Investigation and Comparison of Alternative Oxygen Barrier Coatings for Flexible PP Films as Food Packaging Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Substrate Films
2.1.2. Coating Agents
2.2. Methods
2.2.1. Preparation of CBS004
2.2.2. Preparation of PVOH
2.2.3. Coating Method and Conditions
2.3. Characterization
2.3.1. Determination of Oxygen Transmission Rate
2.3.2. Determination of Degree of Crosslinking
3. Results and Discussion
3.1. Oxygen Barrier Properties
3.1.1. Single Coating
- PP/SiOx, OPP/AlOx
- CPP70
3.1.2. Double Coating
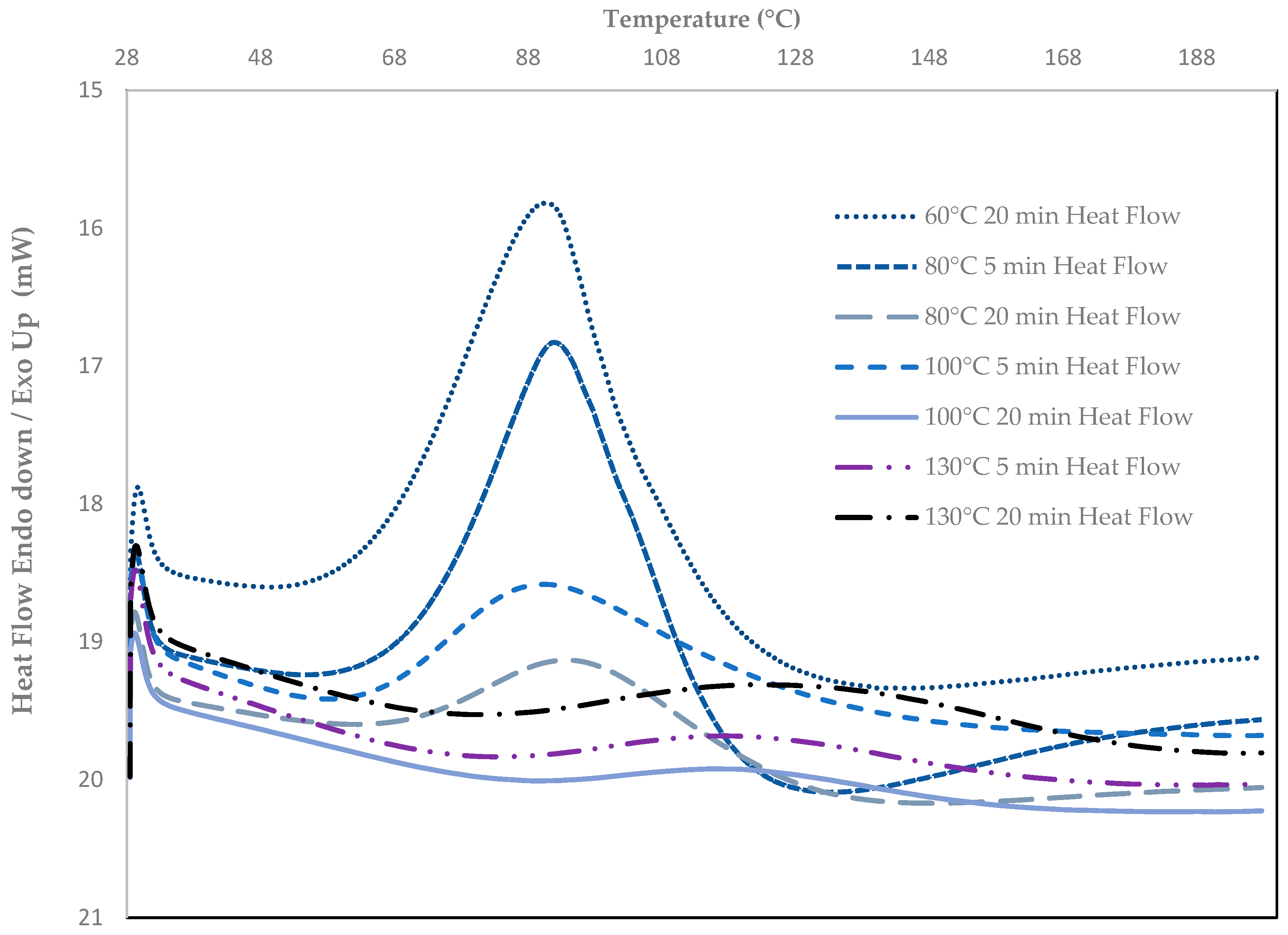
3.1.3. Effect of ORMOCER® Curing Conditions on Oxygen Barrier Properties
3.1.4. DSC Analysis for Degree of Crosslinking
4. Conclusions
- Combining SiOx and AlOx with ORMOCER®, Michem® Flex, and PVOH resulted in high oxygen barrier properties for PP/SiOx and OPP/AlOx films.
