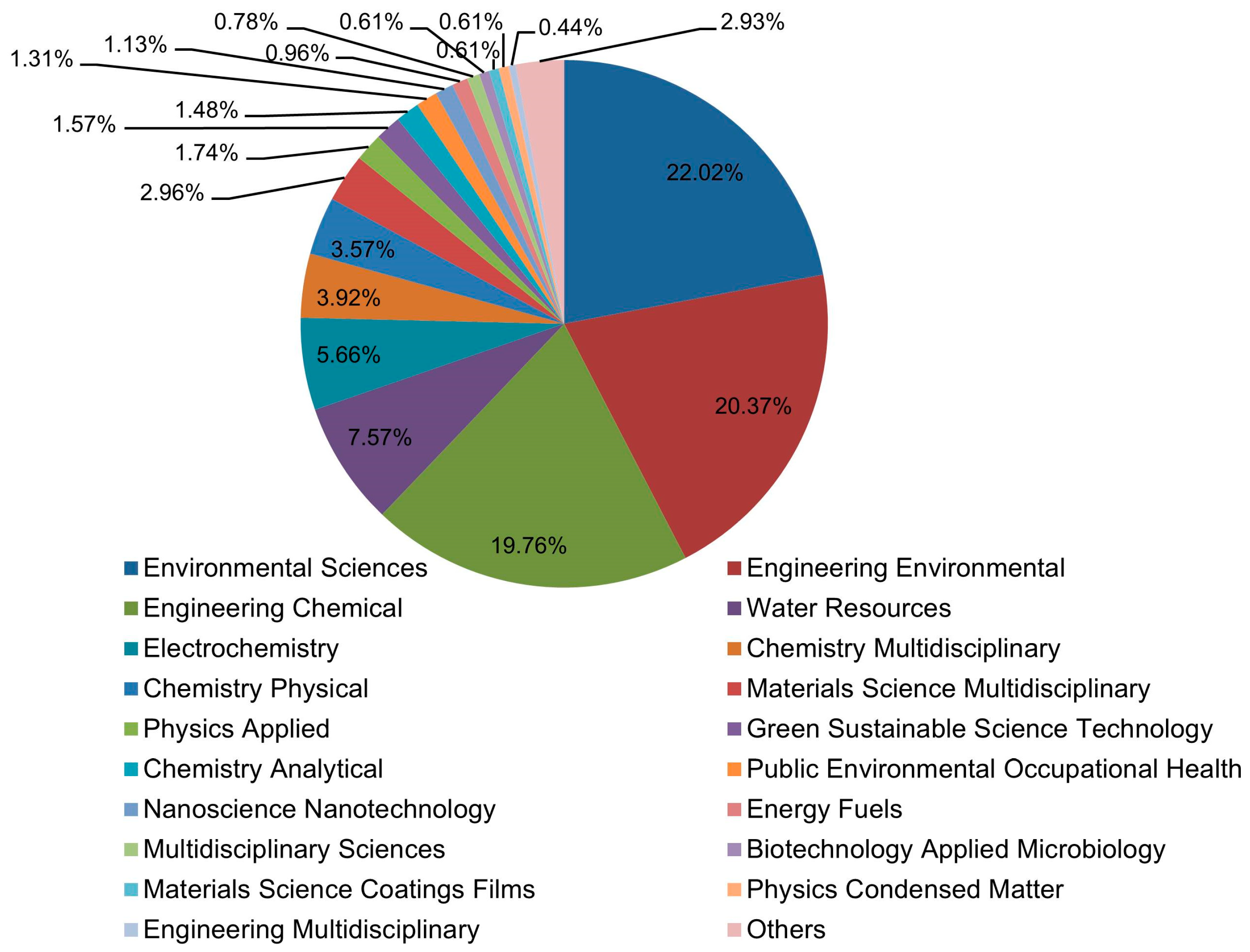
3.5. Keywords Analysis
3.6. Past and Future Research Trends of EAOP Based on Bibliometric Analysis
- Development of Electrode Materials
- Optimization of the EF process
- Integration with Other Treatment Technologies
- Mechanism Studies and ROS Generation
- The Integration and Adaptation of EAOPs
- Scalability and Industrial Applications
- Environmental Impact and Sustainability
- Economic Feasibility and Cost Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital Wastewater as a Source of Environmental Contamination: An Overview of Management Practices, Environmental Risks, and Treatment Processes. J. Water Process Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- Khan, M.T.; Ahmad, R.; Liu, G.; Zhang, L.; Santagata, R.; Lega, M.; Casazza, M. Potential Environmental Impacts of a Hospital Wastewater Treatment Plant in a Developing Country. Sustainability 2024, 16, 2233. [Google Scholar] [CrossRef]
- Koul, B.; Yadav, D.; Singh, S.; Kumar, M.; Song, M. Insights into the Domestic Wastewater Treatment (DWWT) Regimes: A Review. Water 2022, 14, 3542. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Vellido-Pérez, J.A.; González-Hernández, R.; Martínez-Férez, A. Optimization and Modeling of Two-Phase Olive-Oil Washing Wastewater Integral Treatment and Phenolic Compounds Recovery by Novel Weak-Base Ion Exchange Resins. Sep. Purif. Technol. 2020, 249, 117084. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Zhou, S.; Li, T.; Wu, M.; Li, X.; Li, H. Potassium Hydroxide-Treated Walnut Shell Residue Biochar for Wastewater Treatment: Phenol Adsorption and Mechanism Study. Desalin. Water Treat. 2022, 254, 15–24. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic Analysis of the Recovery of Phenols from Olive Mill Wastewater through Membrane Filtration and Resin Adsorption/Desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional Methods and Emerging Wastewater Polishing Technologies for Palm Oil Mill Effluent Treatment: A Review. J. Environ. Manag. 2015, 149, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Moradi, M.; Vasseghian, Y.; Khataee, A.; Kobya, M.; Arabzade, H.; Dragoi, E.-N. Service Life and Stability of Electrodes Applied in Electrochemical Advanced Oxidation Processes: A Comprehensive Review. J. Ind. Eng. Chem. 2020, 87, 18–39. [Google Scholar] [CrossRef]
- Qiao, J.; Xiong, Y. Electrochemical Oxidation Technology: A Review of Its Application in High-Efficiency Treatment of Wastewater Containing Persistent Organic Pollutants. J. Water Process Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Narváez, O.M.; Picos, A.R.; Bravo-Yumi, N.; Pacheco-Alvarez, M.; Martínez-Huitle, C.A.; Peralta-Hernández, J.M. Electrochemical Oxidation Technology to Treat Textile Wastewaters. Curr. Opin. Electrochem. 2021, 29, 100806. [Google Scholar] [CrossRef]
- El-Ghzizel, S.; Tahaikt, M.; Dhiba, D.; Elmidaouia, A.; Taky, M. Desalination and Water Treatment www.Deswater.Com. Desalin. Water Treat. 2021, 231, 1–15. [Google Scholar] [CrossRef]
- Mehrkhah, R.; Park, S.Y.; Lee, J.H.; Kim, S.Y.; Lee, B.H. Prospective Performance Assessment of Enhanced Electrochemical Oxidation Technology: Insights into Fundamentals and Influencing Factors for Reducing Energy Requirements in Industrial Wastewater Treatment. Environ. Technol. Innov. 2023, 32, 103336. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Cotillas, S.; Boaventura, R.A.R.; Moreira, F.C.; Lee, J.; Vilar, V.J.P. Single and Combined Electrochemical Oxidation Driven Processes for the Treatment of Slaughterhouse Wastewater. J. Clean. Prod. 2020, 270, 121858. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S.; Skoumal, M.; Arias, C. Electrochemical Incineration of Diclofenac in Neutral Aqueous Medium by Anodic Oxidation Using Pt and Boron-Doped Diamond Anodes. Chemosphere 2010, 79, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, G.; Pan, Z.; Zhou, X.; Lai, W.; Zheng, L.; Xu, Y. A Novel Pb/PbO2 Electrodes Prepared by the Method of Thermal Oxidation-Electrochemical Oxidation: Characteristic and Electrocatalytic Oxidation Performance. J. Alloys Compd. 2021, 851, 156834. [Google Scholar] [CrossRef]
