Doping and Superhydrophobic Modification for Improving Marine Antifouling Performance of Alkali-Based Geopolymer Coating
Abstract
1. Introduction
2. Materials and Methods
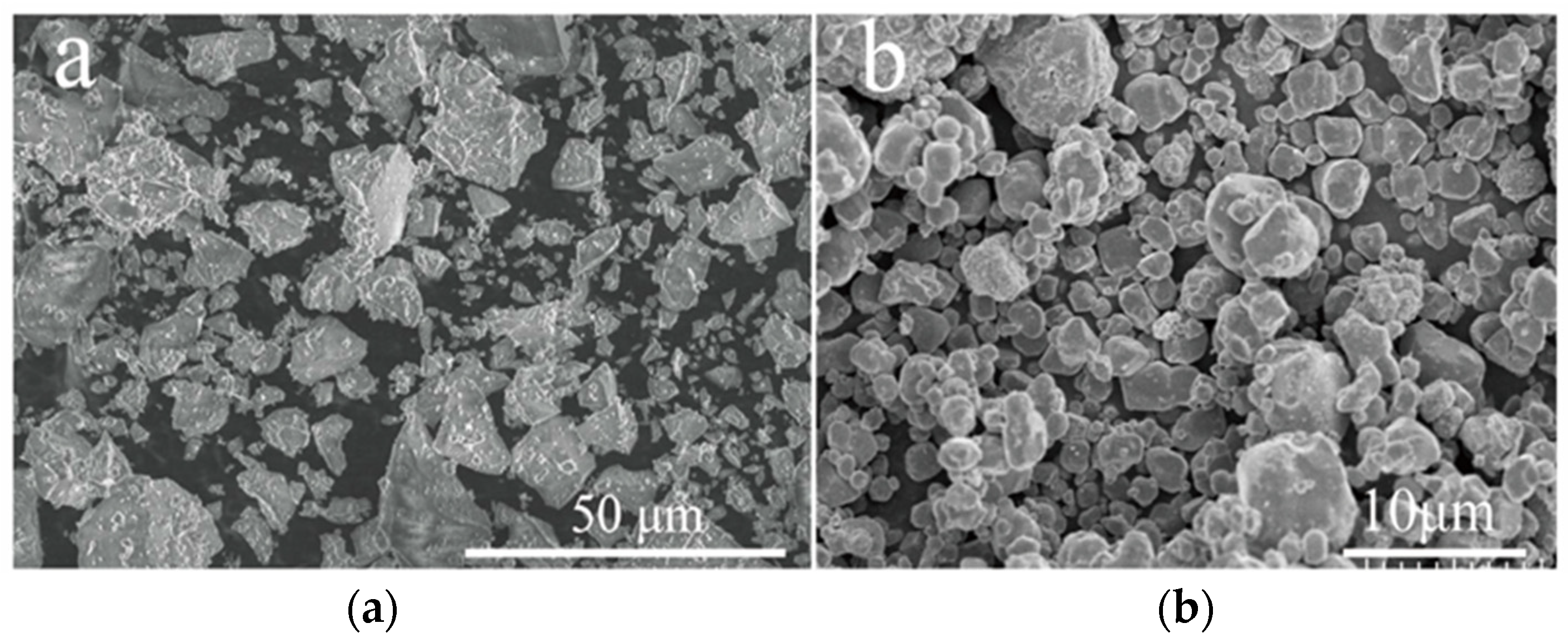
2.1. Materials
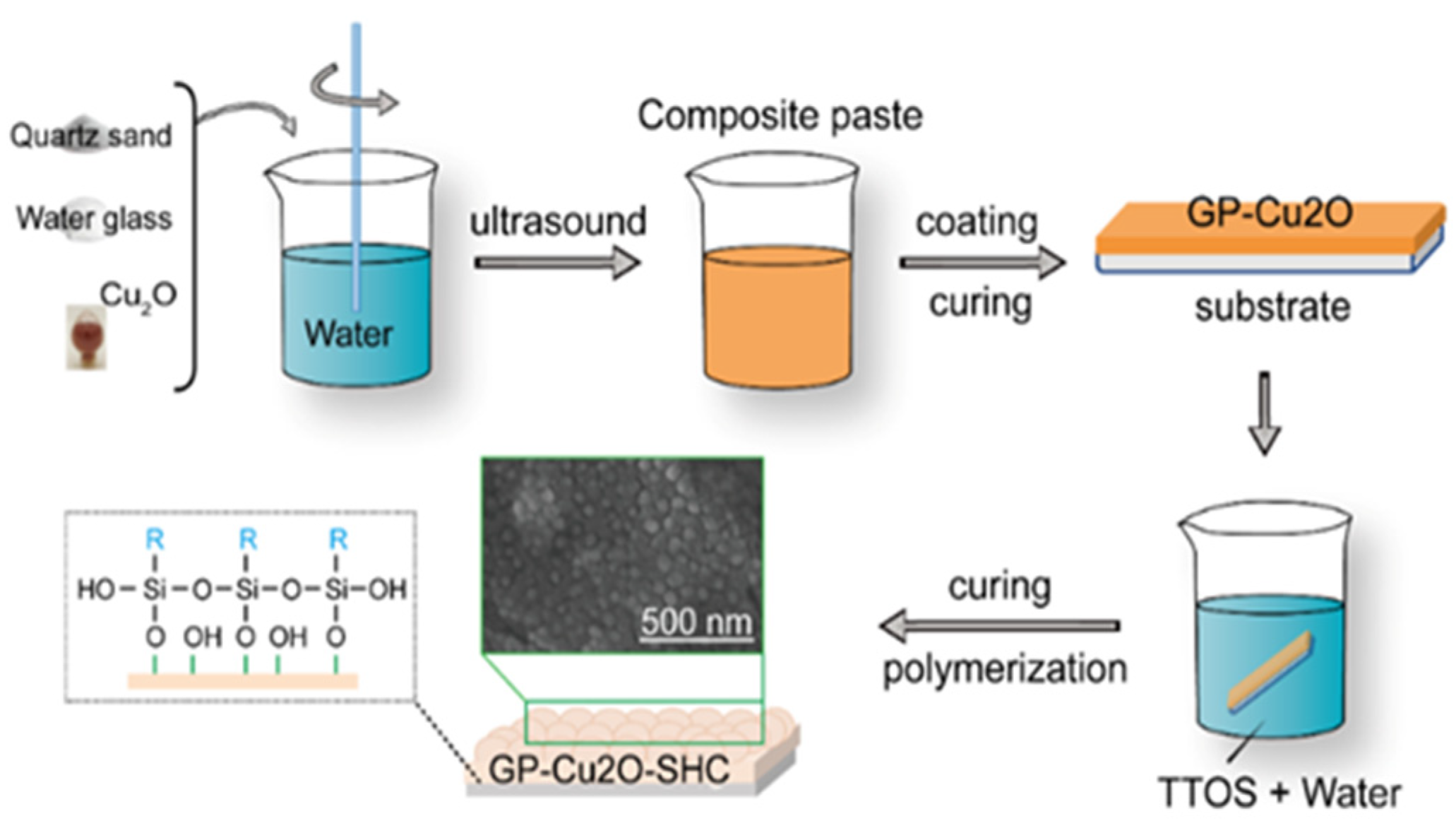
2.2. Fabrication of Superhydrophobic Alkali-Activated Materials Coating
2.3. Preparation of Superhydrophobic Geopolymers
2.4. Characterization
2.5. Antifouling and Antibacterial Analysis
3. Results
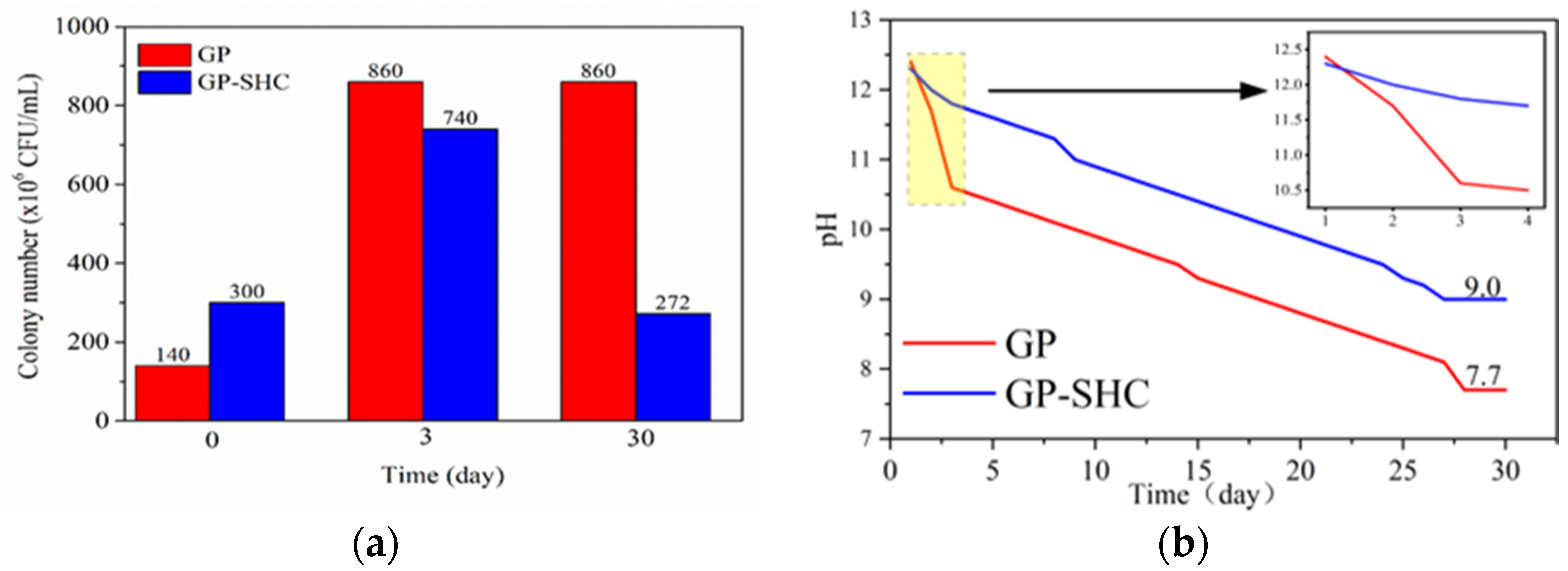
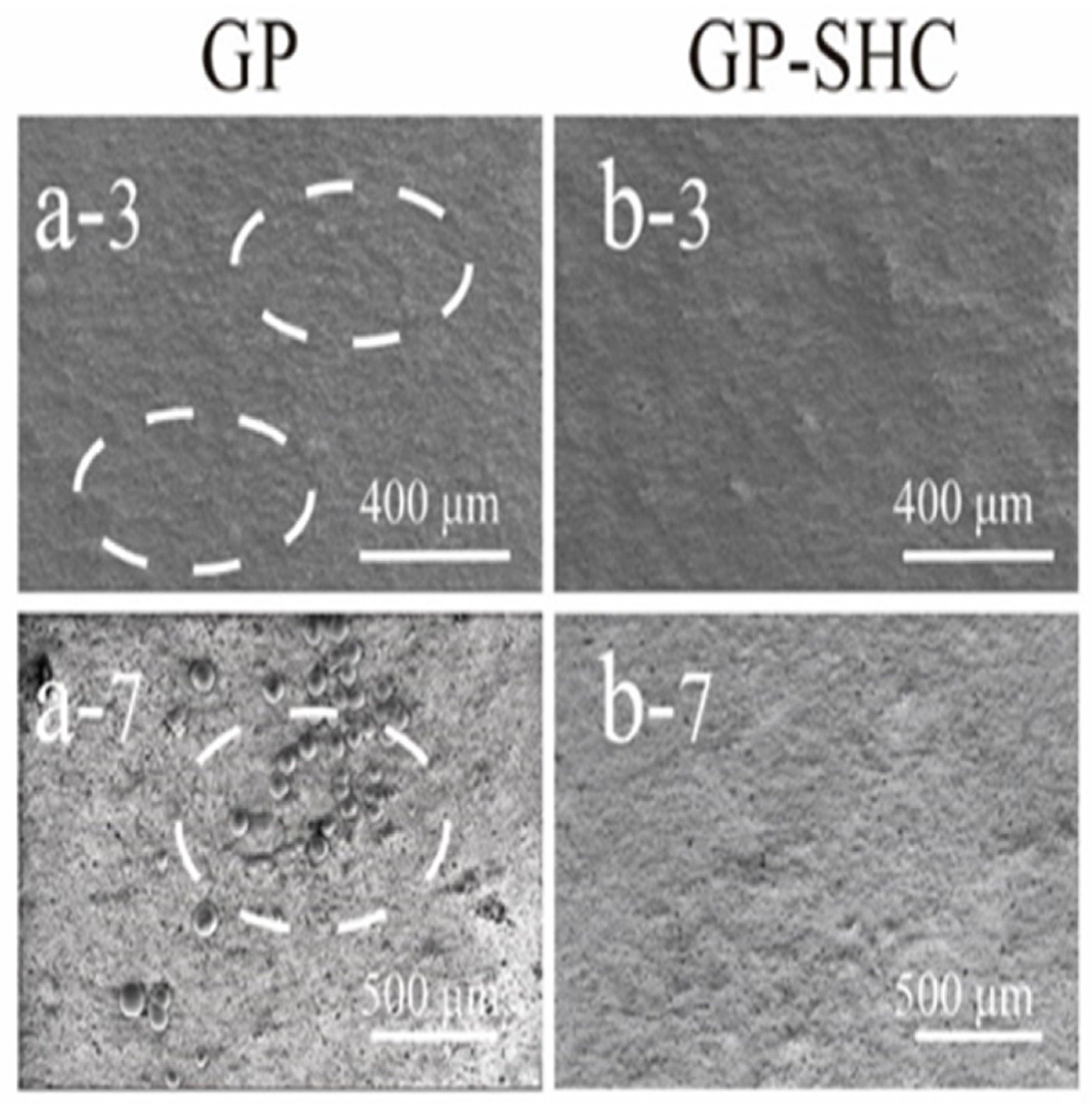
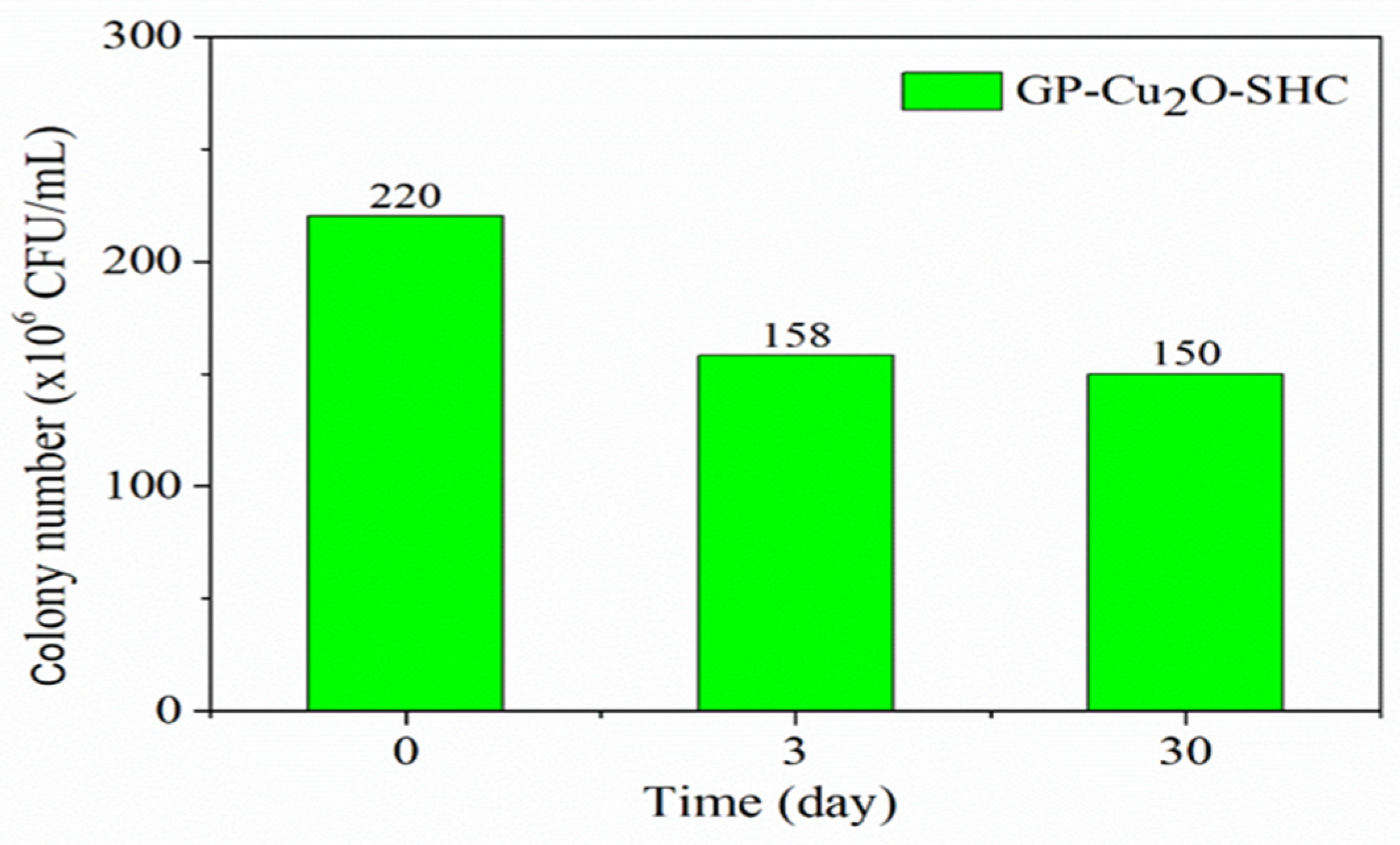
3.1. Antibacterial and Antifouling Performance of GP and GP-SHC
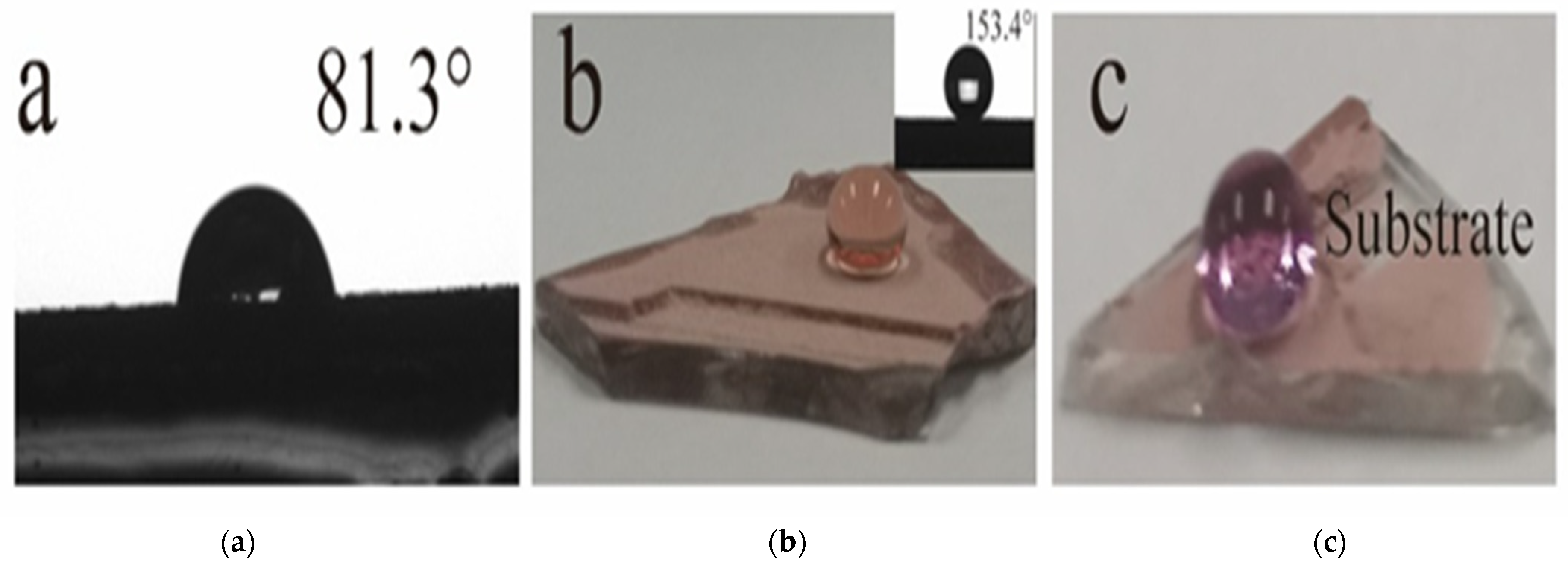
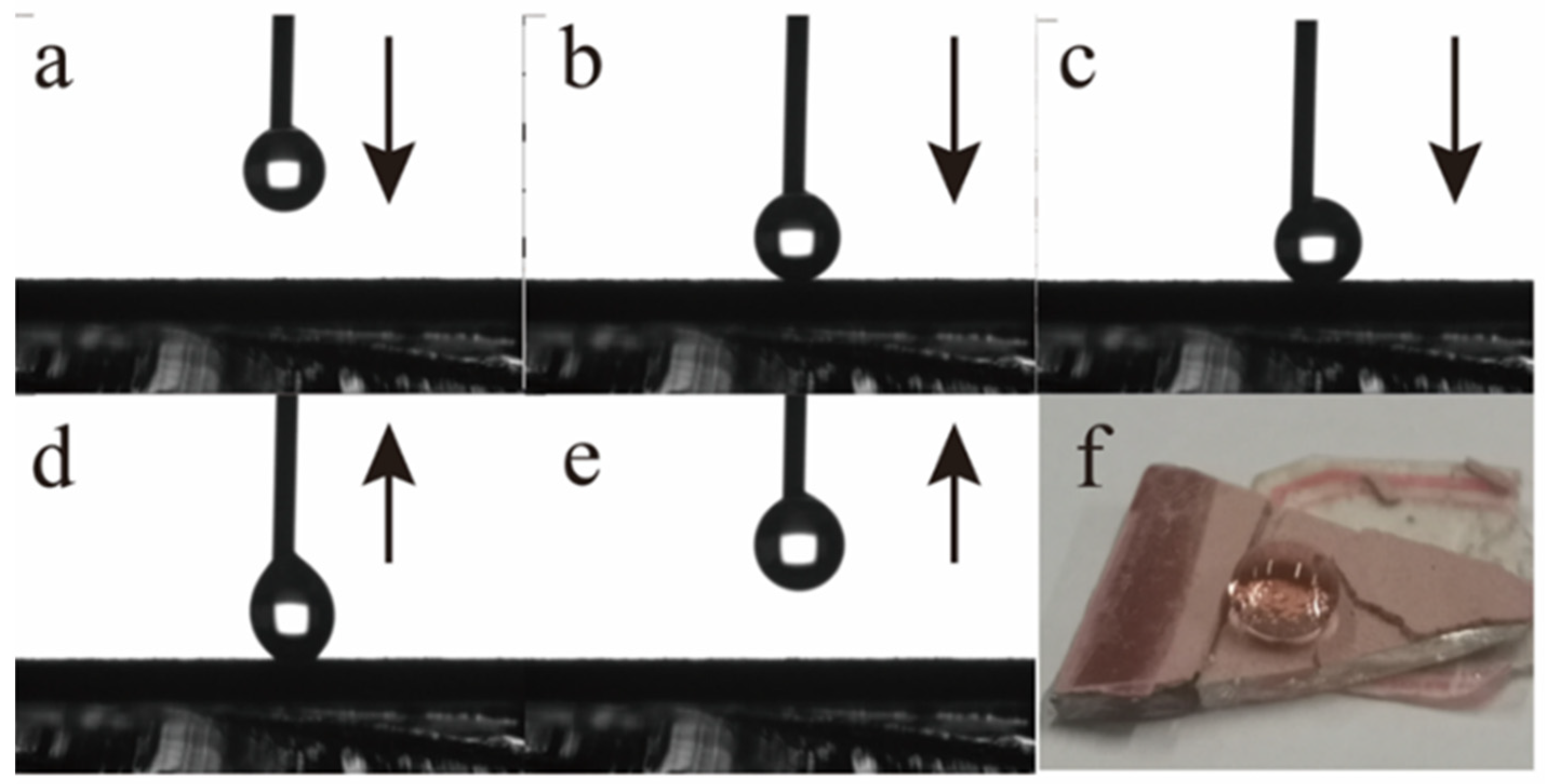
3.2. Wettability of GP-Cu2O and GP-Cu2O-SHC
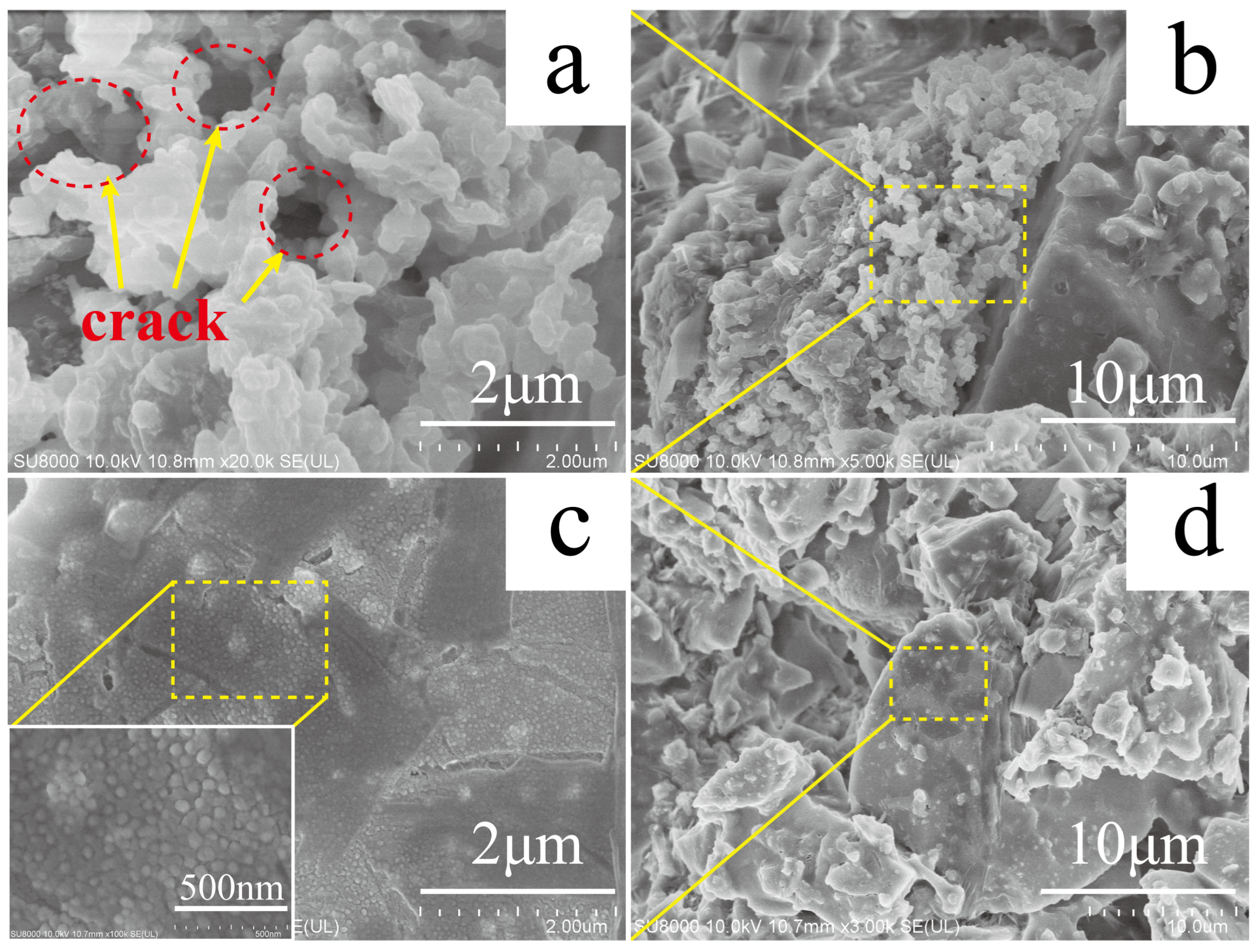
3.3. Surface Structure Characterization of GP-Cu2O and GP-Cu2O-SHC