- Deposition of a single layer of ORMOCER® improved the oxygen barrier property of uncoated CPP70 by approximately 95%. Under different curing conditions, OTR values in the range of 10–100 cm3/m2·d·bar were achieved. Optimization of the curing conditions further improved the barrier performance by approximately 98% and reduced the OTR within this range.
- Increasing the wet thickness of the ORMOCER® coating from 15 µm to 40 µm improved the oxygen barrier performance of CPP70 by a factor of 1.72, with the OTR value changing from 30 cm3/m2·d·bar to 17.41 cm3/m2·d·bar.
- The OTR value at the semi-industrial coating scale correlated well with the laboratory scale, showed consistency between the two processes, and indicated that ORMOCER® could achieve an optimal level of crosslinking under semi-industrial curing conditions.
- Michem® Flex coating resulted in a significant reduction in oxygen permeability and could reduce the OTR value of CPP70 by a factor of 551, from 1516 cm3/m2·d·bar to 2.75 cm3/m2·d·bar.
- The use of ORMOCER® and Michem® Flex as primer coatings in a double coating process improved the adhesion of PVOH and notably improved the oxygen barrier property of CPP70, achieving exceptionally low OTR values (OTR < 0.1 cm3/m2·d·bar).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament and Council Directive 94/62/EC of 20 December 1994 on packaging and packaging waste. J. Environ. Law 1995, 7, 323–337. [CrossRef]
- Niaounakis, M. Flexible Plastic Packaging and Recycling. In Recycling of Flexible Plastic Packaging; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—A Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Gürlich, U.; Kladnik, V.; Katharina, P. Circular Packaging Design Guideline, Design Recommendations for Recyclable Packaging; FH Campus Wien: Wien, Austria, 2022. [Google Scholar]
- Gregorio, F.D.; Potaufeux, J.-E.; Legnini, M. RecyClass Design for Recycling Guidelines; RecyClass: Brussels, Belgium, 2022. [Google Scholar]
- Flexible Packaging Europe. Flexible Packaging Value Chain Position Recycled Content Targets for Packaging. Flexpack-Europe. Available online: www.flexpack-europe.org (accessed on 29 April 2024).
- Cabrera, G.; Li, J.; Maazouz, A.; Lamnawar, K. A Journey from Processing to Recycling of Multilayer Waste Films: A Review of Main Challenges and Prospects. Polymers 2022, 14, 2319. [Google Scholar] [CrossRef] [PubMed]
- Farris, S. Main Manufacturing Processes for Food Packaging Materials. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; p. B9780081005965210238. [Google Scholar] [CrossRef]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Mount, E.M. Extrusion Processes. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 227–266. [Google Scholar] [CrossRef]
- Wooster, J.; Martin, J. Flexible Packaging Applications of Polyethylene. In Handbook of Industrial Polyethylene and Technology, 1st ed.; Spalding, M.A., Chatterjee, A.M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1071–1090. [Google Scholar] [CrossRef]
- Emblem, A. Plastics properties for packaging materials. In Packaging Technology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 287–309. [Google Scholar] [CrossRef]
- Morris, B. Barrier. In The Science and Technology of Flexible Packaging; Morris, B.A., Ed.; William Andrew Publishing: Oxford, UK, 2017; pp. 259–308. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging and Shelf Life: A Practical Guide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Turriziani, B.B.; Vieira, R.P.; Júnior, L.M.; Alves, R.M.V. Mechanical Recycling of Multilayer Flexible Packaging Employing Maleic Anhydride as Compatibilizer. J. Polym. Environ. 2024, 32, 1393–1405. [Google Scholar] [CrossRef]
- Morris, B.A. Commonly Used Resins and Substrates in Flexible Packaging. In The Science and Technology of Flexible Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–119. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- RecyClass Design for Recycling Guidelines, Recyclability of Flexible Polyethylene with PP & PA: Novel Findings. Available online: https://recyclass.eu/news/recyclability-of-flexible-polyethylene-with-pp--pa-novel-findings/ (accessed on 13 August 2024).