- Duan, X.; Ma, F.; Yuan, Z.; Jin, X.; Chang, L. Electrochemical Degradation of Phenol in Aqueous Solution Using PbO2 Anode. J. Taiwan Inst. Chem. Eng. 2013, 44, 95–102. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; De Battisti, A.; Ferro, S.; Reyna, S.; Cerro-López, M.; Quiro, M.A. Removal of the Pesticide Methamidophos from Aqueous Solutions by Electrooxidation Using Pb/PbO2, Ti/SnO2, and Si/BDD Electrodes. Environ. Sci. Technol. 2008, 42, 6929–6935. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, C.; Li, H.; Chen, L.; Zhang, D.; Yang, Z. Electrochemical Degradation of Ciprofloxacin with a Sb-Doped SnO2 Electrode: Performance, Influencing Factors and Degradation Pathways. RSC Adv. 2019, 9, 29796–29804. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Electroplating Wastewater Treatment by Electro-Oxidation Using Synthesized New Electrode: Experimental, Optimization, Kinetics, and Cost Analysis. Process Saf. Environ. Prot. 2024, 183, 735–756. [Google Scholar] [CrossRef]
- Cui, Y.; Li, X.; Chen, G. Electrochemical Degradation of Bisphenol A on Different Anodes. Water Res. 2009, 43, 1968–1976. [Google Scholar] [CrossRef]
- Xiao, H.; Xu, F.; Chen, J.; Hao, Y.; Guo, Y.; Zhu, C.; Luo, S.; Jiang, B. Electrogenerated Oxychlorides Induced Overlooked Negative Effects on Electro-Oxidation Wastewater Treatment in Terms of over-Evaluated COD Removal Efficiency and Biotoxicity. J. Hazard. Mater. 2023, 456, 131667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic Oxidation for the Degradation of Organic Pollutants: Anode Materials, Operating Conditions and Mechanisms. A Mini Review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E. Fenton, Photo-Fenton, Electro-Fenton, and Their Combined Treatments for the Removal of Insecticides from Waters and Soils. A Review. Sep. Purif. Technol. 2022, 284, 120290. [Google Scholar] [CrossRef]
- Marcal, J.; Bishop, T.; Hofman, J.; Shen, J. From Pollutant Removal to Resource Recovery: A Bibliometric Analysis of Municipal Wastewater Research in Europe. Chemosphere 2021, 284, 131267. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Y.; Liu, W.; Dunford, M. Visualizing the Intellectual Structure and Evolution of Innovation Systems Research: A Bibliometric Analysis. Scientometrics 2015, 103, 135–158. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, H.; Duan, Z.; Huang, Z.; Wei, K. Research Progress and Trends of Biochar in the Field of Wastewater Treatment by Electrochemical Advanced Oxidation Processes (EAOPs): A Bibliometric Analysis. J. Hazard. Mater. Adv. 2023, 10, 100305. [Google Scholar] [CrossRef]
- Huang, W.; Liu, S.; Zhang, T.; Wu, H.; Pu, S. Bibliometric Analysis and Systematic Review of Electrochemical Methods for Environmental Remediation. J. Environ. Sci. 2023, 144, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, K.A.; Giwa, S.O.; Adegoke, O.R.; Maxakato, N.W. Bibliometric Evaluation of Nanoadsorbents for Wastewater Treatment and Way forward in Nanotechnology for Clean Water Sustainability. Sci. Afr. 2023, 21, e01753. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, M.; Zhuang, D. Wastewater Treatment and Emerging Contaminants: Bibliometric Analysis. Chemosphere 2022, 297, 133932. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Henao-Aguirre, P.A.; Marín-Flórez, A.; Arredondo-López, S.M.; Sanabria-González, N.R. Bibliometric Analysis of Advanced Oxidation Processes (AOPs) in Wastewater Treatment: Global and Ibero-American Research Trends. Environ. Sci. Pollut. Res. 2021, 28, 23791–23811. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, T.; Qiao, Z.; Sun, P.; Hao, J.; Yang, Y. Application of Artificial Intelligence to Wastewater Treatment: A Bibliometric Analysis and Systematic Review of Technology, Economy, Management, and Wastewater Reuse. Process Saf. Environ. Prot. 2020, 133, 169–182. [Google Scholar] [CrossRef]
