Abstract
With the establishment of a global carbon-neutral target, green and sustainable design concepts, materials, and processes have become the key to scientific and technological innovation, which highlights the development and utilization of environmentally-friendly materials and renewable energy sources. At present, almost all the raw materials in commercial photoresist are from oil resources. In order to implement the policy of “green chemistry”, there is an urgent need to take the place of petroleum with renewable, biodegradable, and green biomass resources in photoresist production. As a kind of green and natural high polymer material from China, lacquer has excellent physical and chemical properties and has great application potential in the field of electronics. In this paper, the potential connections between photoresist and lacquer, especially in the green development of photoresist, are sorted out. The introduction of lacquer and its derivatives may help to improve the environmentally-friendly production of photoresist and its performance. Therefore, in the context of global carbon neutrality, the study of photoresists in the “lacquer” is of great significance in promoting the application of eco-friendly materials in the field of microelectronics.
1. Introduction
With the rapid and sustained development of the electronic information industry, the market demand for integrated circuit components has been growing in recent years, prompting the rapid development of microelectronic technology. The demand for continuous miniaturization of the feature size has driven the development of the photolithography technology, with a great leap from near-ultraviolet light to the deep ultraviolet light and then to extreme ultraviolet light, as shown in Figure 1a. However, the sharp decrease in the wavelength also pushes the photoresist materials to be constantly updated. From near-ultraviolet photoresist (wavelength 436~365 nm) to extreme ultraviolet photoresist (13.5 nm) as shown in Figure 1b, the rapid development of the technology requires more and more sensitivity, resolution, line edge roughness, and other parameters of the photoresist materials [1]. The composition of photoresist usually includes resin, photoinitiator, reactive diluent, and additives. The properties of photoresist, including adhesion, hardness, toughness, thermal stability, and viscosity, are determined by the resin, and common light-cured resins include epoxy resin, phenolic resin, and polyimide. However, it is worth noting that almost all raw materials for commercial light-cured resins are from petroleum, which is a non-renewable resource, and its reserve is decreasing. In addition, in the process of extraction, separation, and purification of petroleum, pollutants such as exhaust gases and wastewater are obviously generated, polluting the environment, which runs counter to the policy of green chemistry. In this connection, how to ensure the performance of photoresist while achieving green and low-carbon production and application is an urgent issue to be solved at present.
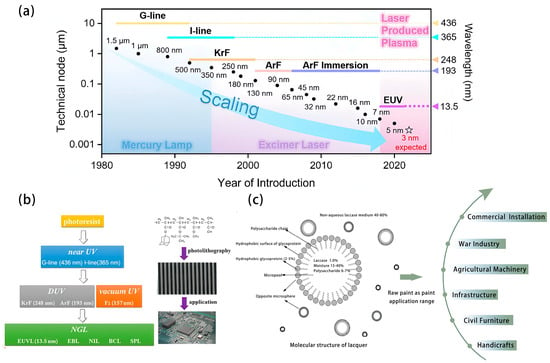
Figure 1.
(a) Scaling of microelectronic technology in technical nodes with the development of the semiconductor industry [2]. (b) Classification, development, and application of photoresist. (c) Raw paint as paint application range.
The lacquer tree, a kind of plant of Toxicodendron genus of the sumac family, belongs to the subtropical flora, and the relic plant in the third period of the Cenozoic era can be dated back to 70 million years ago. From the primitive society, Chinese ancestors discovered the lacquer tree and knew how to use the natural lacquer made from the sap to paint objects and improve the durability. For example, the “lacquer bow” unearthed at the Kuahuqiao (Cross Lake Bridge) Historic Site in Xiaoshan District, Hangzhou City, Zhejiang Province, has a history of 8000 years. Red lacquer accessories have been unearthed at the Upper Cave (Shandingdong) Culture Site, and red lacquer bowls have been excavated at the Hemudu Culture Site. According to historical records, “Lacquer was first used in the bamboo slips. Emperor Shun made food vessels with black lacquer and Emperor Yu made sacrificial vessels with black lacquer outside and vermilion paint inside.” This shows that lacquer was an important material in ancient China. Lacquer art reached its peak in the Ming and Qing dynasties, giving birth to various kinds of lacquer craftwork. Each dynasty played an important role in the development of lacquer art, leading to a wide variety of lacquer works of art.
The “lacquer” here refers to raw lacquer as shown in Figure 1c, and the molecular structure of its main component urushiol contains aliphatic long-side chains and aromatic hydrocarbons, so laccase can catalyze the formation of lachenal polymers. Coupled with the oxidation polymerization of the long side chains, a three-dimensional network structure is formed. The lacquer film formed by chemical reaction has excellent properties such as corrosion resistance, high-temperature resistance, and acid and alkali resistance. In addition, the mixture of lacquer and pigments can show brilliant colors, which are widely used in decoration, anti-corrosion, and cultural relic restoration [3].
Therefore, under the background of global carbon neutrality, this paper draws inspiration from the environmental friendliness, excellent performance, and profound nature of lacquer, and explores the combination of it with modern photoresist technology to develop new environmentally-friendly photoresist with high performance. This interdisciplinary research not only promotes the integration of traditional culture and modern technology, but also meets the demand for environmentally-friendly materials in the microelectronics manufacturing industry, which has a broad market prospect and potential for sustainable development.