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef]
- CEFLEX. CEFLEX—Designing for a Circular Economy (D4ACE) Technical Report—Recyclability of Polyolefin-Based Flexible Packaging. CEFLEX. 2023. Available online: https://guidelines.ceflex.eu/ (accessed on 19 August 2024).
- Saba, N.; Jawaid, M.; Thariq, M. Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Solberg, A.; Zehner, J.; Somorowsky, F.; Rose, K.; Korpela, A.; Syverud, K. Material properties and water resistance of inorganic–organic polymer coated cellulose paper and nanopaper. Cellulose 2023, 30, 1205–1223. [Google Scholar] [CrossRef]
- Amberg-Schwab, S. Functional Barrier Coatings on the Basis of Hybrid Polymers. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Haas, K.-H.; Wolter, H. Synthesis, properties and applications of inorganic–organic copolymers (ORMOCER®s). Curr. Opin. Solid State Mater. Sci. 1999, 4, 571–580. [Google Scholar] [CrossRef]
- Fraunhofer-Institut für, Fraunhofer-Institut für Silicatforschung ISC, and FRAUNHOFER-INSTITUT FÜR SILICATFORSCHUNG ISC, Inorganic-Organic Hybrid Polymers (ORMOCER®s) for Micro Systems Technology. Available online: https://www.isc.fraunhofer.de/content/dam/isc/de/documents/Publikationen/Inorganic-organic_hybrid_polymers_ORMOCER_for_micro_systems_technology.pdf (accessed on 29 April 2024).
- Haas, K.-H.; Amberg-Schwab, S.; Rose, K.; Schottner, G. Functionalized coatings based on inorganic–organic polymers (ORMOCER®s) and their combination with vapor deposited inorganic thin films. Surf. Coat. Technol. 1999, 111, 72–79. [Google Scholar] [CrossRef]
- Emmert, K.; Amberg-Schwab, S.; Braca, F.; Bazzichi, A.; Cecchi, A.; Somorowsky, F. bioORMOCER®—Compostable Functional Barrier Coatings for Food Packaging. Polymers 2021, 13, 1257. [Google Scholar] [CrossRef]
- Kuraray Poval. Barrier Films. Available online: https://www.kuraray-poval.com/applications/barrier-films (accessed on 29 April 2024).
- Idris, A.; Muntean, A.; Mesic, B.; Lestelius, M.; Javed, A. Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions. Coatings 2021, 11, 1253. [Google Scholar] [CrossRef]
- Apicella, A.; Barbato, A.; Garofalo, E.; Incarnato, L.; Scarfato, P. Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers 2022, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Jumah, M.N.B. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Suhag, A.; Biswas, K.; Singh, S.; Kulshreshtha, A. Crosslinking effect on polyvinyl alcohol resin for barrier properties of barrier biaxial orientation films. Prog. Org. Coat. 2022, 163, 106662. [Google Scholar] [CrossRef]
- Schiessl, S.; Kucukpinar, E.; Cros, S.; Miesbauer, O.; Langowski, H.-C.; Eisner, P. Nanocomposite Coatings Based on Polyvinyl Alcohol and Montmorillonite for High-Barrier Food Packaging. Front. Nutr. 2022, 9, 790157. [Google Scholar] [CrossRef] [PubMed]
- Michelman. Available online: https://www.michelman.com/solutions/michem-flex/ (accessed on 24 June 2024).
- Cooper, R. New Oxygen Barrier Coatings for Pouches to Help with Downgauging. michelman.com. 2018. Available online: https://www.researchgate.net/publication/325012527_New_oxygen_barrier_coatings_for_pouches_to_help_with_downgauging (accessed on 30 April 2024).