- Ismail, S.A.; Ang, W.L.; Mohammad, A.W. Electro-Fenton Technology for Wastewater Treatment: A Bibliometric Analysis of Current Research Trends, Future Perspectives and Energy Consumption Analysis. J. Water Process Eng. 2021, 40, 101952. [Google Scholar] [CrossRef]
- Brdarić, T.P.; Aćimović, D.D.; Savić Rosić, B.G.; Simić, M.D.; Stojanović, K.D.; Vranješ, Z.M.; Vasić Anićijević, D. Bibliometric Analysis of Nanostructured Anodes for Electro-Oxidative Wastewater Treatment. Sustainability 2024, 16, 3982. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical Review of Electrochemical Advanced Oxidation Processes for Water Treatment Applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Brosler, P.; Girão, A.V.; Silva, R.F.; Tedim, J.; Oliveira, F.J. Electrochemical Advanced Oxidation Processes Using Diamond Technology: A Critical Review. Environments 2023, 10, 15. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced Oxidation Processes: Performance, Advantages, and Scale-up of Emerging Technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Liu, Z.; Hu, Z.; Wang, X. Methodological Functions of CiteSpace Knowledge Graphs. Stud. Sci. Sci. 2015, 33, 242–253. [Google Scholar]
- Zhang, Q.; Zhou, M.; Ren, G.; Li, Y.; Li, Y.; Du, X. Highly Efficient Electrosynthesis of Hydrogen Peroxide on a Superhydrophobic Three-Phase Interface by Natural Air Diffusion. Nat. Commun. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesh, P.V.; Syam Babu, D.; Gopinath, A.; Suresh Kumar, M. Treatment of Dyeing Wastewater by Combined Sulfate Radical Based Electrochemical Advanced Oxidation and Electrocoagulation Processes. Sep. Purif. Technol. 2021, 254, 117570. [Google Scholar] [CrossRef]
- Olvera-Vargas, H.; Gore-Datar, N.; Garcia-Rodriguez, O.; Mutnuri, S.; Lefebvre, O. Electro-Fenton Treatment of Real Pharmaceutical Wastewater Paired with a BDD Anode: Reaction Mechanisms and Respective Contribution of Homogeneous and Heterogeneous OH. Chem. Eng. J. 2021, 404, 126524. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Cai, J.; Zhou, M. Internal-Micro-Electrolysis-Enhanced Heterogeneous Electro-Fenton Process Catalyzed by Fe/Fe3C@PC Core–Shell Hybrid for Sulfamethazine Degradation. Chem. Eng. J. 2020, 398, 125681. [Google Scholar] [CrossRef]
- Ghanbari, F.; Wang, Q.; Hassani, A.; Wacławek, S.; Rodríguez-Chueca, J.; Lin, K.-Y.A. Electrochemical Activation of Peroxides for Treatment of Contaminated Water with Landfill Leachate: Efficacy, Toxicity and Biodegradability Evaluation. Chemosphere 2021, 279, 130610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, S.; Feng, H.; Hu, T.; Wu, Y.; Luo, T.; Tang, W.; Wang, D. Efficient Degradation of Tetracycline Using Core–Shell Fe@Fe2O3-CeO2 Composite as Novel Heterogeneous Electro-Fenton Catalyst. Chem. Eng. J. 2022, 428, 131403. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Wen, R.; Zhang, H.; Elumalai, P.; Zheng, Q.; Chen, H.; Yang, Y.; Huang, M.; Ying, G. Heterogeneous Electro–Fenton Using Three–Dimension NZVI–BC Electrodes for Degradation of Neonicotinoid Wastewater. Water Res. 2020, 182, 115975. [Google Scholar] [CrossRef]
- Ghanbari, F.; Wu, J.; Khatebasreh, M.; Ding, D.; Lin, K.-Y.A. Efficient Treatment for Landfill Leachate through Sequential Electrocoagulation, Electrooxidation and PMS/UV/CuFe2O4 Process. Sep. Purif. Technol. 2020, 242, 116828. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Kumar, A.; Syam Babu, D.; Scaria, J.; Suresh Kumar, M. Treatment of Mixed Industrial Wastewater by Electrocoagulation and Indirect Electrochemical Oxidation. Chemosphere 2020, 251, 126437. [Google Scholar] [CrossRef]
- García, O.; Isarain-Chávez, E.; El-Ghenymy, A.; Brillas, E.; Peralta-Hernández, J.M. Degradation of 2,4-D Herbicide in a Recirculation Flow Plant with a Pt/Air-Diffusion and a BDD/BDD Cell by Electrochemical Oxidation and Electro-Fenton Process. J. Electroanal. Chem. 2014, 728, 1–9. [Google Scholar] [CrossRef]
