Influence of Oxygen Flow Rate on the Phase Structures and Properties for Copper Oxide Thin Films Deposited by RF Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cu2O Thin Films
2.2. Characterization Techniques
3. Results and Discussion
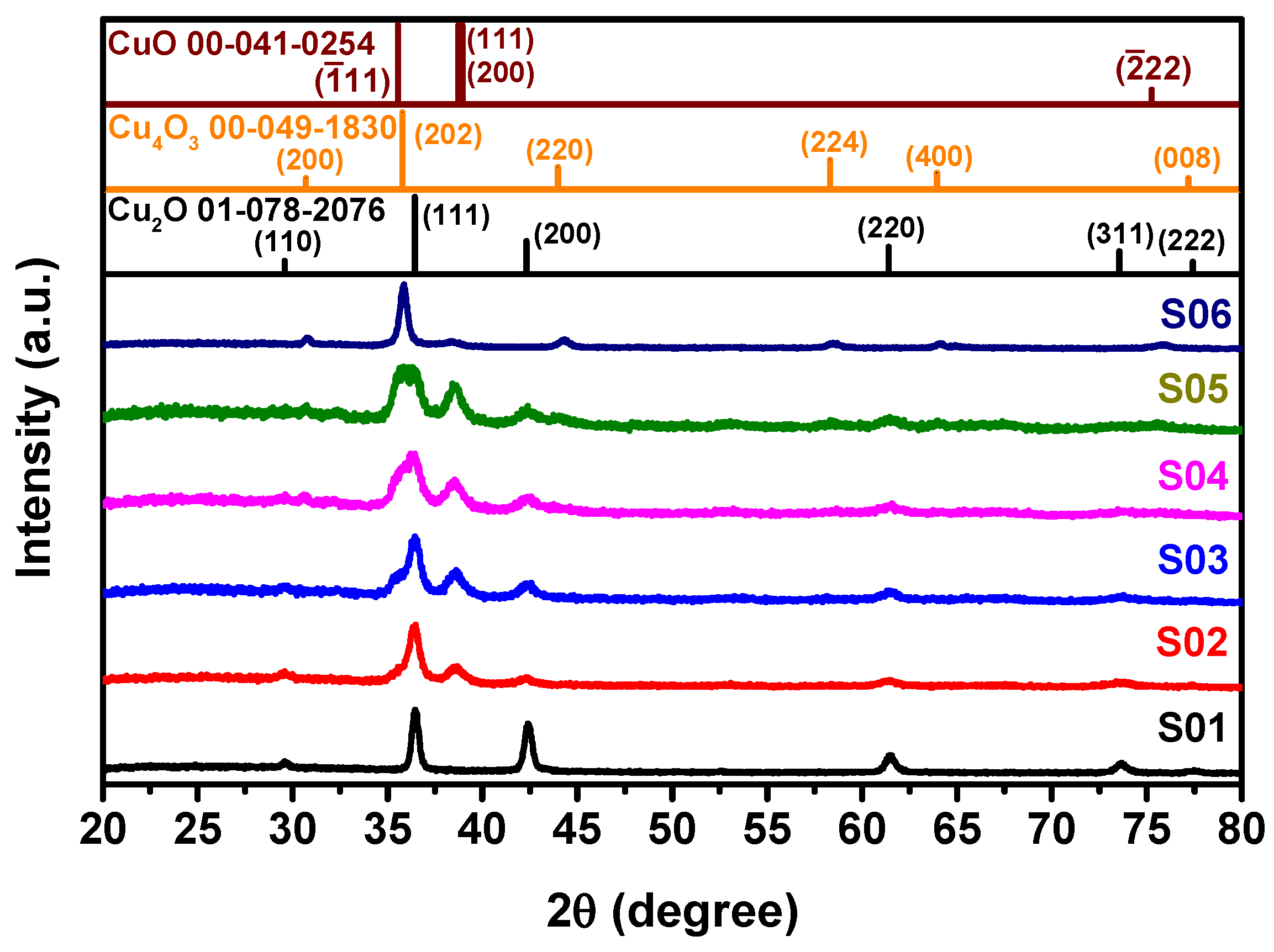
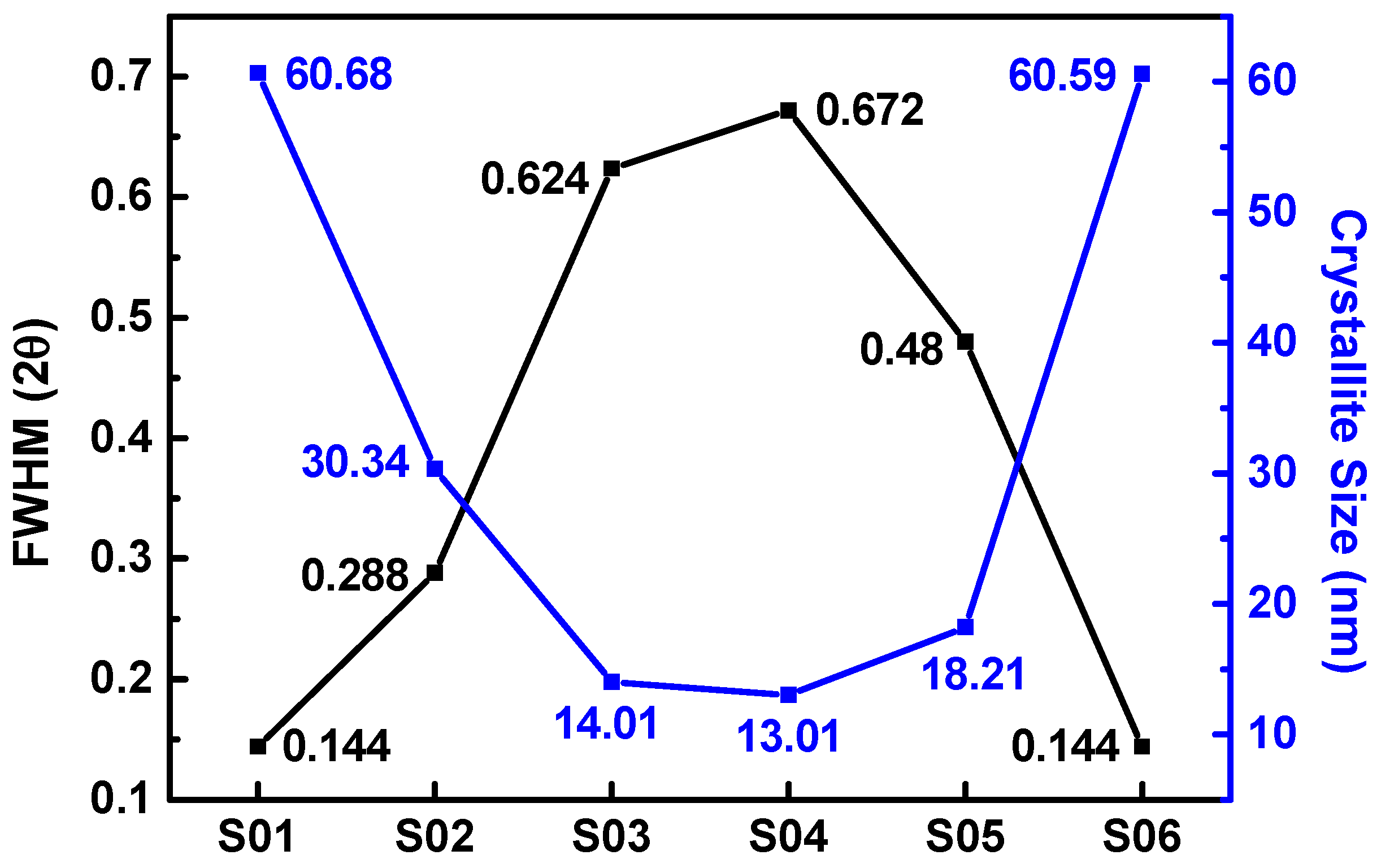
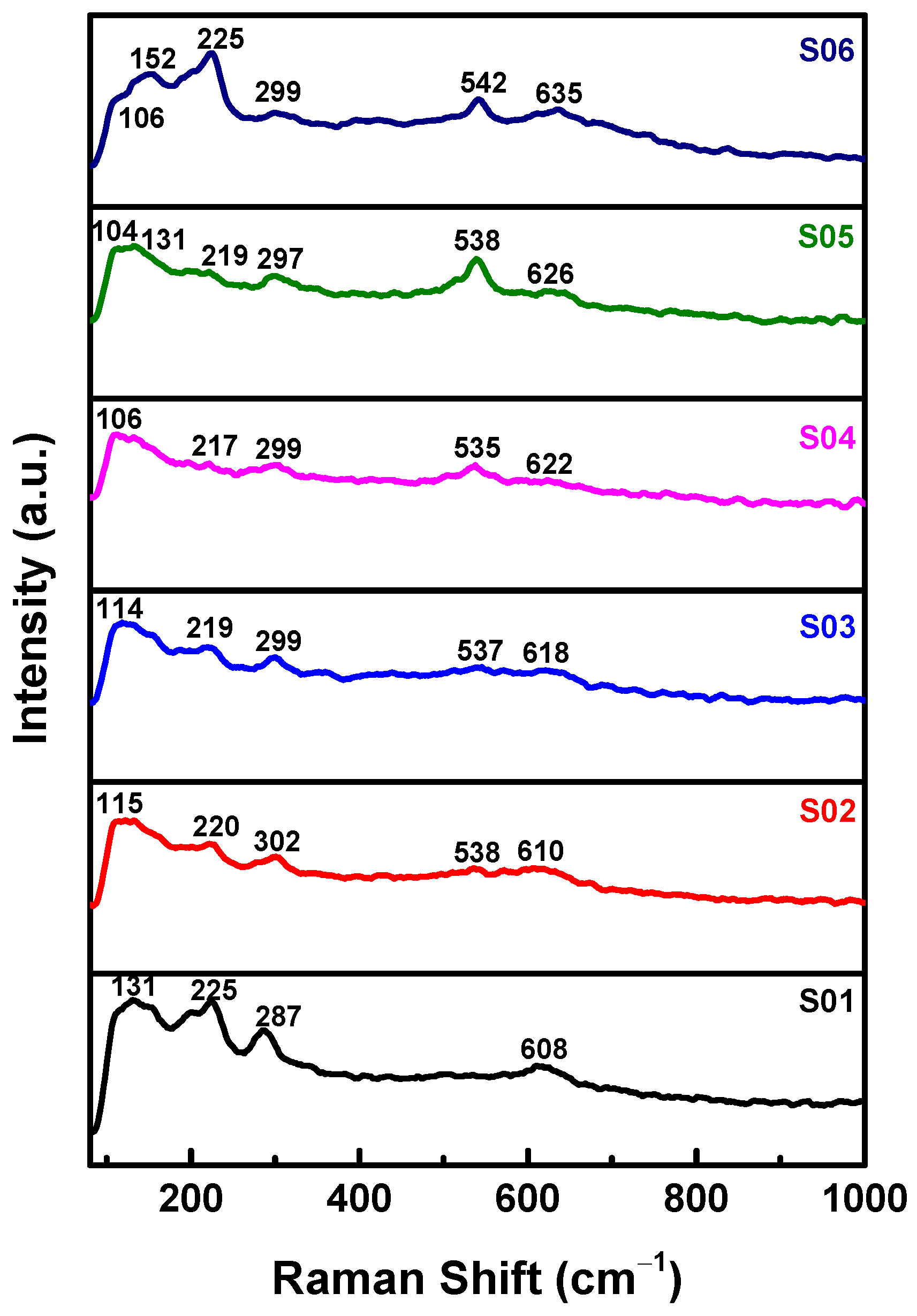
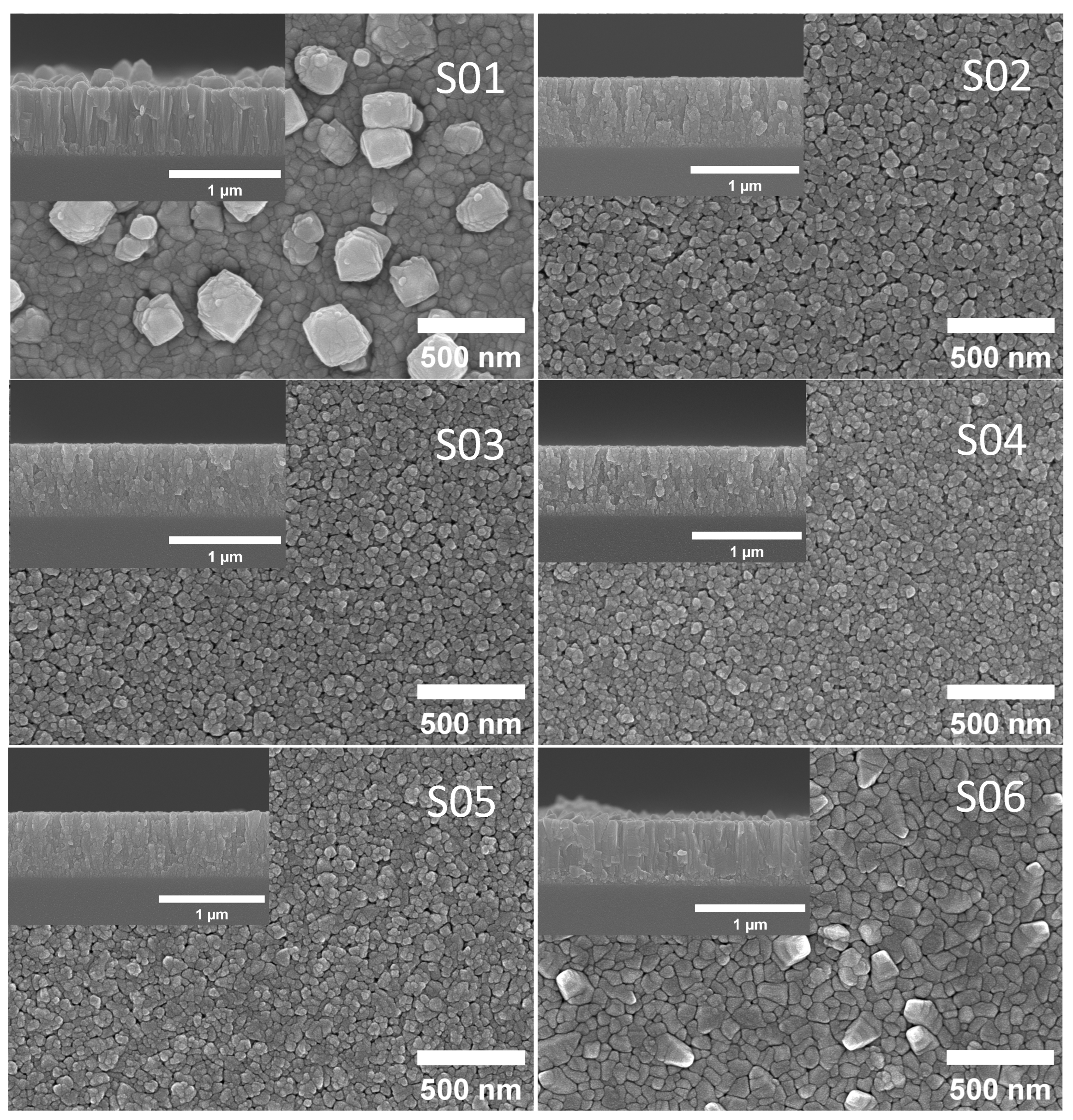
3.1. Structural Properties of Cu2O Thin Films
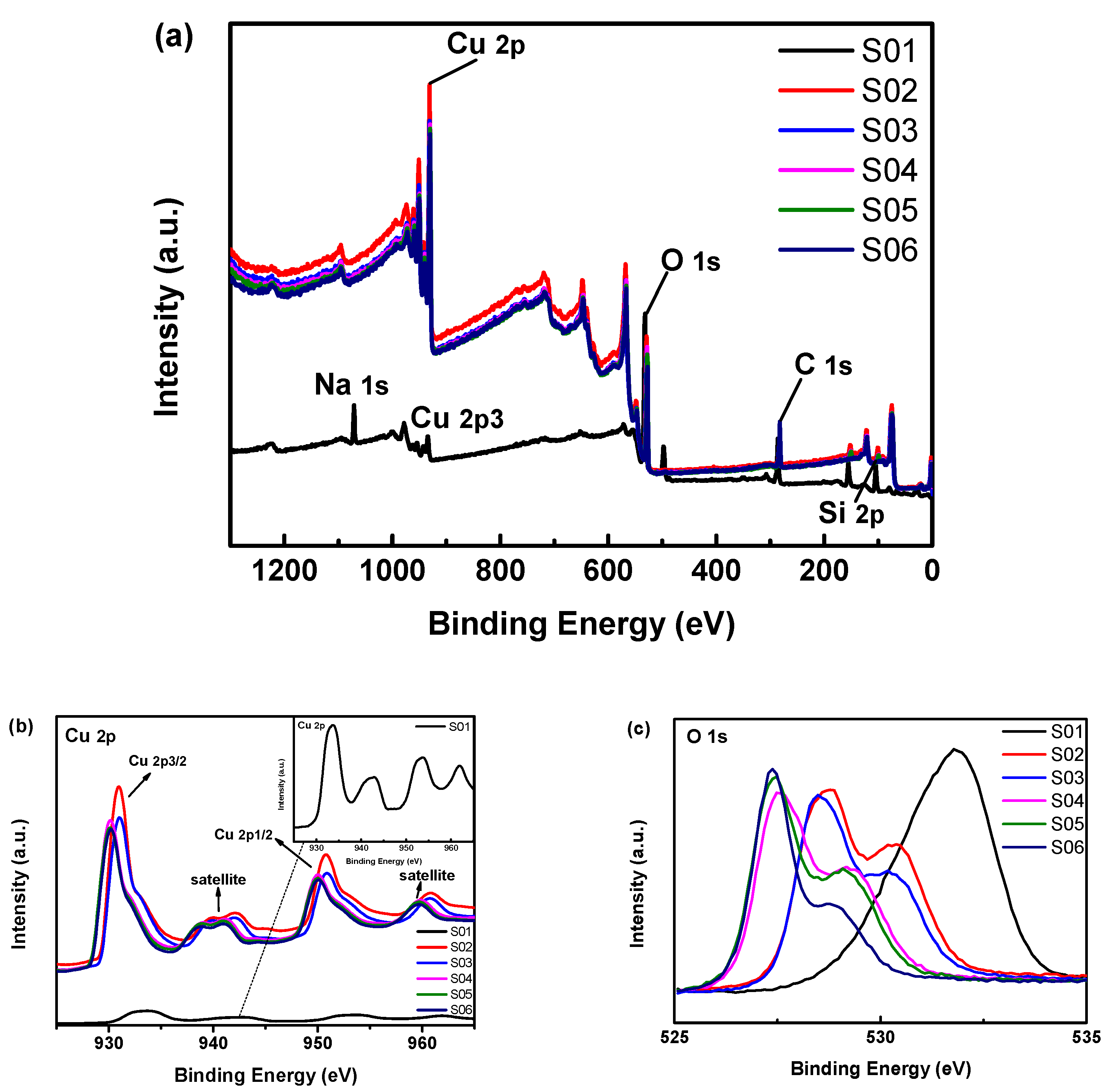
3.2. Compositional Properties of Cu2O Thin Films
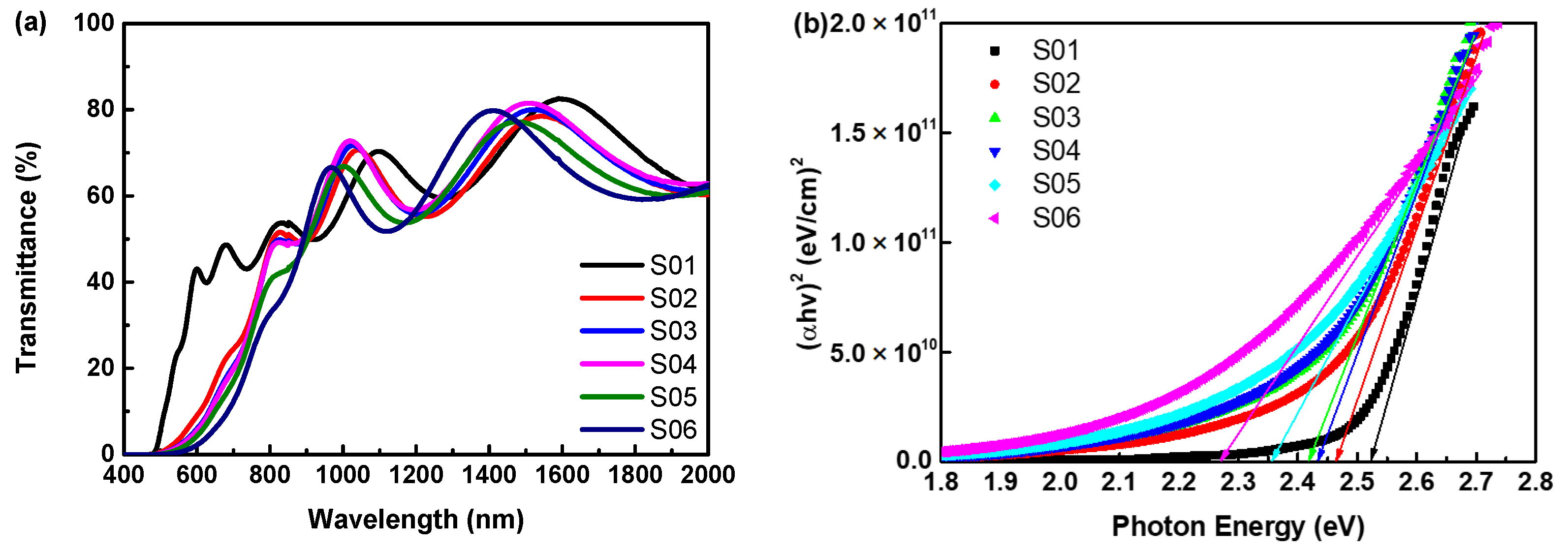
3.3. Optical Properties of Cu2O Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jo, J.; Lenef, J.D.; Mashooq, K.; Trejo, O.; Dasgupta, N.P.; Peterson, R.L. Causes of the Difference between Hall Mobility and Field-Effect Mobility for p-Type RF Sputtered Cu2O Thin-Film Transistors. IEEE Trans. Electron. Devices 2020, 67, 5557–5563. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Yang, Q.; Liang, S.; Zhang, X.; Yang, Z. Cuprous oxide (Cu2O) crystals with tailored architectures: A comprehensive review on synthesis, fundamental properties, functional modifications and applications. Prog. Mater. Sci. 2018, 96, 111–173. [Google Scholar] [CrossRef]
- Houng, B.; Wu, J.K.; Yeh, P.C.; Yeh, W.L.; Sun, C.K. Effect of Cu addition on the properties of the RF magnetron-sputtered Cu2O thin films. J. Electroceram. 2021, 45, 129–134. [Google Scholar] [CrossRef]
- Kardarian, K.; Nunes, D.; Maria Sberna, P.; Ginsburg, A.; Keller, D.A.; Vaz Pinto, J.; Deuermeier, J.; Anderson, A.Y.; Zaban, A.; Martins, R.; et al. Effect of Mg doping on Cu2O thin films and their behavior on the TiO2/Cu2O heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 27–36. [Google Scholar] [CrossRef]
- Дёмин В, Г.; Brungardt, M.V.; Goncharova, E.A.; Fedorov, L.Y.; Karpov, I.V.; Ushakov, A.V. Investigation of the effect of oxygen partial pressure on the phase composition of copper oxide nanoparticles by vacuum arc synthesis. Tech. Phys. 2022, 67, 2410. [Google Scholar]