- Bayus, J.; Ge, C.; Thorn, B. A preliminary environmental assessment of foil and metallized film centered laminates. Resour. Conserv. Recycl. 2016, 115, 31–41. [Google Scholar] [CrossRef]
- Selke, S.E.M. Packaging: Polymers in Flexible Packaging. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019; p. B9780128035818021688. [Google Scholar] [CrossRef]
- Sanetuntikul, J.; Ketpang, K.; Naknaen, P.; Narupai, B.; Petchwattana, N. A circular economy use of waste metalized plastic film as a reinforcing filler in recycled polypropylene packaging for injection molding applications. Clean. Eng. Technol. 2023, 17, 100683. [Google Scholar] [CrossRef]
- Gürlich, U.; Kladnik, V.; Tacker, M.; Kreuzinger, M. Packaging Design for Recycling; World Packaging Organisation: Vienna, Austria, 2021. [Google Scholar] [CrossRef]
- RecyClass. Design for Recycling Guidelines, Natural PP Flexible Films for Household and Commercial Packaging. Available online: https://recyclass.eu/guidelines/natural-pp-flexible-films/ (accessed on 13 August 2024).
- Struller, C.F.; Kelly, P.J.; Copeland, N.J.; Liauw, C.M. Characterization studies of aluminum oxide barrier coatings on polymeric substrates. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 041502. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater. Sci. 2003, 48, 1–55. [Google Scholar] [CrossRef]
- Hedenqvist, M.S. Barrier Packaging Materials. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 559–581. [Google Scholar] [CrossRef]
- Guerritore, M.; Olivieri, F.; Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M.E.; Galdi, M.R.; Carfagna, C.; Gentile, G. Recyclable-by-design mono-material flexible packaging with high barrier properties realized through graphene hybrid coatings. Resour. Conserv. Recycl. 2022, 179, 106126. [Google Scholar] [CrossRef]
- Hedenqvist, M.; Johansson, K. Barrier properties of SiOx-coated polymers: Multi-layer modelling and effects of mechanical folding. Surf. Coat. Technol. 2003, 172, 7–12. [Google Scholar] [CrossRef]
- Van Velzen, U.T.; De Weert, L.; Molenveld, K. Flexible Laminates within the Circular Economy; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Pauer, E.; Tacker, M.; Gabriel, V.; Krauter, V. Sustainability of flexible multilayer packaging: Environmental impacts and recyclability of packaging for bacon in block. Clean. Environ. Syst. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Unsbo, H.; Strömberg, E.; Almasi, A.M. Increasing the Circularity of High Barrier Flexible Plastic Packaging; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2023. [Google Scholar]
- Felts, J.T. Transparent Barrier Coatings Update: Flexible Substrates. J. Plast. Film. Sheeting 1993, 9, 139–158. [Google Scholar] [CrossRef]
- Struller, C.F.; Kelly, P.J.; Copeland, N.J. Aluminum oxide barrier coatings on polymer films for food packaging applications. Surf. Coat. Technol. 2014, 241, 130–137. [Google Scholar] [CrossRef]
- Lahtinen, K.; Lahti, J.; Johansson, P.; Seppänen, T.; Cameron, D.C. Influence of substrate contamination, web handling, and pretreatments on the barrier performance of aluminum oxide atomic layer-deposited BOPP film. J. Coat. Technol. Res. 2014, 11, 775–784. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent innovations in barrier technologies for plastic packaging—A review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Amberg-Schwab, S.; Hoffmann, M.; Bader, H.; Gessler, M. Inorganic-Organic Polymers with Barrier Properties for Water Vapor, Oxygen and Flavors. J. Sol-Gel Sci. Technol. 1998, 13, 141–146. [Google Scholar] [CrossRef]