2. Green and Pollution-Free Photoresist
The main components of photoresist include resin, photoinitiators, solvents, and additives, which determine its performance and application effect in a photolithographic process. Most of the components need to be synthesized by using petroleum or other non-renewable resources. However, there are some components that can be synthesized by green materials. For example, the alkaline soluble biobased epoxy acrylic resins were successfully prepared by Zhou et al. [4] via a ring-opening reaction of epoxidized soybean oil (ESO) with the modified acrylic precursor (MMHEA), followed by diisocyanate modifying to introduce a small amount of polyurethane, as shown in Figure 2a. The epoxy acrylate resins produced in this way have excellent mechanical properties and thermal stability as shown in Figure 2d,e. A new kind of castor oil-based methacrylate prepolymer (MCOG) was successfully prepared from castor oil by Liang et al. [5], as shown in Figure 2b. MCOG was used as the main component compounded with additives to form UV-curable coatings, and the resulting cured film showed relatively high mechanical properties as in Figure 2f, high thermal stability as in Figure 2g, high pencil hardness, and excellent resistance to acidic and alkaline solutions. The rigidity and flexibility of the cured film could also be adjusted by changing the content of each component in the formulation. In addition, digital images showed that the cured film had high transparency. Acrylic acid was successfully introduced into the double bond of the long chain of linseed oil by Su et al. [6] with the help of the catalyst boron trifluoride ethyl ether. This enabled the preparation of UV-cured acrylate esterified linseed oil prepolymer could be finished in a single step and provided a new way to synthesize UV-curable materials from plant oil in one step. The prepared high-performance film has the advantages of using economic and renewable resources as well as high performance, ensuring a broad application prospect.
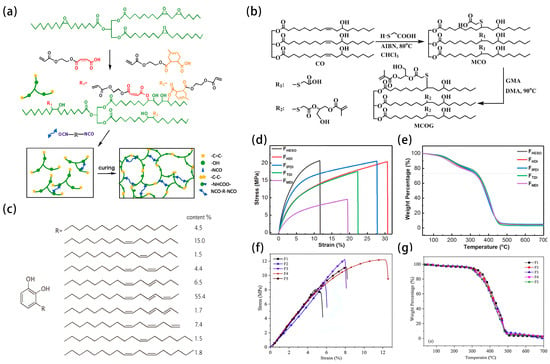
Figure 2.
(a) Epoxy soybean oil acrylate resin, diisocyanate modified epoxy soybean oil acrylate resin, UV-cured film, (b) synthetic route of MCOG, (c) structural formula of urushiol, (d–g) Stress-strain and TGA curves of FHESO, FHDI, FIPDI, FTDI and FMDI cured films, and Stress-strain and TGA curves of MCOG/PETA/B-215 cured films.
Lacquer is a kind of natural resin whose main components include urushiol, laccase, lacquer polysaccharide, glycoprotein, water, and other trace elements, with properties of anti-corrosion, strong acid resistance, strong alkali resistance, corrosion resistance, insulation, environmental friendliness, excellent adhesion, etc. The adhesion is mainly due to its own unique molecular structure, and there is no need to use a large amount of harmful solvents during use. The main component urushiol, as shown in Figure 2c, can be oxidized by oxygen, and ultimately produces a brownish-black polymer, especially in the presence of laccase. The phenolic hydroxyl group generates oxygen radicals by dissociation, and further loses electrons to become orthoquinone, which can undergo polymerization. In the air, the molecules of urushiol bond with each other to form a lacquer film with high stability, and the film-forming reaction is mainly divided into three steps.
In the first step, the urushiol is catalyzed and oxidized by laccase in oxygen, generating semiquinone radicals, of which the transfer forms polymers; in the second step, there is an auto-oxidation reaction on the aliphatic unsaturated side chains to build cross-links and continue to polymerize; in the third step, the compound of three-dimensional network structure is formed, and then cured into a film. Xiao et al. [7] prepared modified lacquer coatings by copolymerizing a formaldehyde-urea prepolymer with lacquer, and the results showed that the incorporation of the formaldehyde-urea prepolymer significantly improved the properties of the lacquer—the drying time of the lacquer was significantly shortened, the gloss, pencil hardness, and impact properties were all significantly enhanced, and the thermal stability and surface smoothness were also improved. On the reaction modification of lacquer with metal element compounds, Ko et al. [8] prepared natural lacquer phenol organogels with high mechanical strength through coordination bonds between lacquer phenol and Fe3+. Fe3+ ions acted as a catalyst for the polymerization of lacquer phenol, solving the problem that lacquer enzymes could not be controlled. The formation of coordination bonds increased chemical cross-linking and polymerization of the long alkyl side chains of the lacquer phenols, which in turn enhanced the tensile strength and durability of the large lacquer films.
In contrast, many of the components in traditional photoresists are synthetic, and the solvents commonly used are potentially harmful to the environment and human health. Epoxy resins consist of epoxy compounds and curing agents, and are characterized by high strength, abrasion, and corrosion resistance, but their high-temperature resistance is poor. Polyimide resin has a very high heat resistance and mechanical strength, excellent film-forming properties, and can form a high-temperature resistant film, but it needs to be modified or added photosensitizer to achieve photosensitive properties. As a natural polymer material, lacquer has unique environmental characteristics and excellent mechanical properties. Common photosensitizing resins such as epoxy, acrylic, and polyimide resins perform even better in terms of mechanical properties, film formation, and photosensitizing properties. However, the varnish itself has weak photosensitive properties and needs to be chemically modified or added with photosensitizers to enhance its photosensitive properties for more industrial applications.
