Surface Decontamination of Titanium Dental Implants Subjected to Implantoplasty by Treatment with Citric Acid Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
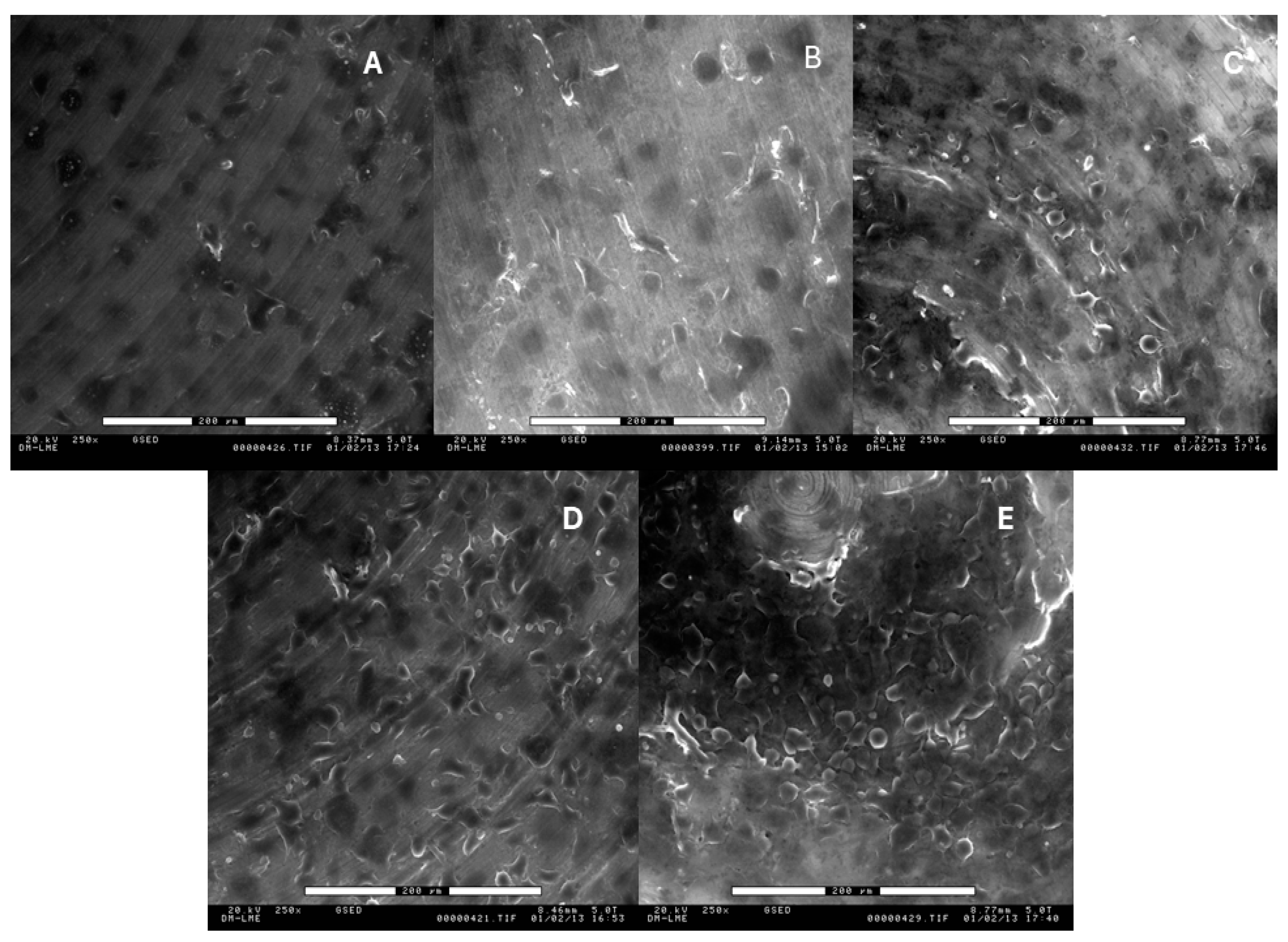
2.2. Roughness Analysis