- Roberts, A.; Henry, B.; Sutton, A.; Grovenor, C.; Briggs, G.; Miyamoto, T.; Kano, M.; Tsukahara, Y.; Yanaka, M. Gas permeation in silicon-oxide/polymer (SiOx/PET) barrier films: Role of the oxide lattice, nano-defects and macro-defects. J. Membr. Sci. 2002, 208, 75–88. [Google Scholar] [CrossRef]
- Da Silva Sobrinho, A.S.; Czeremuszkin, G.; Latrèche, M.; Dennler, G.; Wertheimer, M.R. A study of defects in ultra-thin transparent coatings on polymers. Surf. Coat. Technol. 1999, 116–119, 1204–1210. [Google Scholar] [CrossRef]
- Jacques, F.; Gillie, K.; Chester, W. Coated Film Structures with an Aluminum Oxide Intermediate Layer. U.S. Patent 10766228B2, 30 December 2016. [Google Scholar]
- Johansson, P.; Teisala, H.; Lahtinen, K.; Kuusipalo, J. Protecting an atomic layer deposited aluminum oxide barrier coating on a flexible polymer substrate. Thin Solid Film 2017, 621, 151–155. [Google Scholar] [CrossRef]
- Körner, L.; Sonnenfeld, A.; Heuberger, R.; Waller, J.H.; Leterrier, Y.; Månson, J.A.E.; von Rohr, P.R. Oxygen permeation, mechanical and structural properties of multilayer diffusion barrier coatings on polypropylene. J. Phys. D Appl. Phys. 2010, 43, 115301. [Google Scholar] [CrossRef]
- Amberg-Schwab, S.; Katschorek, H.; Weber, U.; Hoffmann, M.; Burger, A. Barrier Properties OF Inorganic-Organic Polymers: Influence of Starting Compounds, Curing Conditions and Storage-Scaling-Up to Industrial Application. J. Sol-Gel Sci. Technol. 2000, 19, 125–129. [Google Scholar] [CrossRef]
- Amberg-Schwab, S.; Müller, K.; Somorowsky, F.; Sängerlaub, S. UV-Activated, Transparent Oxygen Scavenger Coating Based on Inorganic–Organic Hybrid Polymer (ORMOCER®) with High Oxygen Absorption Capacity. Coatings 2023, 13, 473. [Google Scholar] [CrossRef]
- Sawada, T.; Ohhasi, S.; Yoshida, S. Laminated Moistureproof Film with Silicon Oxide Core Layer. US5112673A, 12 May 1992. Available online: https://patents.google.com/patent/US5112673A/en#patentCitations (accessed on 15 April 2024).
- Ettridge, P.; Christopherson, R.; Isabel, B.; Lohwasser, W.; Skov, S.; Jensen, S. Flexible Multilayer Packaging Film with Ultra-High Barrier Properties. WO2017005597A1, 12 January 2017. Available online: https://patents.google.com/patent/WO2017005597A1/en (accessed on 7 April 2024).
- Rashad, M.; Abd-Elnaiem, A.M.; Hanafy, T.A.; Shaalan, N.M.; Shamekh, A.M.A. Optical properties of functional Al2O3 nano-filler in eco-friendly PVA polymer for flexible optoelectronic devices. Opt. Mater. 2023, 141, 113990. [Google Scholar] [CrossRef]
- Kim, S.W. Preparation and barrier property of poly(vinyl alcohol)/SiO2 hybrid coating films. Korean J. Chem. Eng. 2008, 25, 1195–1200. [Google Scholar] [CrossRef]
- Riley, A. Basics of polymer chemistry for packaging materials. In Packaging Technology, Fundamentals, Materials and Processes; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 262–286. [Google Scholar] [CrossRef]
- Selke, S.E.M.; Culter, J.D. Major Plastics in Packaging. In Plastics Packaging, 3rd ed; Selke, S.E.M., Culter, J.D., Eds.; Hanser: Billings, MT, USA, 2016; pp. 101–157. [Google Scholar] [CrossRef]
- Lim, M.; Kwon, H.; Kim, D.; Seo, J.; Han, H.; Khan, S.B. Highly-enhanced water resistant and oxygen barrier properties of cross-linked poly(vinyl alcohol) hybrid films for packaging applications. Prog. Org. Coat. 2015, 85, 68–75. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, X.; Wang, Y. Study on the Behavior of BOPP Film Treated by Corona Discharge. Coatings 2020, 10, 1195. [Google Scholar] [CrossRef]

| Substrate Film | Description | Substrate Thickness (µm) |
|---|---|---|
| CPP70 | Cast PP with corona discharge | 70 |