In terms of industrial applications, lacquer and these photosensitive resins each have their own advantages and potential. As a natural, renewable material, lacquer meets the requirements of environmental protection and sustainable development, and is particularly suitable for use in areas with stringent environmental requirements. Due to its excellent corrosion and abrasion resistance, lacquer can be used in high-performance coatings to replace some synthetic resins. In addition, through modification to improve photosensitive properties, varnish is expected to be used in the photoresist field to reduce the use of petroleum-based materials and reduce environmental pollution. The insulating and heat-resistant properties of varnish also give it potential in the field of electronic packaging materials. The chemical reaction in the process of lacquer curing has some similarities with the photoinitiation reaction of photoresist, so it can provide a reference for the development of photoresists with low or non-toxic solvents [9].
3. Etch Resistance of Photoresist
Etch resistance of photoresist refers to its ability to withstand the photolithography process when it is subject to etching. With the development of photolithographic technology, photoresist continues to evolve. At present, what matters most is to develop a low-resolution resist to meet the requirements of the International Technology Roadmap for Semiconductors (ITRS) for sensitivity, resolution, and line width roughness. Therefore, the researchers solved this problem and produced photoresists by using chemically amplified resist [10]. Typically, a chemically amplified resist (CAR) system consists of a polymer matrix, a phosphoric acid generator (PAG), a base quencher, and a solvent. During the process of extreme ultraviolet lithography, CAR films (mainly polymeric matrix) absorb extreme ultraviolet photons to generate photoelectrons and subsequently secondary electrons. As a result, they undergo an ionization reaction with the PAG to produce photic acid [11,12,13,14,15]. During the process of baking after exposure, the generated acid diffuses throughout the resist film at random, triggering a variety of catalytic reactions and leading to an increase in solubility. A brief process flow is shown in Figure 3a [2]. Every detail in the lithography process can affect the final performance, which can be continuously improved by optimizing the photoresist formulation and controlling the physical and chemical changes during the lithography process. So far, chemically amplified photoresists have been widely used. It has been reported that a new type of chemically amplified positive-tone photoresist based on the bisphenol A structure (BPA) can be used in extreme ultraviolet lithography [16]. On this basis, a negative-tone molecular glass photoresist based on BPA-6OH for electron beam lithography has been developed [17]. This material has a high glass transition temperature and excellent thermal stability. After exposure in an e-beam lithography system, the negative-tone molecular glass photoresist achieves a sharp linear of a good contrast of about 73.4 nm and a sensitivity of 52 µC cm−2, which suggests that the negative-tone molecular glass photoresist has a promising future for application in e-beam lithography.
The common chemical amplification resists include polymer resist, molecular glass, and metal-containing resist. Figure 3c shows a comparison of the three kinds of resists. the photoresist prepared by the combination of organic and inorganic materials has significant advantages in terms of processability and etch resistance. Figure 3b shows the photoresist consisting of an inorganic metal core and an organic shell. The research made by Jiang et al. showed that the metal sensitizer can increase the sensitivity by 17% with little influence on linewidth roughness and local critical dimension uniformity [18].
In addition, compensating for the local critical dimension uniformity results in a significant increase of 30% in sensitivity and line width roughness. Pozo et al. produced a supramolecular cholesteric liquid crystal photonic photoresists for fabricating four-dimensional photonic microactuators which are shaped like flowers (Figure 3d) [19]. This review mainly focuses on the research of applying lacquer in photoresist. Lacquer shows good physical stability, and the research shows that the hardness of the lacquer film (lacquer film value/glass transition value) is up to 0.78–0.89, and the electrical breakdown strength reaches 50–80 kV/mm. Even if it is soaked in water for a long time, the strength can still be as high as 50 kV/mm. The lacquer film can withstand temperatures up to 250 °C in a short time, and the temperature should be below 150 °C during long-term use. If an equal amount of body pigments (such as porcelain powder) is added to the lacquer, the bond between it and the steel plate can be more than 70 kg/cm2 [20]. It is because of the thermal stability and corrosion resistance of lacquer that the lacquer might be used in photoresist. As the lacquer material is green and environmentally friendly, the lacquer material can be used as a kind of synthetic adhesive, which is not only in line with the green and environmentally-friendly development strategy under the background of dual-carbon goals, but also improves the stability of the photoresist to a certain extent.
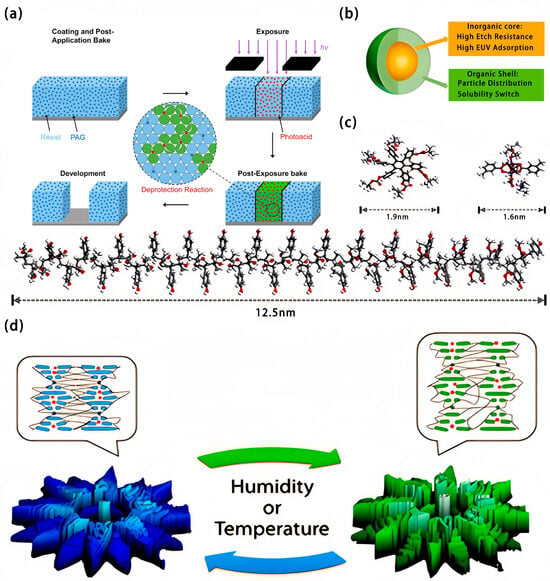
Figure 3.