- Assumpção, M.H.M.T.; De Souza, R.F.B.; Reis, R.M.; Rocha, R.S.; Steter, J.R.; Hammer, P.; Gaubeur, I.; Calegaro, M.L.; Lanza, M.R.V.; Santos, M.C. Low Tungsten Content of Nanostructured Material Supported on Carbon for the Degradation of Phenol. Appl. Catal. B Environ. 2013, 142–143, 479–486. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, N.; Olvera-Vargas, H.; Brillas, E.; Gadri, A.; Ammar, S.; Oturan, M.A. Pyrite as a Sustainable Catalyst in Electro-Fenton Process for Improving Oxidation of Sulfamethazine. Kinetics, Mechanism and Toxicity Assessment. Water Res. 2016, 94, 52–61. [Google Scholar] [CrossRef]
- Luo, T.; Feng, H.; Tang, L.; Lu, Y.; Tang, W.; Chen, S.; Yu, J.; Xie, Q.; Ouyang, X.; Chen, Z. Efficient Degradation of Tetracycline by Heterogeneous Electro-Fenton Process Using Cu-Doped Fe@Fe2O3: Mechanism and Degradation Pathway. Chem. Eng. J. 2020, 382, 122970. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Ra, S.; Cretin, M.; Causserand, C.; Oturan, M.A. Efficiency of Plasma Elaborated Sub-Stoichiometric Titanium Oxide (Ti4O7) Ceramic Electrode for Advanced Electrochemical Degradation of Paracetamol in Di Ff Erent Electrolyte Media. Sep. Purif. Technol. 2018, 208, 142–152. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical Oxidation of Organic Pollutants for Wastewater Treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Esmilaire, R.; van Hullebusch, E.; Esposito, G.; Oturan, M.A. Sub-Stoichiometric Titanium Oxide (Ti4O7) as a Suitable Ceramic Anode for Electrooxidation of Organic Pollutants: A Case Study of Kinetics, Mineralization and Toxicity Assessment of Amoxicillin. Water Res. 2016, 106, 171–182. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, M.; Ren, G.; Yang, W.; Liang, L. A Highly Energy-Efficient Flow-through Electro-Fenton Process for Organic Pollutants Degradation. Electrochim. Acta 2016, 200, 222–230. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.; Oturan, M.A. Effect of the Anode Materials on the Efficiency of the Electro-Fenton Process for the Mineralization of the Antibiotic Sulfamethazine. Appl. Catal. B Environ. 2016, 199, 331–341. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z. Electro-Fenton Process for Water and Wastewater Treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong Le, T.X.; Bechelany, M.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A Hierarchical CoFe-Layered Double Hydroxide Modified Carbon-Felt Cathode for Heterogeneous Electro-Fenton Process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Porous Graphite Felt Electrode with Catalytic Defects for Enhanced Degradation of Pollutants by Electro-Fenton Process. Chem. Eng. J. 2021, 403, 126270. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A Review of the Recent Advances on the Treatment of Industrial Wastewaters by Sulfate Radical-Based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Yin, K.; Liu, C.; Wei, Y. Selective H2O2 Production on N-Doped Porous Carbon from Direct Carbonization of Metal Organic Frameworks for Electro-Fenton Mineralization of Antibiotics. Chem. Eng. J. 2020, 383, 123184. [Google Scholar] [CrossRef]
- Hasani, K.; Hosseini, S.; Gholizadeh, H.; Dargahi, A.; Vosoughi, M. Degradation of Oxytetracycline by Electrochemical, Fenton and Electro-Fenton Processes Using SS316 and SS316/β-PbO2 Anodes: Process Optimization Using Rsm-Ccd, Bioassay Test and Degradation Pathway. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Iglesias, O.; Meijide, J.; Bocos, E.; Sanromán, M.Á.; Pazos, M. New Approaches on Heterogeneous Electro-Fenton Treatment of Winery Wastewater. Electrochim. Acta 2015, 169, 134–141. [Google Scholar] [CrossRef]
- Simić, M.D.; Savić, B.G.; Ognjanović, M.R.; Stanković, D.M.; Relić, D.J.; Aćimović, D.D.; Brdarić, T.P. Degradation of Bisphenol A on SnO2-MWCNT Electrode Using Electrochemical Oxidation. J. Water Process Eng. 2023, 51, 103416. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Y.; Zhang, C.; Li, Y.; Li, F.; He, Y.; Zhai, L.; Li, L.; Chen, X. The Efficiency and Mechanism of Electrochemical Oxidation of Levofloxacin Using Ti/RuO2-TiO2-SnO2 Anodes. Water Air Soil Pollut. 2023, 234, 637. [Google Scholar] [CrossRef]