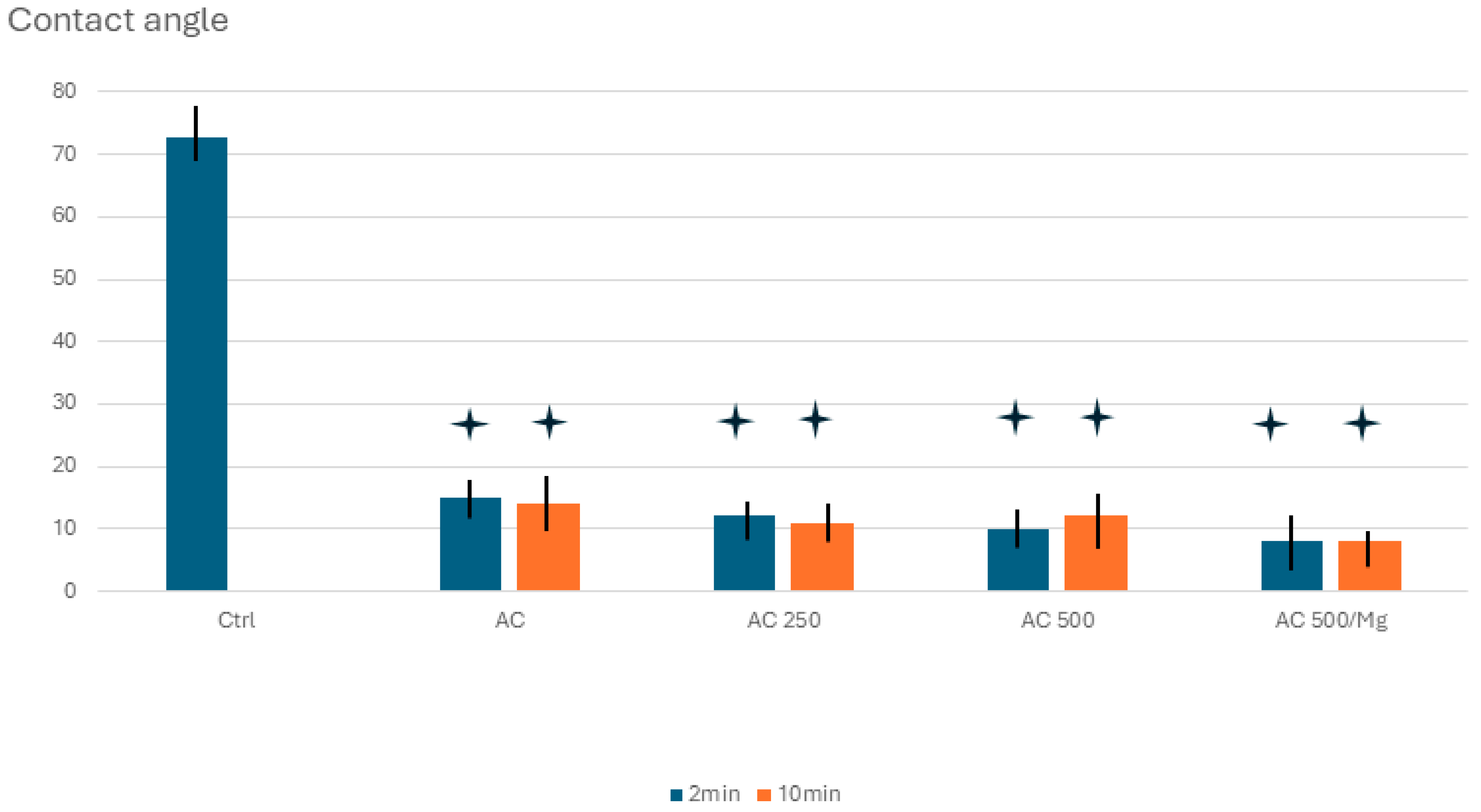
2.3. Wettability
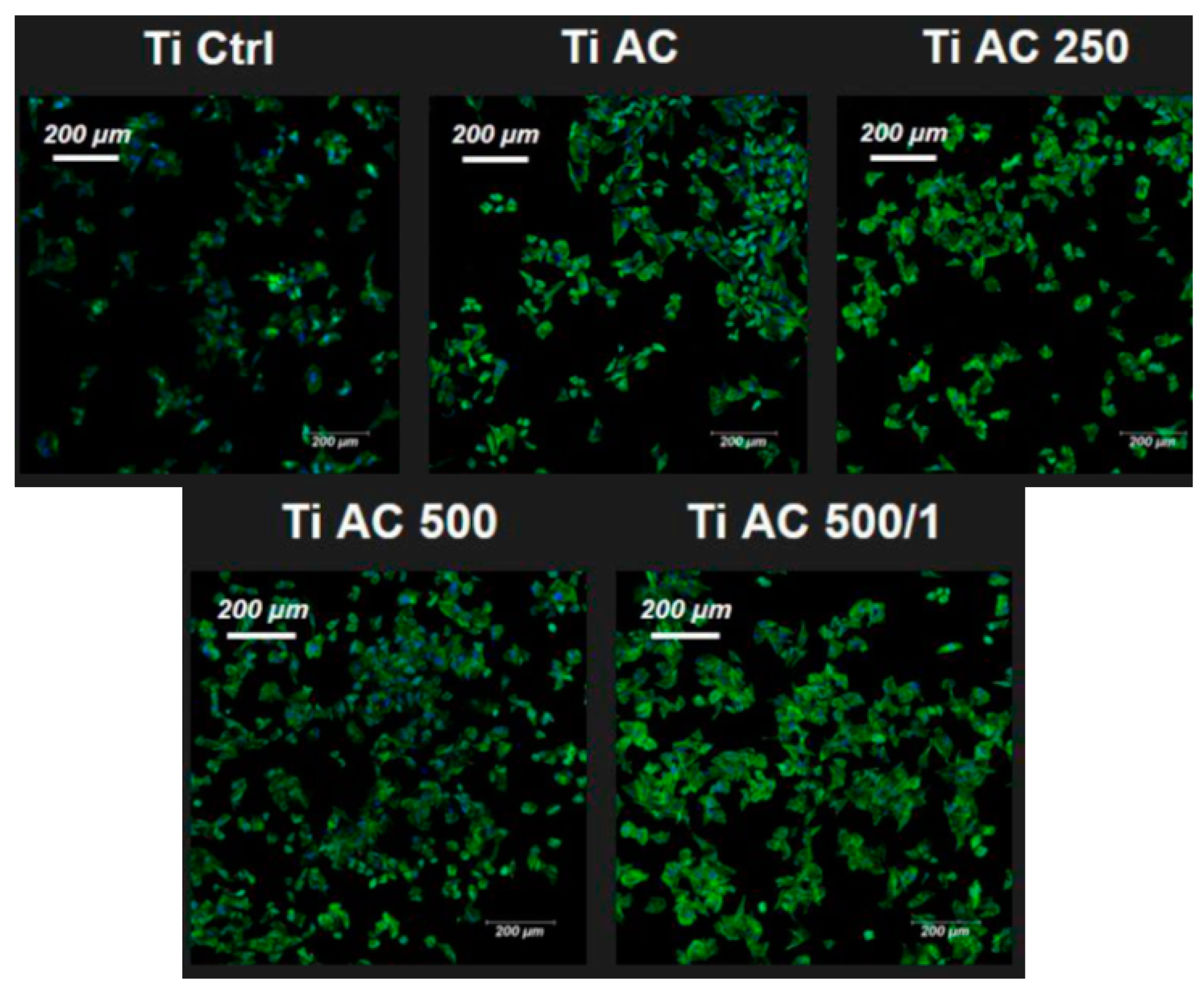
2.4. Fibroblast Culture
2.5. Osteoblasts Culture