(a) Schematic diagrams of CAR brief process flow and chemically amplified mechanism. (b) Schematic diagram of metal nanoparticles or metal oxide clusters. (c) Comparison of polymer resists, molecular glasses, and metal-containing resists. Lower one: 60 alternating repeating units of polymethyl methacrylate-4-hydroxystyrene [21]; upper left one: representative molecular glass hexa(m-tert-butoxycarbonyloxyphenyl)benzene [22]; upper right one: representative metal-resist Zn2(CH3C6H4COO)4(CH3COOHN(CH2CH3)3)2. (d) Three-dimensional profiles depicting direct (triggered by humidity) or indirect (triggered by temperature) initiation.
4. Curing Performance of Photoresists
The process of photoresist curing to film is a complex physical and chemical reaction process, involving coating, pre-drying, exposure, post-drying, development, and other steps. After these steps, the photoresist forms a thin film with a specific pattern on the surface of the substrate. Exposure is the key step in the curing of photoresist film, and the exposure time and coating thickness have a very important influence on its curing effect. If the light is not enough or the coating is too thick, the surface layer is cured yet the bottom layer is not cured completely.
As an adhesion agent, the resin also plays a vital role, because it determines the adhesion strength, hardness, heat resistance, and developability of the photoresist. Lee et al. [23] synthesized an acrylate copolymer resin as a binder for photoresist by means of free radical polymerization, and the surface-modified silicon dioxide particles prepared by the sol-gel method were introduced into the photoresist. The modified photoresist has a hardness of 5H, an adhesion of 100%, and a resolution of 10 μm on glass substrates. The photoresist can be developed smoothly in 60 s, and the mosaic and resolution test results shown in Figure 4a,b exhibit good performance. The compatibility of most of the other components with the resin also affects the performance of photoresist [24,25,26,27,28]. Tang et al. [29] attained a completely insoluble film by mixing polysiloxane and epoxy methacrylate resins with an amine curing agent, followed by direct irradiation curing at room temperature.
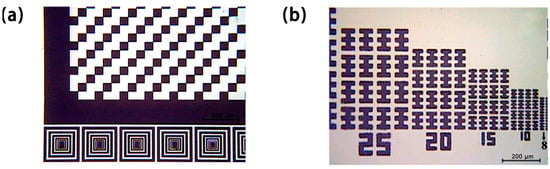
Figure 4.
Optical microscopy images of patterns formed by using photoresist. (a) Mosaic image. (b) Resolution test.
The curing film of natural lacquer can be formed naturally at room temperature, and it is hard and wear-resistant with good physical and chemical properties. The hardness of the film is highly controllable by changing thickness and the number of layers. The applicability of naturally formed films can also be improved by physical and chemical modification. Without adding photoinitiators, Xia [30] prepared UV-cured natural lacquer films by UV irradiation to study the curing mechanism, performance of the cured films, and morphology control, and found that this method can not only accelerate the formation of cured films while keeping the excellent physical and chemical properties of the naturally curing films, but also improve the heat resistance, adhesion to metals, and flexibility, as well the morphology control. For example, Ogawa et al. [31] improved the weather fastness by brominating the lacquer film and found that the brominated lacquer film showed better photostability in terms of gloss and color. It was found that regardless of the degree of bromination, the oxygen content on the surface of the lacquer film still increases gradually with the extension of ultraviolet illumination time.
This modification research has successfully improved the performance of lacquer, helped it to meet the needs of modern industry and life, and extended its scope of application. Whether it is a natural film or a modified film, it has high hardness and abrasion resistance. Through modifications, the lacquer can be applied to different electronic components to form a stronger and more durable coating.
5. Surface Adhesion of Photoresist
Adhesion strength is one of the important factors to ensure the reliability of photolithography, because poor adhesion performance of photoresist will lead to the peeling and collapse of the photoresist pattern in the photolithography process [32]. Many research groups are also trying to explore how to improve the adhesion of photoresist. For example, Hasegawa et al. proposed a method to directly analyze the adhesion in the nanoscale area [33]. The peeling test is a direct assessment of the failure modes of the resist and substrate adhesion, which are mainly categorized into interface failure and cohesive failure [34]. The adhesive force can be quantitatively assessed by peeling test and free energy analysis of the material surface, and adhesive energy can be assessed by using AFM. By demonstrating the peeling test of the adhesion between the photoresist film and the silicon substrate, the difference in the failure modes due to the surface condition of the substrate can be confirmed. The interaction energy (J) between the tip of the AFM and the surface-modified silicon substrate can be evaluated in a region with a diameter of less than 10 nm, as shown in Figure 5a. The interaction energy (J) between the tip of AFM and the surface of the silicon substrate is positively correlated with the adhesion work between the photoresist and the surface of the silicon substrate. This evaluation method would help to minimize the patterning defects within the diameter of 10 nm due to poor adhesion. Chen et al. also developed a comprehensive semiconductor photoresist with a nano-interpenetrating structure, as shown in Figure 5b [35]. Besides, Cao et al. also conducted research on the regulation of the viscosity, mechanical strength, volume shrinkage, and polymerization reaction rate of the photoresist [36]. The two-color photosensitive direct laser writing (TCS-DLW) is a novel nanolithography technique that can break through the optical diffraction limit and realize the fabrication of 2D/3D micro-nano structures with sub-hundred-nanometer resolution [37], as shown in Figure 5c. Polyvinylpyrrolidone (PVP), as a hybrid component, is introduced into the pentaerythritol triacrylate (PETA) active monomer, and the result shows that PVP had hydrogen-bond interactions with PETA, which can be used as a cross-linking site to improve the cross-linking degree of photoresist. Meanwhile, the introduction of PVP increases the system viscosity, which can reduce the oxygen barrier polymerization effect and effectively enhance the photopolymerization monomer conversion of PETA in photoresist (30.1%), obtaining higher sensitivity and a lower polymerization threshold (6.5 mW, 20 wt% PVP).