- Aćimović, D.D.; Karić, S.D.; Nikolić, Ž.M.; Brdarić, T.P.; Tasić, G.S.; Marčeta Kaninski, M.P.; Nikolić, V.M. Electrochemical Oxidation of the Polycyclic Aromatic Hydrocarbons in Polluted Concrete of the Residential Buildings. Environ. Pollut. 2017, 220, 393–399. [Google Scholar] [CrossRef]
- Simić, M.D.; Brdarić, T.P.; Savić Rosić, B.G.; Švorc, Ľ.; Relić, D.J.; Aćimović, D.D. Degradation of Bisphenol A via the Electro–Fenton Process Using Nanostructured Carbon-Metal Oxide Anodes: Intermediates and Reaction Mechanisms Study. J. Environ. Chem. Eng. 2024, 12, 113369. [Google Scholar] [CrossRef]
- Martínez-Sánchez, C.; Robles, I.; Godínez, L.A. Review of Recent Developments in Electrochemical Advanced Oxidation Processes: Application to Remove Dyes, Pharmaceuticals, and Pesticides. Int. J. Environ. Sci. Technol. 2022, 19, 12611–12678. [Google Scholar] [CrossRef]
- Trellu, C.; Olvera Vargas, H.; Mousset, E.; Oturan, N.; Oturan, M.A. Electrochemical Technologies for the Treatment of Pesticides. Curr. Opin. Electrochem. 2021, 26, 100677. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Medina-Orendain, D.A.; Karri, R.R.; Rodríguez-Narvaez, O.M. Electrochemical Advanced Oxidation Processes for the Removal of Pesticides from Water and Wastewater. A Review. In Pesticides Remediation Technologies from Water and Wastewater; Elsevier: Amsterdam, The Netherlands, 2022; pp. 99–126. ISBN 9780323908931. [Google Scholar]
- Panizza, M.; Cerisola, G. Direct And Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Savić, B.G.; Stanković, D.M.; Živković, S.M.; Ognjanović, M.R.; Tasić, G.S.; Mihajlović, I.J.; Brdarić, T.P. Electrochemical Oxidation of a Complex Mixture of Phenolic Compounds in the Base Media Using PbO2-GNRs Anodes. Appl. Surf. Sci. 2020, 529, 147120. [Google Scholar] [CrossRef]
- Ječmenica Dučić, M.; Krstić, A.; Zdolšek, N.; Aćimović, D.; Savić, B.; Brdarić, T.; Vasić Anićijević, D. Low-Cost Graphene-Based Composite Electrodes for Electrochemical Oxidation of Phenolic Dyes. Crystals 2023, 13, 125. [Google Scholar] [CrossRef]
- Ječmenica Dučić, M.; Aćimović, D.; Savić, B.; Rakočević, L.; Simić, M.; Brdarić, T.; Vasić Anićijević, D. Is It Possible to Restrain OER on Simple Carbon Electrodes to Efficiently Electrooxidize Organic Pollutants? Molecules 2022, 27, 5203. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, C.; Liu, W.; Zhao, X.; Chang, L. Fabrication of a Novel PbO2 Electrode with a Graphene Nanosheet Interlayer for Electrochemical Oxidation of 2-Chlorophenol. Electrochim. Acta 2017, 240, 424–436. [Google Scholar] [CrossRef]
- Tong, S.P.; Ma, C.A.; Feng, H. A Novel PbO2 Electrode Preparation and Its Application in Organic Degradation. Electrochim. Acta 2008, 53, 3002–3006. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, K.; Han, W.; Wang, L.; Sun, X.; Li, J. Electrochemical Degradation of Tricyclazole in Aqueous Solution Using Ti/SnO2-Sb/PbO2 Anode. J. Electroanal. Chem. 2013, 705, 68–74. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Prasad, K.; Yoo, J.; Kim, J.; Yoo, K. CuO-SnO2 Nanocomposites: Efficient and Cost-Effective Electrocatalysts for Urea Oxidation. Mater. Lett. 2023, 353, 10–13. [Google Scholar] [CrossRef]
- Duan, X.; Sui, X.; Wang, W.; Bai, W.; Chang, L. Fabrication of PbO2/SnO2 Composite Anode for Electrochemical Degradation of 3-Chlorophenol in Aqueous Solution. Appl. Surf. Sci. 2019, 494, 211–222. [Google Scholar] [CrossRef]
- Dhanabalan, A.; Yu, Y.; Li, X.; Chen, W.; Bechtold, K.; Gu, L.; Wang, C. Porous SnO2/CNT Composite Anodes: Influence of Composition and Deposition Temperature on the Electrochemical Performance. J. Mater. Res. 2010, 25, 1554–1560. [Google Scholar] [CrossRef]