- Al-Kuhaili, M.F. Characterization of copper oxide thin films deposited by the thermal evaporation of cuprous oxide (Cu2O). Vacuum 2008, 82, 623–629. [Google Scholar] [CrossRef]
- Ungeheuer, K.; Marszalek, K.W.; Mitura-Nowak, M.; Jelen, P.; Perzanowski, M.; Marszalek, M.; Sitarz, M. Cuprous Oxide Thin Films Implanted with Chromium Ions-Optical and Physical Properties Studies. Int. J. Mol. Sci. 2022, 23, 8358. [Google Scholar] [CrossRef]
- Liau, L.C.-K.; Tang, C.-H. Effect of a Cu2O buffer layer on the efficiency in p-Cu2O/ZnO hetero-junction photovoltaics using electrochemical deposition processing. J. Appl. Electrochem. 2022, 52, 1459–1467. [Google Scholar] [CrossRef]
- Patwary, M.A.M.; Saito, K.; Guo, Q.; Tanaka, T. Influence of oxygen flow rate and substrate positions on properties of Cu-oxide thin films fabricated by radio frequency magnetron sputtering using pure Cu target. Thin Solid. Films 2019, 675, 59–65. [Google Scholar] [CrossRef]
- Reddy, A.S.; Uthanna, S.; Reddy, P.S. Properties of dc magnetron sputtered Cu2O films prepared at different sputtering pressures. Appl. Surf. Sci. 2007, 253, 5287–5292. [Google Scholar] [CrossRef]
- Dolai, S.; Dey, R.; Das, S.; Hussain, S.; Bhar, R.; Pal, A.K. Cupric oxide (CuO) thin films prepared by reactive d.c. magnetron sputtering technique for photovoltaic application. J. Alloys Compd. 2017, 724, 456–464. [Google Scholar] [CrossRef]
- Wee, S.H.; Huang, P.S.; Lee, J.K.; Goyal, A. Heteroepitaxial Cu2O thin film solar cell on metallic substrates. Sci. Rep. 2015, 5, 16272. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Kumar, M.D.; Park, W.-H.; Patel, M.; Kim, J. Cu4O3-based all metal oxides for transparent photodetectors. Sens. Actuators A Phys. 2017, 253, 35–40. [Google Scholar] [CrossRef]
- Patwary, M.A.M.; Ho, C.Y.; Saito, K.; Guo, Q.; Yu, K.M.; Walukiewicz, W.; Tanaka, T. Effect of oxygen flow rate on properties of Cu4O3 thin films fabricated by radio frequency magnetron sputtering. J. Appl. Phys. 2020, 127, 085302. [Google Scholar] [CrossRef]