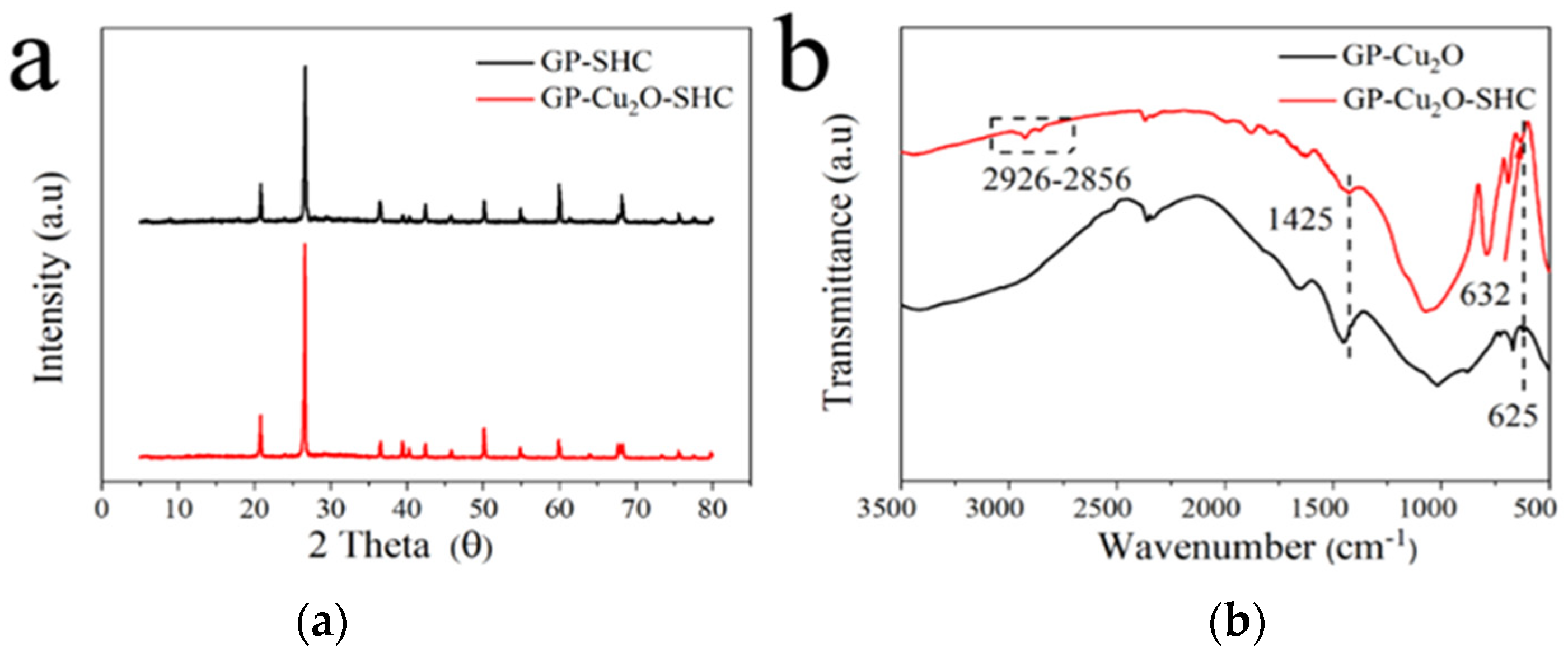
3.4. Physical and Chemical Characteristic Analysis
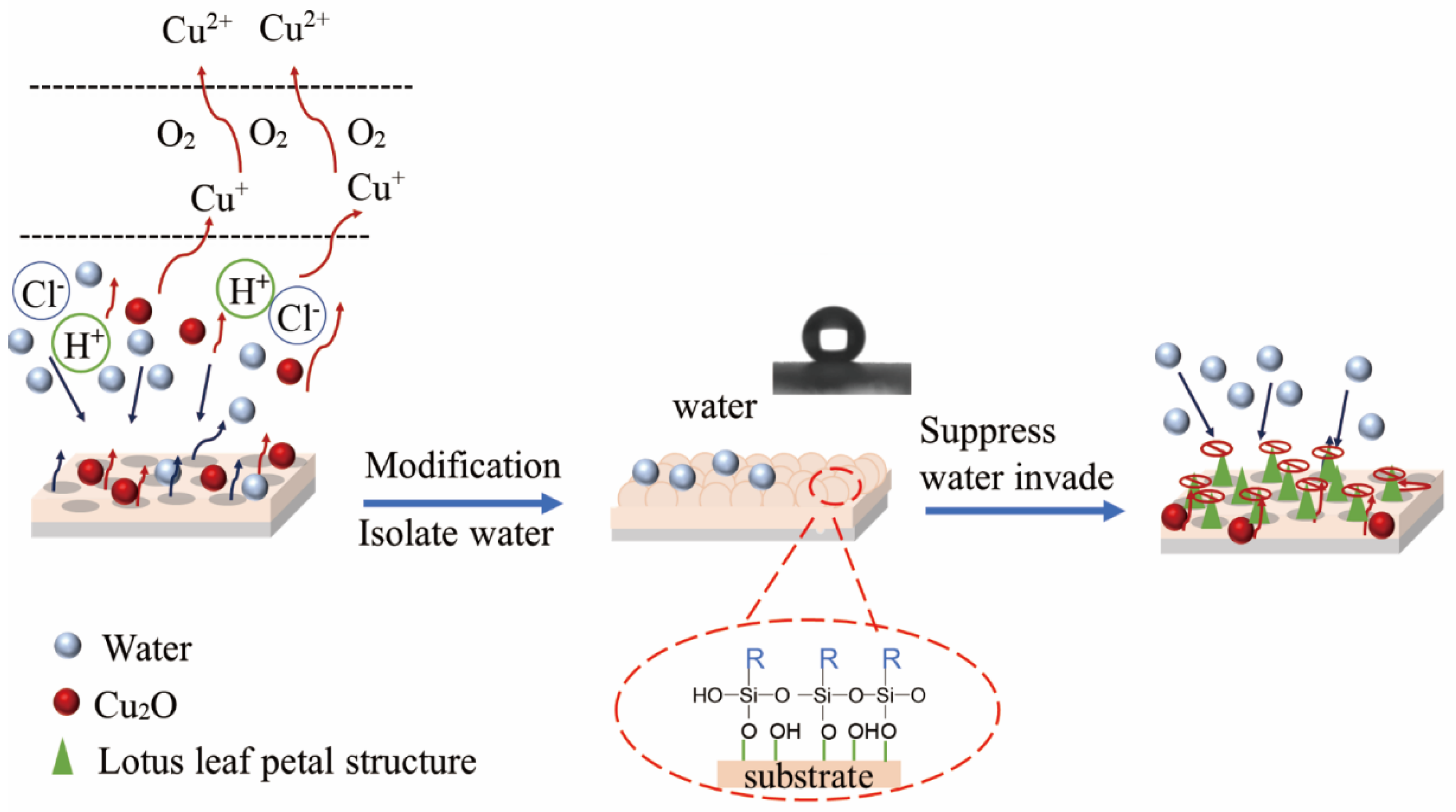
3.5. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Ali, A.; Jamil, M.I.; Jiang, J. An overview of controlled-biocide-release coating based on polymer resin for marine antifouling applications. J. Polym. Res. 2020, 27, 85. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, J.; Zhao, W.; Xu, T.; Lin, C.; Zhang, J.; Zhang, L. Direct formation of amphiphilic crosslinked networks based on PVP as a marine anti-biofouling coating. Chem. Eng. J. 2019, 374, 1353–1363. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Lu, G.; Xu, C.; Yu, H.; Yu, J. Preparation and properties of polyvinylpyrrolidone-cuprous oxide microcapsule antifouling coating. Prog. Org. Coat. 2020, 141, 105317. [Google Scholar] [CrossRef]