- Ramalho, A.M.Z.; Martínez-Huitle, C.A.; Silva, D.R. da Application of Electrochemical Technology for Removing Petroleum Hydrocarbons from Produced Water Using a DSA-Type Anode at Different Flow Rates. Fuel 2010, 89, 531–534. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, Z.; Zhou, M.; Zhou, L.; Xi, B. Efficient Degradation of P-Nitrophenol by Electro-Oxidation on Fe Doped Ti/TiO2 Nanotube/PbO2 Anode. Sep. Purif. Technol. 2014, 128, 67–71. [Google Scholar] [CrossRef]
- Sun, Z.; Ni, Y.; Wu, Y.; Yue, W.; Zhang, G.; Bai, J. Electrocatalytic Degradation of Methyl Orange and 4-Nitrophenol on a Ti/TiO2-NTA/La-PbO2 Electrode: Electrode Characterization and Operating Parameters. Environ. Sci. Pollut. Res. 2023, 30, 6262–6274. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.; Hu, Z.-T.; He, C.; Lim, T. High Performance Duplex-Structured SnO2-Sb-CNT Composite Anode for Bisphenol A Removal. Sep. Purif. Technol. 2017, 179, 25–35. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.; Zhou, X.; Li, J.; Sun, X.; Shen, J.; Wang, L.; Han, W. Electrochemical Degradation of Pyridine by Ti/SnO2-Sb Tubular Porous Electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; He, J.; Zhang, J. Preparation of Ti/SnO2-Sb Electrodes Modified by Carbon Nanotube for Anodic Oxidation of Dye Wastewater and Combination with Nanofiltration. Electrochim. Acta 2014, 117, 192–201. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Wang, X.; Wang, X.; Zhang, C.; Zhang, Y.; Sun, Q. Electrochemical Oxidation of Hydroquinone Using Eu-Doped PbO2 Electrodes: Electrode Characterization, Influencing Factors and Degradation Pathways. J. Electroanal. Chem. 2021, 895, 115493. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Hao, C.; Zhang, Y.; Chai, S.; Han, G.; Xu, H.; Lu, J.; Dang, Y.; Sun, X.; et al. Electrocatalysis Enhancement of α, β-PbO2 Nanocrystals Induced via Rare Earth Er(III) Doping Strategy: Principle, Degradation Application and Electrocatalytic Mechanism. Electrochim. Acta 2020, 333, 135535. [Google Scholar] [CrossRef]
- Yang, C.; Shang, S.; Li, X. Fabrication of Sulfur-Doped TiO2 Nanotube Array as a Conductive Interlayer of PbO2 Anode for Efficient Electrochemical Oxidation of Organic Pollutants. Sep. Purif. Technol. 2021, 258, 118035. [Google Scholar] [CrossRef]
- Xia, Y.; Bian, X.; Xia, Y.; Zhou, W.; Wang, L.; Fan, S.; Xiong, P.; Zhan, T.; Dai, Q.; Chen, J. Effect of Indium Doping on the PbO2 Electrode for the Enhanced Electrochemical Oxidation of Aspirin: An Electrode Comparative Study. Sep. Purif. Technol. 2020, 237, 116321. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like Processes with in-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Degradation of Emerging Contaminants: Advances and Prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical Advanced Oxidation Processes for Wastewater Treatment: Advances in Formation and Detection of Reactive Species and Mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Olvera-Vargas, H.; Oturan, N.; Oturan, M.A. Heterogeneous Electro-Fenton Process: Principles and Applications. In Electro-Fenton Process: New Trends and Scale-Up; Zhou, M., Oturan, M.A., Sirés, I., Eds.; Springer: Singapore, 2017; pp. 85–110. ISBN 978-981-10-6406-7. [Google Scholar]
- Brillas, E. Progress of Homogeneous and Heterogeneous Electro-Fenton Treatments of Antibiotics in Synthetic and Real Wastewaters. A Critical Review on the Period 2017–2021. Sci. Total Environ. 2022, 819, 153102. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, X.; Lu, M.; He, F.; Lin, F.; Zhang, Y.; Zhang, J.; Dong, P.; Zhao, C.; Sun, H. Melamine Foam Derived Nitrogen and Boron Co-Doped Metal-Free Electrode for Enhanced Electro-Fenton Degradation of Metronidazole. Chem. Eng. J. 2023, 455, 140593. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Song, G.; Du, X.; Lu, X. Efficient H2O2 Generation and Spontaneous OH Conversion for In-Situ Phenol Degradation on Nitrogen-Doped Graphene: Pyrolysis Temperature Regulation and Catalyst Regeneration Mechanism. J. Hazard. Mater. 