- Patwary, M.A.M.; Saito, K.; Guo, Q.; Tanaka, T.; Man Yu, K.; Walukiewicz, W. Nitrogen Doping Effect in Cu4O3 Thin Films Fabricated by Radio Frequency Magnetron Sputtering. Phys. Status Solidi (b) 2019, 257, 1900363. [Google Scholar] [CrossRef]
- Cruz Almazán, M.A.; Vigueras Santiago, E.; López, R.; Hernández López, S.; Castrejón Sánchez, V.H.; Esparza, A.; Encarnación Gómez, C. Cu4O3 thin films deposited by non-reactive rf-magnetron sputtering from a copper oxide target. Rev. Mex. De Física 2021, 67, 495–499. [Google Scholar]
- Rahmat, R.; Heryanto, H.; Ilyas, S.; Fahri, A.N.; Mutmainna, I.; Rahmi, M.H.; Tahir, D. The relation between structural, optical, and electronic properties of composite CuO/ZnO in supporting photocatalytic performance. Desalination Water Treat. 2022, 270, 289–301. [Google Scholar] [CrossRef]
- Purusottam-Reddy, B.; Sivajee-Ganesh, K.; Jayanth-Babu, K.; Hussain, O.M.; Julien, C.M. Microstructure and supercapacitive properties of rf-sputtered copper oxide thin films: Influence of O2/Ar ratio. Ionics 2015, 21, 2319–2328. [Google Scholar] [CrossRef]
- Solache-Carranco, H.; Juarez-Diaz, G.; Galvan-Arellano, M.; Martinez-Juarez, J.; Romero-Paredes, R.G.; Pena-Sierra, R. Raman scattering and photoluminescence studies on Cu2O. In Proceedings of the 5th International Conference on Electrical Engineering, Computing Science and Automatic Control, Mexico City, Mexico, 12–14 November 2008. [Google Scholar]
- Jagadish, K.A.; Kekuda, D. Thermal annealing effect on phase evolution, physical properties of DC sputtered copper oxide thin films and transport behavior of ITO/CuO/Al Schottky diodes. Appl. Phys. A Mater. Sci. Process 2024, 130, 315. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Yu, C.-H.; Chen, Y.-C.; Lin, Y.