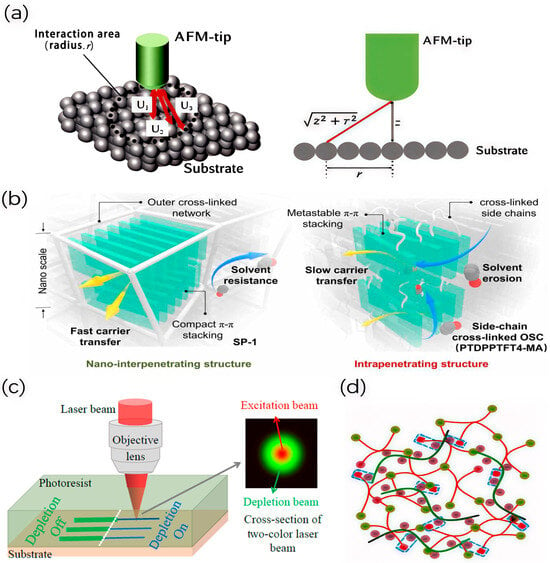
Figure 5.
(a) Diagram of tip-substrate interactions. (b) Diagram of nano-interpenetrating structure. (c) Diagram of the TCS-DLW process. (d) Molecular structure of photoresist.
These studies provide more ideas for improving the interface adhesion of photoresists, and the paper also proposes a conception of using lacquer for photoresists, as the urushiol component has strong adhesion that can form a strong adhesive layer on the surface of a variety of substrates. In the application of photoresists, good adhesion helps the photoresists to be uniformly coated and stably adhered to the substrate, and reduces the defects in the process of pattern transfer. After curing, lacquer shows strong chemical resistance and durability, and it is able to resist the erosion of acids, alkalis, and solvents. This property is especially important in photoresist, because it needs to resist various chemicals in the process of etching and developing to ensure the integrity and precision of the pattern. The writer applied the lacquer on ceramic, wood, glass, and metal to observe the adhesion properties between the lacquer and different substrates by using a scanning electron microscope (SEM) and found that the good adhesion and coatability of the lacquer helped it to be uniformly coated on the substrate to form a flat, smooth surface. This can not only be applied on electronic components, but also improve the precision and reliability of the production. More experiments can be done to analyze the guiding role of the adhesion performance on photoresist, thus improving environmental friendliness, abrasion resistance, acid and alkali resistance, and water and moisture resistance, and promoting the further development of photolithography.
6. Spin-Coating Process for Photoresists
In the manufacturing of microelectronic devices, the formation of thin films on wafers by PR coating is a very important process as shown in Figure 6a. An even and uniform film is formed by rotating the substrate at high speed to spread the photoresists evenly under centrifugal force. By controlling the key parameters, such as rotation speed, rotation time, photoresist viscosity, and environment conditions, the desired film thickness and uniformity can be achieved. There are two main reasons why it is necessary to reduce the amount of photoresist. The first reason is for environmental friendliness, as photoresists contain chemically hazardous substances. The second reason is cost. As the semiconductor industry is seeking smaller devices with higher capacity, the photoresists are shifting from i-line photoresists to deep UV photoresists [38], whose cost is several times higher than the older ones. The studies have shown that about 85%–90% of the total photoresist is wasted during the spinning stage. Therefore, the efficiency of spin-coating can be greatly improved by eliminating waste at this stage [39]. The spin-coating process has been thoroughly investigated in previous literature. Through relevant studies, it was found that the dry film thickness (D) is affected by the rotation speed, which is shown in this formula: D = kω−1/2, (k is a constant).
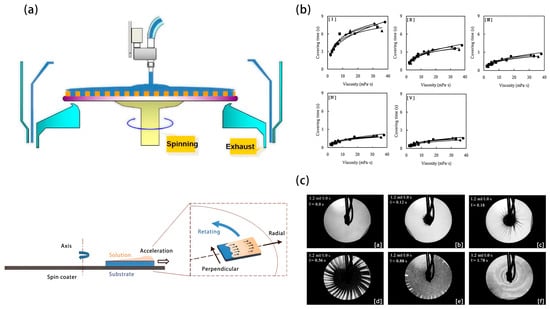
Figure 6.