2020, 397, 122681. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Duan, X.; Zhan, M.; Ji, L.; Li, X.; Wang, S.; Yan, J. Biochar Cathode: Reinforcing Electro-Fenton Pathway against Four-Electron Reduction by Controlled Carbonization and Surface Chemistry. Sci. Total Environ. 2021, 754, 142136. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; de Araújo, M.J.G.; de Araújo Costa, E.C.T.; Santos, J.E.L.; dos Santos, E.V.; Martínez-Huitle, C.A.; Pergher, S.B.C. Design of Highly Efficient Porous Carbon Foam Cathode for Electro-Fenton Degradation of Antimicrobial Sulfanilamide. Appl. Catal. B Environ. 2021, 283, 119652. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Zhu, Y.; Zhou, C.; Yang, Y.; Miao, J.; Zhou, W.; Shao, Z. The Recent Progress of Cathode Materials for Heterogeneous Electro-Fenton Reactions. Surf. Interfaces 2024, 44, 103820. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, X.; Xie, H.; Zhang, Z. Overcoming the Activity–Stability Trade-Off in Heterogeneous Electro-Fenton Catalysis: Encapsulating Carbon Cloth-Supported Iron Oxychloride within Graphitic Layers. ACS Catal. 2022, 12, 13334–13348. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X.; Su, Y.; Qin, X.; Chen, S.; Yu, H. Enhanced Chlorinated Pollutant Degradation by the Synergistic Effect between Dechlorination and Hydroxyl Radical Oxidation on a Bimetallic Single-Atom Catalyst. Environ. Sci. Technol. 2021, 55, 14194–14203. [Google Scholar] [CrossRef]
- Shen, X.; Xiao, F.; Zhao, H.; Chen, Y.; Fang, C.; Xiao, R.; Chu, W.; Zhao, G. In Situ-Formed PdFe Nanoalloy and Carbon Defects in Cathode for Synergic Reduction–Oxidation of Chlorinated Pollutants in Electro-Fenton Process. Environ. Sci. Technol. 2020, 54, 4564–4572. [Google Scholar] [CrossRef]
- Souza, F.L.; Lanza, M.R.V.; Llanos, J.; Sáez, C.; Rodrigo, M.A.; Cañizares, P. A Wind-Powered BDD Electrochemical Oxidation Process for the Removal of Herbicides. J. Environ. Manag. 2015, 158, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.C.; Pinheiro, V.S.; Joca, J.F.S.; de Souza, R.A.S.; Gentil, T.C.; Lanza, M.R.V.; de Oliveira, H.P.M.; Neto, A.M.P.; Gaubeur, I.; Santos, M.C. Removal of Orange II (OII) Dye by Simulated Solar Photoelectro-Fenton and Stability of WO2.72/Vulcan XC72 Gas Diffusion Electrode. Chemosphere 2020, 239, 124670. [Google Scholar] [CrossRef]
- Khaleel, G.F.; Ismail, I.; Abbar, A.H. Application of Solar Photo-Electro-Fenton Technology to Petroleum Refinery Wastewater Degradation: Optimization of Operational Parameters. Heliyon 2023, 9, e15062. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E. Solar Photoelectro-Fenton: A Very Effective and Cost-Efficient Electrochemical Advanced Oxidation Process for the Removal of Organic Pollutants from Synthetic and Real Wastewaters. Chemosphere 2023, 327, 138532. [Google Scholar] [CrossRef] [PubMed]
- Clematis, D.; Panizza, M. Electro-Fenton, Solar Photoelectro-Fenton and UVA Photoelectro-Fenton: Degradation of Erythrosine B Dye Solution. Chemosphere 2021, 270, 129480. [Google Scholar] [CrossRef]
- Murrieta, M.F.; Brillas, E.; Nava, J.L.; Sirés, I. Solar Photoelectro-Fenton-like Process with Anodically-Generated HClO in a Flow Reactor: Norfloxacin as a Pollutant with a Particular Structure. Sep. Purif. Technol. 2023, 308, 122893. [Google Scholar] [CrossRef]
- Herrera-Muñoz, J.; Cabrera-Reina, A.; Miralles-Cuevas, S.; Piña, S.; Salazar-González, R. Simultaneous Degradation of Contaminants of Emerging Concern and Disinfection by Solar Photoelectro-Fenton Process at Circumneutral PH in a Solar Electrochemical Raceway Pond Reactor. Chemosphere 2023, 341, 139978. [Google Scholar] [CrossRef] [PubMed]
- Radeef, A.Y.; Najim, A.A. Microbial Fuel Cell: The Renewable and Sustainable Magical System for Wastewater Treatment and Bioenergy Recovery. Energy 360 2024, 1, 100001. [Google Scholar] [CrossRef]