-G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactivelysputtered copper oxide electrodes for supercapacitor and electrocatalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Kang, Y.; Yang, J.C.; Zhou, G. Effect of oxygen gas pressure on orientations of Cu2O nuclei during the initial oxidation of Cu(100), (110) and (111). Surf. Sci. 2012, 606, 1790–1797. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface Oxidation and Reduction of CuO and Cu2O Studied Using XPS and XAES. Surf. Interface Anal. 1998, 24, 811–820. [Google Scholar] [CrossRef]
- Messaoudi, O.; Assaker, I.B.; Gannouni, M.; Souissi, A.; Makhlouf, H.; Bardaoui, A.; Chtourou, R. Structural, morphological and electrical characteristics of electrodeposited Cu2O: Effect of deposition time. Appl. Surf. Sci. 2016, 366, 383–388. [Google Scholar] [CrossRef]
- Wang, Y.; Miska, P.; Pilloud, D.; Horwat, D.; Mücklich, F.; Pierson, J.F. Transmittance enhancement and optical band gap widening of Cu2O thin films after air annealing. J. Appl. Phys. 2014, 115, 073505. [Google Scholar] [CrossRef]
- Alajlani, Y.; Placido, F.; Barlow, A.; Chu, H.O.; Song, S.; Ur Rahman, S.; De Bold, R.; Gibson, D. Characterisation of Cu2O, Cu4O3, and CuO mixed phase thin films produced by microwave-activated reactive sputtering. Vacuum 2017, 144, 217–228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Son, Y.-G.; Son, C.-S.; Hwang, D. Influence of Oxygen Flow Rate on the Phase Structures and Properties for Copper Oxide Thin Films Deposited by RF Magnetron Sputtering. Coatings 2024, 14, 930. https://doi.org/10.3390/coatings14080930
Park J, Son Y-G, Son C-S, Hwang D. Influence of Oxygen Flow Rate on the Phase Structures and Properties for Copper Oxide Thin Films Deposited by RF Magnetron Sputtering. Coatings. 2024; 14(8):930. https://doi.org/10.3390/coatings14080930
Chicago/Turabian StylePark, Junghwan, Young-Guk Son, Chang-Sik Son, and Donghyun Hwang. 2024. "Influence of Oxygen Flow Rate on the Phase Structures and Properties for Copper Oxide Thin Films Deposited by RF Magnetron Sputtering" Coatings 14, no. 8: 930. https://doi.org/10.3390/coatings14080930