(a) Schematic diagram of spin-coating and off-center spin-coating processes. (b) Flow visualization at various pre-spin speeds for covering time (I) 1000 rpm, (II) 2000 rpm, (III) 3000 rpm, (IV) 4000 rpm, (V) 5000 rpm, (c) 3000 rpm.
Photoresist samples can be represented by the constant k because there is nothing to do with their composition. Figure 6b(I–Ⅴ) shows the covering time at different pre-spin velocities [40]. With the increasing flow rate and decreasing viscosity, the covering time becomes shorter. The type of solvent and solute has less influence on it. Since the photoresist dispensing time becomes shorter, it is expected to decrease the consumption of photoresist by decreasing the coverage time. That is, the photoresist viscosity should be reduced or the pre-spin velocity should be increased. When the pre-spin speed is optimized to reduce the consumption, the same k (amount of photoresist) leads to the same consumption. Consumption decreases as k becomes smaller. With a fixed pre-spin velocity, the consumption becomes smaller when the samples have low solvent viscosity and high solvent evaporation rate [40]. Notably, the true spin-coating flow, which includes the injection of the photoresist onto the wafer, the formation and growth of fingers, and the spreading of the photoresist film, is illustrated in Figure 6c for Q = 1.2 mL/1.0 s and ω = 3000 rpm as shown in Figure 6c.
In addition to the traditional spin-coating method, vapor phase treatment of atomic layer deposition (ALD) also appears. Area-selective deposition (ASD) is an additive deposition method that helps to form thin films on a specific area within the substrate surface via ALD. The technology has been widely applied in the semiconductor industry. Vapor-phase infiltration (VPI), a technique derived from ALD, is an emerging approach to hybridizing polymer-based materials by incorporating inorganic elements into an organic matrix.
7. The Enlightenment of Lacquer to the Development of Modern Photoresists
China is the first country to use lacquer, so the lacquer material can no longer be regarded as a single handicraft, but as a symbol of national culture and the wisdom of the laboring people. Chinese lacquer culture reached its peak in the pre-Qin and Han dynasties, and played an important role in utensils for the Imperial Court in the Tang and Song dynasties. In addition to serving as furnishings in the ancient noble class, lacquer artworks also walked into the life of the general public, and became an important intangible cultural heritage with profound aesthetic value, as shown in Figure 7. Pure natural lacquer film formed after drying boasts hard texture, heat resistance, corrosion resistance, and so on, but it also has some disadvantages. For example, the film can only be formed when it is dried at a specific temperature and humidity; it is difficult to spray and apply; its color is dark; it has poor alkali resistance; it is easy to cause skin allergies; and so on. Since urushiol is the main film-forming substance in lacquer, its performance and film-forming process by drying will directly affect the performance of the lacquer film. By utilizing the special characteristics of the structure of lacquer phenol, the film-forming process by drying can be modified to accelerate the process or to obtain a modified film with excellent performance. Therefore, the research on lacquer and its modification mainly focuses on the modification of urushiol, the main film-forming substance urushiol [3], and the modified lacquer also plays a leading role in the modern photolithography.
Figure 7.
The schematic of lacquer in various application scenarios.
Lacquer culture has a far-reaching influence on the processing and preparation of electronic devices. Lacquer, as an inseparable part of China’s traditional crafts, has been handed down to the present day, and now it has its own unique cultural meaning. Exploring the origin of the lacquer civilization has great significance in the protection and inheritance of the traditional culture. With the improvement of the life of the people as well as the development of culture and technology, there will be new requirements for the development and inheritance of lacquer to meet the needs. This oldest and excellent natural polymer composite material needs to be innovated and its application fields should be expanded continuously, so that it can play a greater role. Applying lacquer in photoresists is a new perspective. The lacquer wares are famous for their durability and stability, which inspired us to research and develop photoresists with high chemical resistance and thermal stability, thus improving the reliability and service life of microelectronic devices. Lacquer can be integrated with more advanced technologies, materials, and media to achieve a broader range of application, which can make life better with natural and low-carbon materials.