- Arun, J.; SundarRajan, P.; Grace Pavithra, K.; Priyadharsini, P.; Shyam, S.; Goutham, R.; Hoang Le, Q.; Pugazhendhi, A. New Insights into Microbial Electrolysis Cells (MEC) and Microbial Fuel Cells (MFC) for Simultaneous Wastewater Treatment and Green Fuel (Hydrogen) Generation. Fuel 2024, 355, 129530. [Google Scholar] [CrossRef]
- Islam, A.K.K.; Dunlop, P.S.; Bhattacharya, G.; Mokim, M.; Hewitt, N.J.; Huang, Y.; Gogulancea, V.; Zhang, K.; Brandoni, C. Comparative Performance of Sustainable Anode Materials in Microbial Fuel Cells (MFCs) for Electricity Generation from Wastewater. Results Eng. 2023, 20, 101385. [Google Scholar] [CrossRef]
- Zahran, M. Iron- and Carbon-Based Nanocomposites as Anode Modifiers in Microbial Fuel Cells for Wastewater Treatment and Power Generation Applications. J. Water Process Eng. 2024, 64, 105679. [Google Scholar] [CrossRef]
- Santos, J.S.; Tarek, M.; Sikora, M.S.; Praserthdam, S.; Praserthdam, P. Anodized TiO2 Nanotubes Arrays as Microbial Fuel Cell (MFC) Electrodes for Wastewater Treatment: An Overview. J. Power Sources 2023, 564, 232872. [Google Scholar] [CrossRef]



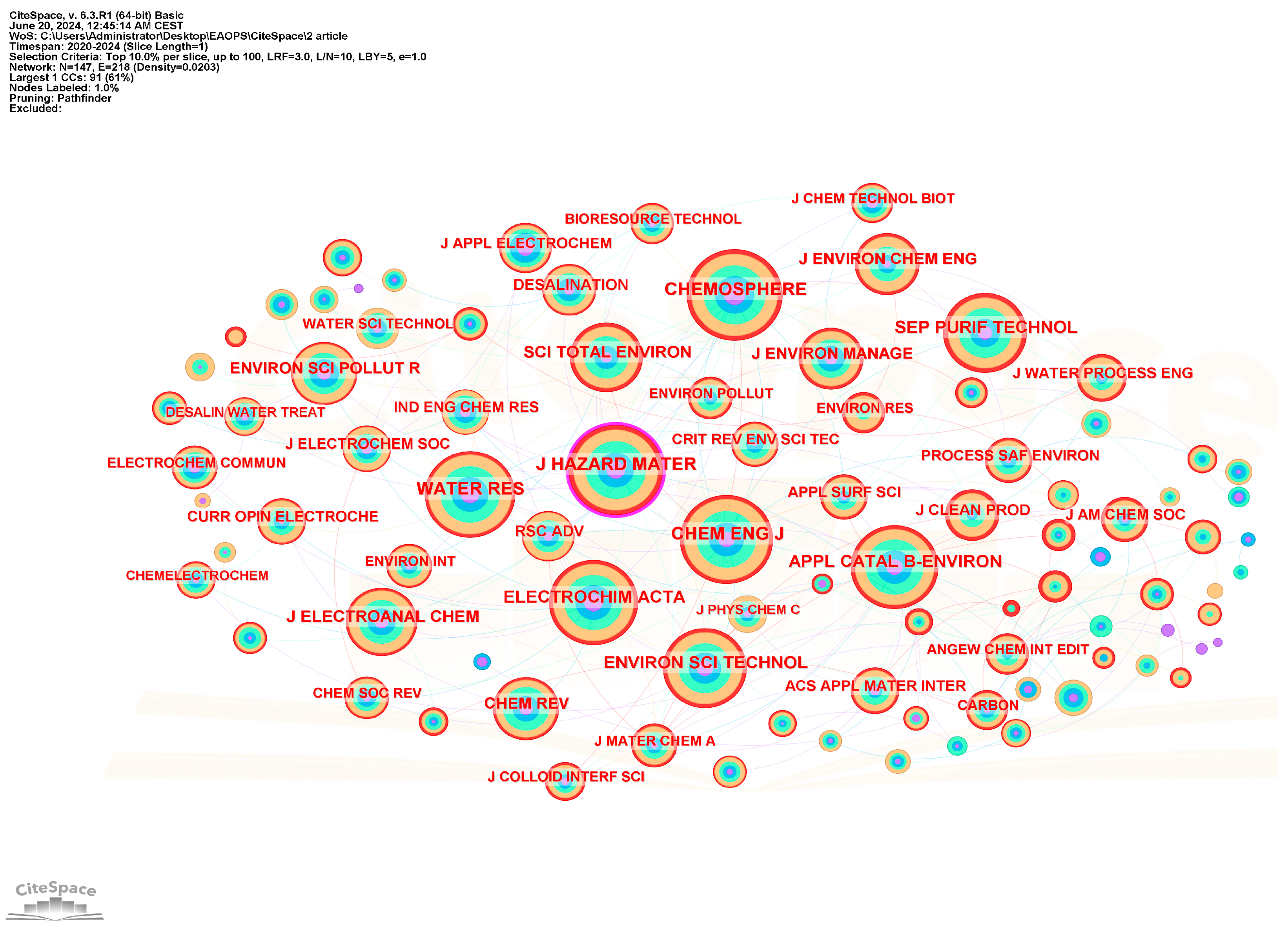
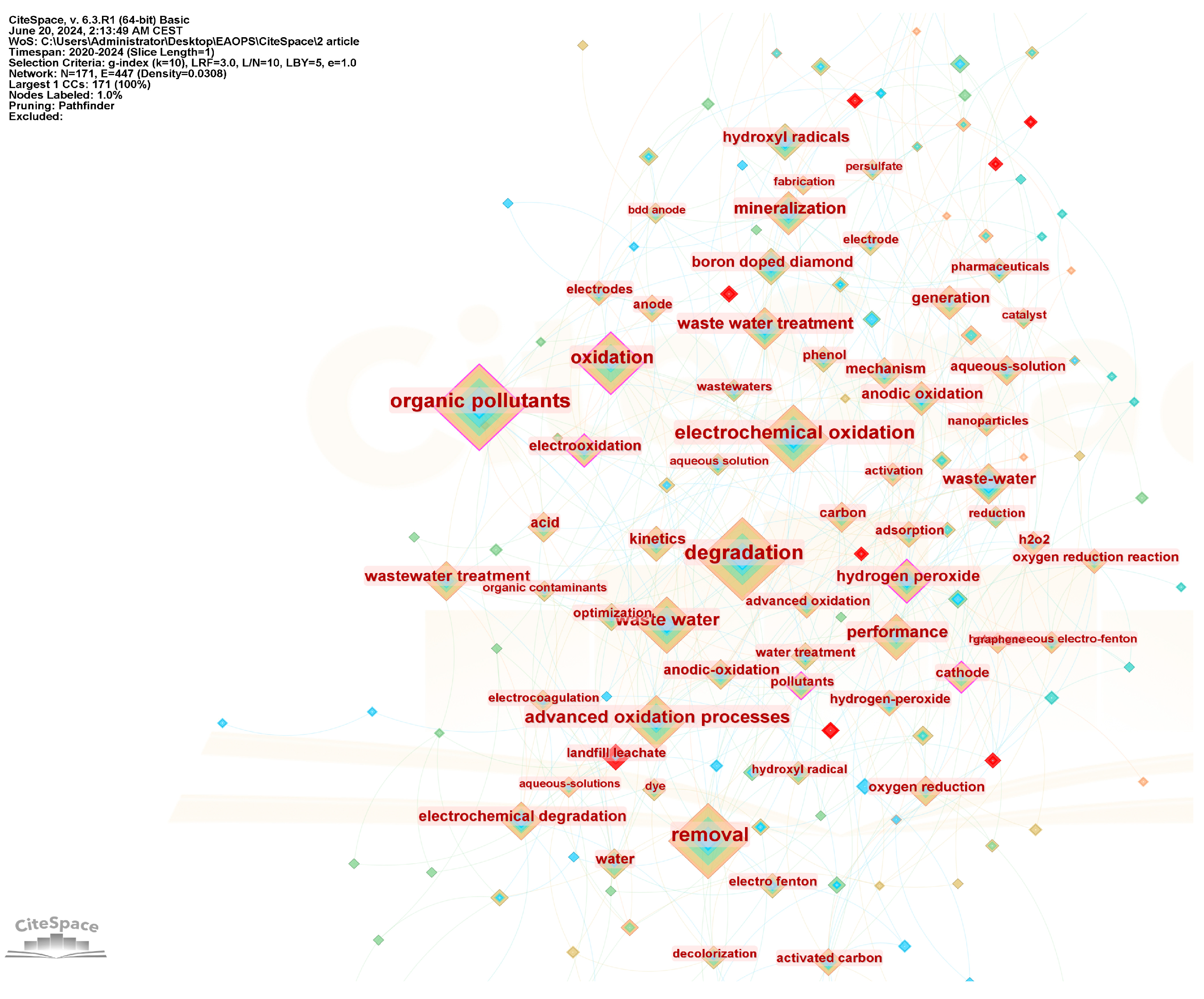
| Nb. | Document | Title | Journal | Citations | Links |
|---|---|---|---|---|---|
| 1 | Zhang (2020d) [43] | Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion | Nature Communications, 11 (2020), 1731 | 414 | 18 |
| 2 | Chanikya (2021) [44] | Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes | Separation and Purification Technology 254 (2021) 117570 | 144 | 5 |
| 3 | Olvera-Vargas (2021) [45] | Electro-Fenton treatment of real pharmaceutical wastewater paired with a BDD anode: Reaction mechanisms and respective contribution of homogeneous and heterogeneous OH | Chemical Engineering Journal, 404 (2021), 126524 | 133 | 15 |
| 4 | Du (2020) [46] | Internal-micro-electrolysis-enhanced heterogeneous electro-Fenton process catalyzed by Fe/Fe3C@PC core–shell hybrid for sulfamethazine degradation | Chemical Engineering Journal 398 (2020) 125681 | 119 | 7 |
| 5 | Ghanbari (2020a) [47] | Efficient treatment for landfill leachate through sequential electrocoagulation, electrooxidation and PMS/UV/CuFe2O4 process | Separation and Purification Technology 242 (2020) 116828 | 115 | 2 |
| 6 | Zhang (2022a) [48] | Efficient degradation of tetracycline using core–shell Fe@Fe2O3-CeO2 composite as novel heterogeneous electro-Fenton catalyst | Chemical Engineering Journal 428 (2022) 131403 | 111 | 6 |
| 7 | Zhang (2020a) [49] | Heterogeneous electro–Fenton using three–dimension NZVI–BC electrodes for degradation of neonicotinoid wastewater | Water Research 182 (2020) 115975 | 102 | 4 |
| 8 | Ghanbari (2021) [50] | Electrochemical activation of peroxides for treatment of contaminated water with landfill leachate: Efficacy, toxicity and biodegradability evaluation | Chemosphere 279 (2021) 130610 | 99 | 2 |
| 9 | Nidheesh (2020) [51] | Electrochemical oxidation of ofloxacin using a TiO2-based SnO2-Sb/polytetrafluoroethylene resin-PbO2 electrode: Reaction kinetics and mass transfer impact | Chemosphere 251 (2020) 126437 | 93 | 4 |
| Technology | Advantages | Disadvantages | Safety |
|---|---|---|---|
| Anodic oxidation |
|
|
|
| Electro-Fenton |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brdarić, T.P.; Aćimović, D.D.; Švorc, Ľ.; Vasić Anićijević, D.D. Bibliometric Study of Electrochemical Advanced Oxidation Processes (EAOPs) for Wastewater Treatment. Coatings 2024, 14, 1060. https://doi.org/10.3390/coatings14081060
Brdarić TP, Aćimović DD, Švorc Ľ, Vasić Anićijević DD. Bibliometric Study of Electrochemical Advanced Oxidation Processes (EAOPs) for Wastewater Treatment. Coatings. 2024; 14(8):1060. https://doi.org/10.3390/coatings14081060
Chicago/Turabian StyleBrdarić, Tanja P., Danka D. Aćimović, Ľubomír Švorc, and Dragana D. Vasić Anićijević. 2024. "Bibliometric Study of Electrochemical Advanced Oxidation Processes (EAOPs) for Wastewater Treatment" Coatings 14, no. 8: 1060. https://doi.org/10.3390/coatings14081060