| PP/SiOx | PP films vapor deposition coated with silicon oxide | 22 |
| OPP/AlOx | Biaxially stretched PP film vapor deposition coated with aluminum oxide | 20 |
| Substrate | Coating Agent (40 µm wet) | Thermal Treatment |
|---|---|---|
| PP/SiOx, OPP/AlOx, CPP70 | CBS004 | 100 °C (20 min) |
| MCHB3513 | 70 °C (30 min) | |
| PVOH | 50 °C (10 min) |
| Substrate | Coating Agent | Thermal Treatment | |
|---|---|---|---|
| First Coating (15 µm wet) | Second Coating (40 µm wet) | First Coating/Second Coating | |
| CPP70 | CBS004 | CBS004 | 100 °C (20 min)/100 °C (20 min) |
| MCHB3513 | 100 °C (20 min)/70 °C (30 min) | ||
| PVOH | 100 °C (20 min)/50 °C (10 min) | ||
| MCHB3513 | CBS004 | 70 °C (30 min)/100 °C (20 min) | |
| MCHB3513 | 70 °C (30 min)/70 °C (30 min) | ||
| PVOH | 70 °C (30 min)/50 °C (10 min) | ||
| Coating Scale | Laboratory | Semi-Industrial | ||
|---|---|---|---|---|
| Coating speed | 10 mm/s | 83 mm/s (5 m/min) | ||
| Curing conditions | Temperature (°C) | Time (min) | Temperature (°C) | Time (s) |
| 60 | 20 | 100 | 20 | |
| 80 | 5 and 20 | |||
| 100 | 5 and 20 | |||
| 130 | 5 and 20 | |||
| Substrate | Coating Scale | Wet Coating Thickness (µm) | Coating Agent | OTR (cm3/m2·d·Bar) |
|---|---|---|---|---|
| PP/SiOx | Laboratory | 40 | Uncoated | 0.536 ± 0.259 |
| CBS004 | 0.061 ± 0.061 | |||
| MCHB3513 | 0.005 ± 0.002 | |||
| PVOH | 0.006 ± 0.001 | |||
| OPP/AlOx | Laboratory | 40 | Uncoated | 6.134 ± 1.802 |
| CBS004 | 0.076 ± 0.054 | |||
| MCHB3513 | 0.035 ± 0.050 | |||
| PVOH | 0.279 ± 0.470 | |||
| CPP70 | Laboratory | 40 | Uncoated | 1516.64 ± 39.17 |
| CBS004 | 17.41 ± 1.17 | |||
| MCHB3513 | 2.75 ± 1.49 | |||
| PVOH | 259.99 ± 69.01 * | |||
| Semi-industrial | No information provided | CBS004 | 35.59 ± 7.66 |
| Substrate | Coating Agent | OTR | |
|---|---|---|---|
| First Coating (15 µm wet) | Second Coating (40 µm wet) | (cm3/m2·d·bar) | |
| CPP70 | CBS004 | CBS004 | 9.314 ± 1.79 |
| MCHB3513 | 0.129 ± 0.04 | ||
| PVOH | 0.075 ± 0.10 | ||
| MCHB3513 | CBS004 | 0.211 ± 0.33 | |
| MCHB3513 | 0.101 ± 0.10 | ||
| PVOH | 0.040 ± 0.06 | ||
| Substrate | Coating Scale | Curing Conditions | OTR Improvement | |
|---|---|---|---|---|
| Temperature (°C) | Time | Percentage (%) | ||
| CPP70 | Laboratory | 80 | 5 (min) | 95.0 |
| 20 (min) | 96.9 | |||
| 100 | 5 (min) | 97.5 | ||
| 20 (min) | 98.0 | |||
| 130 | 5 (min) | 98.2 | ||
| 20 (min) | 98.0 | |||
| Semi-industrial | 100 | 20 (s) | 97.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharbafian, F.; Tosic, K.; Schmiedt, R.; Novak, M.; Krainz, M.; Rainer, B.; Apprich, S. Investigation and Comparison of Alternative Oxygen Barrier Coatings for Flexible PP Films as Food Packaging Material. Coatings 2024, 14, 1086. https://doi.org/10.3390/coatings14091086
Sharbafian F, Tosic K, Schmiedt R, Novak M, Krainz M, Rainer B, Apprich S. Investigation and Comparison of Alternative Oxygen Barrier Coatings for Flexible PP Films as Food Packaging Material. Coatings. 2024; 14(9):1086. https://doi.org/10.3390/coatings14091086
Chicago/Turabian StyleSharbafian, Farshad, Katharina Tosic, Romana Schmiedt, Martin Novak, Michael Krainz, Bernhard Rainer, and Silvia Apprich. 2024. "Investigation and Comparison of Alternative Oxygen Barrier Coatings for Flexible PP Films as Food Packaging Material" Coatings 14, no. 9: 1086. https://doi.org/10.3390/coatings14091086
APA StyleSharbafian, F., Tosic, K., Schmiedt, R., Novak, M., Krainz, M., Rainer, B., & Apprich, S. (2024). Investigation and Comparison of Alternative Oxygen Barrier Coatings for Flexible PP Films as Food Packaging Material. Coatings, 14(9), 1086. https://doi.org/10.3390/coatings14091086