8. Summary and Prospect
Lacquer has a history of thousands of years, and its unique aesthetic value and functionality still shine in modern times. In lacquer arts and crafts, natural lacquer, the main material, turns into works of art and articles of utility with a high degree of luster, durability, and aesthetics after a fine coating and curing process. The natural and environmental friendliness of lacquer is highly matched with the concept of green manufacturing. Natural lacquer is from the lacquer tree, and it enjoys the advantages of being renewable, non-toxic, and harmless, which is of great practical significance in the context of environmental protection and sustainable development. Lacquer products can still maintain stable physical and chemical properties in harsh environments such as high temperature and high humidity, and this excellent durability is an important revelation for the development of photoresist materials. However, at the same time, lacquer itself also has some defects, such as the weak photosensitive property of lacquer itself, which needs to be improved by chemical modification or the addition of photosensitizer. The modern photolithography process has very strict requirements for photoresist, including exposure, development, etching, and other aspects. The film-forming process of lacquer is somewhat different from that of traditional photoresist, which requires process adjustment and optimization to ensure its operability in practical applications. The extraction and processing costs of lacquer are high and the production process is complicated, which makes it difficult to realize large-scale and low-cost industrialized production. New extraction and processing processes need to be developed to improve the yield and quality of lacquer and reduce the production cost to meet the demands of industrial applications. Developing a new type of green photoresist material by researching and utilizing the greenness, strong adhesion, chemical resistance and mechanical strength of lacquer is not only an inheritance and innovation of traditional culture, but also an important contribution to the development of modern science and technology.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 52102291).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, W.; Liu, J.; Li, H.; Mu, Q.; Liu, X. Development and Application of Microelectronic Photoresist. Prog. Chem. 2014, 26, 1867–1888. [Google Scholar]
- Wang, X.; Tao, P.; Wang, Q.; Zhao, R.; Liu, T.; Hu, Y.; Hu, Z.; Wang, Y.; Wang, J.; Tang, Y.; et al. Trends in photoresist materials for extreme ultraviolet lithography: A review. Mater. Today 2023, 67, 299–319. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Fang, J. Research Progress on Modification and Application of Raw Lacquer. ChemistrySelect 2022, 7, e202200943. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, Y.; Zhou, X.; Wen, Q.; Ye, C.; Ye, Z.; Li, P.; Yang, S.; Yang, Z. A solvent-free and scalable method to prepare alkali soluble soybean oil-based epoxy acrylic resin for photoresist application. Ind. Crops Prod. 2023, 191, 115877. [Google Scholar] [CrossRef]
- Liang, B.; Li, R.; Zhang, C.; Yang, Z.; Yuan, T. Synthesis and characterization of a novel tri-functional bio-based methacrylate prepolymer from castor oil and its application in UV-curable coatings. Ind Crop. Prod 2019, 135, 170–178. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Chen, Y.; Yuan, T.; Yang, Z. One-step synthesis of novelrenewable multi-functional linseed oil-based acrylate prepolymers and its +application in UV-curable coatings. Prog. Org. Coat. 2020, 148, 105820. [Google Scholar] [CrossRef]
- Xiao, Q.; Cao, Y.; Zheng, W.; Hou, T.; Gao, S.; Lyu, J.; Xiao, H.; Chen, Y.; Chen, M. Kinetics of thermal degradation of raw lacquer enhanced by formaldehyde urea prepolymer. Sci. Rep. 2023, 13, 1649. [Google Scholar] [CrossRef]
- Ko, M.; Jung, J.; Hwang, S.S.; Won, J. Design and development of trivalent fe ion-induced novel urushi organogels. Polymer 2020, 205, 122835. [Google Scholar] [CrossRef]
- Wang, Y.; Su, T.; Shen, L.; Gao, J.; Wu, Q. The “Chemical” Talk about National Lacquer-Popularizing Lacquer Chemistry, Transmitting Lacquer Art and Intangible Cultural Heritage. Univ. Chem. 2024, 39, 371–379. [Google Scholar]
- Ito, H.I.H. Advances in chemical amplification resist systems. Jpn. J. Appl. Phys. 1992, 31, 4273. [Google Scholar]
- Kozawa, T.; Tagawa, S. Radiation chemistry in chemically amplified resists. Jpn. J. Appl. Phys. 2010, 49, 030001. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Duan, C.; Gu, Y.; Zhang, Z. Application and Research Progress of Copper Current Collector in Alkali Metal Batteries. Copp. Eng. 2023, 1–13. [Google Scholar]
- Zhang, Y.; Wang, W.; Wang, Y.; Zhang, Y. ZnS Encapsulated in N/S Co-Doped Carbon Fiber as a Composite Material for Li Storage. Copp. Eng. 2023, 23–31. [Google Scholar]
- Wei, S.; Jia, X.; Guo, J.; Li, D. Biomass-Based Carbon Derived from Poplar Catkins as Sodium Ion Anode Ma-terial. Copp. Eng. 2023, 32–38. [Google Scholar]
- Thackeray, J.; Cameron, J.; Jain, V.; Labeaume, P.; Coley, S.; Ongayi, O.; Wagner, M.; Rachford, A.; Biafore, J. Progress in resolution, sensitivity, and critical dimensional uniformity of EUV chemically amplified resists. Proc. SPIE 2013, 8682, 277–288. [Google Scholar]