- Mohammad, A.A.; El-Sayed, M.S.; Saeed, M.A.-Z. Fabrication of Various Epoxy Coatings for Offshore Applications and Evaluating Their Mechanical Properties and Corrosion Behavior. Int. J. Electrochem. Sci. 2013, 8, 3121–3131. [Google Scholar]
- Ehsan, B.; Ali, J.; Zahra, R.; Sarah, S.; Mohammad, R.S. Anti-corrosion hybrid coatings based on epoxy–silica nano-composites: Toward relationship between the morphology and EIS data. Prog. Org. Coat. 2014, 77, 1169–1183. [Google Scholar]
- Price, S.J.; Figueira, R.B. Corrosion Protection Systems and Fatigue Corrosion in Offshore Wind Structures: Current Status and Future Perspectives. Coatings 2017, 7, 25. [Google Scholar] [CrossRef]
- Valkirs, A.O.; Seligman, P.F.; Haslbeck, E. Measurement of copper release rates from antifouling paint under laboratory and in situ conditions: Implications for loading estimation to marine waterbodies. Mar. Pollut. Bull. 2003, 46, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Li, C. ZnO/Acrylic Polyurethane Nanocomposite Superhydrophobic Samples on Aluminum Substrate Obtained via Spraying and Co-Curing for the Control of Marine Biofouling. Surf. Interfaces 2021, 22, 100833. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Qiu, J.; Cao, J.; Zou, Y.; Wang, S.; Jia, D.; Zhou, Y. Robust Inorganic Daytime Radiative Cooling Samples Based on a Phosphate Alkali activated materials. ACS Appl. Mater. Interfaces 2020, 12, 54963–54971. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.H.; Abdullah, M.M.A.B.; Che Pa, F. Phase Transformation of Kaolin-Ground Granulated Blast Furnace Slag from Alkali activated materialsization to Sintering Process. Magnetochemistry 2021, 7, 32. [Google Scholar] [CrossRef]