- Peng, X.; Wang, Y.; Xu, J.; Yuan, H.; Wang, L.; Zhang, T.; Guo, X.; Wang, S.; Li, Y.; Yang, G. Molecular glass photoresists with high resolution, low LER, and high sensitivity for EUV lithography. Macromol. Mater. Eng. 2018, 303, 1700654. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yu, J.; Guo, X.; Wang, S.; Yang, G. Negative-tone molecular glass photoresist for high-resolution electron beam lithography. R. Soc. Open Sci. 2021, 8, 202132. [Google Scholar] [CrossRef]
- Jiang, J.; De Simone, D.; Vandenberghe, G. Difference in EUV photoresist design towards reduction of LWR and LCDU. Proc. SPIE 2017, 10146, 64–71. [Google Scholar]
- del Pozo, M.; Delaney, C.; Bastiaansen, C.W.M.; Diamond, D.; Schenning, A.P.H.J.; Florea, L. Direct laser writing of four-dimensional structural color microactuators using a photonic photoresist. ACS Nano 2020, 14, 9832–9839. [Google Scholar] [CrossRef] [PubMed]
- Liu, B. About the quality inspection of raw lacquer. Biomass Chem. Eng. 1980, 148–153. [Google Scholar]
- Hirose, R.; Kozawa, T.; Tagawa, S.; Shimizu, D.; Kai, T.; Shimokawa, T. Difference between acid generation mechanisms in poly (hydroxystyrene)-and polyacrylate-based chemically amplified resists upon exposure to extreme ultraviolet radiation. Jpn. J. Appl. Phys. 2008, 47, 7125. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Chang, S.W.; Felix, N.M.; Ueda, M.; Ober, C.K. Lithography based on molecular glasses. J. Photopolym. Sci. Technol. 2005, 18, 431–434. [Google Scholar] [CrossRef]
- Lee, C.-K.; Don, T.-M.; Lai, W.-C.; Chen, C.-C.; Lin, D.-J.; Cheng, L.-P. Preparation and properties of nano-silica modified negative acrylate photoresist. Thin Solid Film. 2008, 516, 8399–8407. [Google Scholar] [CrossRef]
- Schonhorn, H.; Torza, S.; Albarino, R.V.; Vazirani, H.N.; Wang, T.T. Organic Polymeric Coatins for Silica-Fibers.1. UV-Curing Epocy Acryate. J. Appl. Polym. Sci. 1979, 23, 75–84. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Gu, Y.; Duan, C.; Zhang, Z. Research Progress of Cu Doping in Sodium-Ion Battery Cathode. Copp. Eng. 2023, 14–22. [Google Scholar]
- Li, H.; Chang, S.; Zhang, M. Research Progress on Properties Tuning and Products of Cu-Based Catalyst in Electrocatalytic CO2 Reduction. Copp. Eng. 2023, 38–50. [Google Scholar]
- Alagar, M.; Ashok Kumar, A.; Anand Prabu, A.; Rajendran, A. Modification of Siliconized Epoxy Resin Using Multifuctional Silanes. Int. J. Polym. Mater. 2004, 53, 45–58. [Google Scholar] [CrossRef]
- Si, Q.F.; Fan, X.D.; Liu, Y.Y.; Kong, J.; Wang, S.J.; Qiao, W.Q. Synthesis and Characterization of Hyperbranched-Poly(Siloxysilane)-basedPolymeric Photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3261–3270. [Google Scholar] [CrossRef]
- Tang, C.Y.; Liu, W.Q. Preparation of dual-curable polysiloxane and the properties of its cured films with epoxy resin. J. Plast. Film Sheeting 2010, 26, 241–257. [Google Scholar] [CrossRef]
- Xia, J. Research on UV Curing Natural Raw Lacquer and Its Composite System. Ph.D. Thesis, Fujian Normal University, Fuzhou, China, 2011. [Google Scholar]
- Ogawa, T.; Jinnai, H.; Osawa, S. Stability in Appearance of Oriental Lacquer Film under Various Atmospheres. Kobunshi Ronbunshu 2001, 58, 29–35. Available online: https://www.semanticscholar.org/paper/Stability-in-Appearance-of-Oriental-Lacquer-Film-Ogawa-Jinnai/1c5df05946d2eba62aa90da8da03421b8f00f4f7 (accessed on 12 July 2024).
- Kulmala, T.S.; Vockenhuber, M.; Buitrago, E.; Fallica, R.; Ekinci, Y. Toward 10 nm half-pitch in EUV lithography: Results on resist screeningand pattern collapse mitigation techniques. Proc. SPIE 2015, 9422, 942204. [Google Scholar]
- Hasegawa, K.; Kawai, A. Adhesion improvement of photoresist: Destruction mode analysis. Proc. SPIE 2020, 11326, 373–382. [Google Scholar]
- Chalvin, F.; Nakamura, N.; Tochino, T.; Yasuda, M.; Kawata, H.; Hirai, Y. Resist behaviour during peeling release in nano-imprint lithography. Proc. SPIE 2016, 9984, 128–133. [Google Scholar]
- Chen, R.; Wang, X.; Li, X.; Wang, H.; He, M.; Yang, L.; Guo, Q.; Zhang, S.; Zhao, Y.; Li, Y.; et al. A comprehensive nano-interpenetrating semiconducting photoresist toward all-photolithography organic electronics. Sci. Adv. 2021, 7, eabg0659. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Qiu, Y.; Liu, J.; Zhu, D.; Ding, C.; Yang, Z.; Kuang, C.; Liu, X. Study of poly(vinylpyrrolidone)-hybridized two-color photosensitive laser direct-write photoresists. Acta Polym. Sin. 2022, 53, 608–616. [Google Scholar]
- Müller, P.; Müller, R.; Hammer, L.; Barner-Kowollik, C.; Wegener, M.; Blasco, E. STED-Inspired Laser Lithography Based on Photoswitchable Spirothiopyran Moieties. Chem Mater. 2019, 31, 1966–1972. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.; Choi, S.; Yoon, H.J. Organometallic and coordinative photoresist materials for EUV lithography and related photolytic mechanisms. Coord. Chem. Rev. 2023, 493, 215307. [Google Scholar] [CrossRef]
- Chou, F.-C.; Wang, M.-W.; Gong, S.-C.; Yang, Z.-G. Reduction of photoresist usage during spin coating. J. Electron. Mater. 2001, 30, 432–438. [Google Scholar] [CrossRef]
- Sanada, M.; Nakano, K.; Matsunaga, M. Characteristics of material for photoresist spin coating: Property for reduction of photoresist consumption. Jpn. J. Appl. Phys. Part 2-Lett. 1998, 37, L1448–L1451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).