- Lv, X.S.; Wang, K.; He, Y.; Cui, X.M. A green drying powder inorganic samples based on alkali activated materials technology. Constr. Build. Mater. 2019, 214, 441–448. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.-L.; Dai, J.-G. Inhibiting efflorescence formation on fly ash–based alkali activated materials via silane surface modification. Cem. Concr. Compos. 2018, 94, 43–52. [Google Scholar] [CrossRef]
- Kuang, L.H.; Xiang, J.C.; Li, G.H.; Ma, W.J.; Cui, X.M. Effect of seawater on the properties and microstructure of metakaolin/slag-based geopolymers. Constr. Build. Mater. 2023, 397, 132418. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, Y.L.; He, L.; Feng, Y.J.; Tan, H.Y.; Chen, X.; Yang, W.S. Simultaneous detection of multiple neuroendocrine tumor markers in patient serum with an ultrasensitive and antifouling electrochemical. Biosens. Bioelectron. 2021, 194, 113603. [Google Scholar] [CrossRef]
- Lan, W.Q.; Xie, J.; Mao, F.; Chen, W.Y. Antibacterial effect of composite natural preservatives against Staphylococcus aureus. J. Food Sci. Biotechnol. 2014, 33, 1673–1689. [Google Scholar]
- Nostro, A.; Cellini, L.; Di Giulio, M.; D’Arrigo, M.; Marino, A.; Blanco, A.R.; Favaloro, A.; Cutroneo, G.; Bisignano, G. Effect of alkaline pH on staphylococcal biofilm formation. APMIS 2012, 120, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/graphene oxide sheet-alumina nanorod ternary composite for superhydrophobic antifouling coating. Prog. Org. Coat. 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Selim, M.S.; Yang, H.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A.; Wang, F.Q.; Huang, Y. Superhydrophobic coating of silicone/β–MnO2 nanorod composite for marine antifouling. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 518–530. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, B. A novel colorful sepiolite-based superhydrophobic samples with excellent mechanical and chemical stability and self-cleaning property. Mater. Lett. 2019, 254, 340–343. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Lin, Z.X.; Tian, Z.K.; Cui, X.M. Inhibition of Efflorescence in Na-Based Geopolymer Inorganic Coating. ACS Omega 2020, 5, 14822–14830. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cai, C.; Long, Y. Cu2O-clay composites with sub-micrometer-sized Cu2O particles for marine antifouling paints. J. Coat. Technol. Res. 2018, 16, 25–30. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Wu, Y.; Zhou, C.; Li, L. Antibacterial behaviors of Cu2O particles with controllable morphologies in acrylic samples. Appl. Surf. Sci. 2019, 465, 279–287. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Qin, Y.; Tan, J.; Liu, J.; Li, J.; Cui, X. Doping and Superhydrophobic Modification for Improving Marine Antifouling Performance of Alkali-Based Geopolymer Coating. Coatings 2024, 14, 974. https://doi.org/10.3390/coatings14080974
Sun M, Qin Y, Tan J, Liu J, Li J, Cui X. Doping and Superhydrophobic Modification for Improving Marine Antifouling Performance of Alkali-Based Geopolymer Coating. Coatings. 2024; 14(8):974. https://doi.org/10.3390/coatings14080974
Chicago/Turabian StyleSun, Mingyang, Yao Qin, Jianli Tan, Jiazheng Liu, Jing Li, and Xuemin Cui. 2024. "Doping and Superhydrophobic Modification for Improving Marine Antifouling Performance of Alkali-Based Geopolymer Coating" Coatings 14, no. 8: 974. https://doi.org/10.3390/coatings14080974
APA StyleSun, M., Qin, Y., Tan, J., Liu, J., Li, J., & Cui, X. (2024). Doping and Superhydrophobic Modification for Improving Marine Antifouling Performance of Alkali-Based Geopolymer Coating. Coatings, 14(8), 974. https://doi.org/10.3390/coatings14080974