2.6. Immunofluorescence
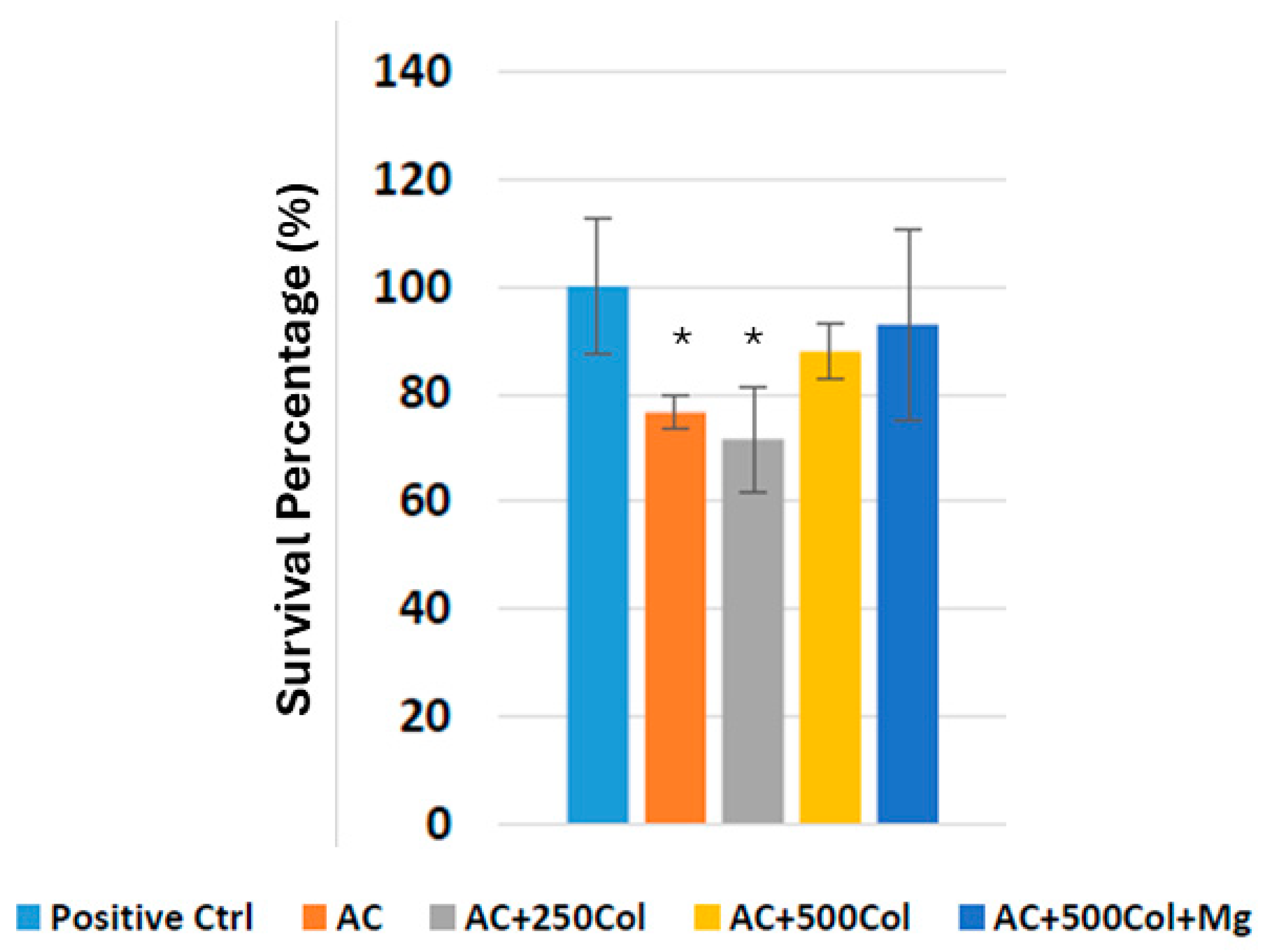
2.7. Bacterial Culture
2.8. Statistical Analysis
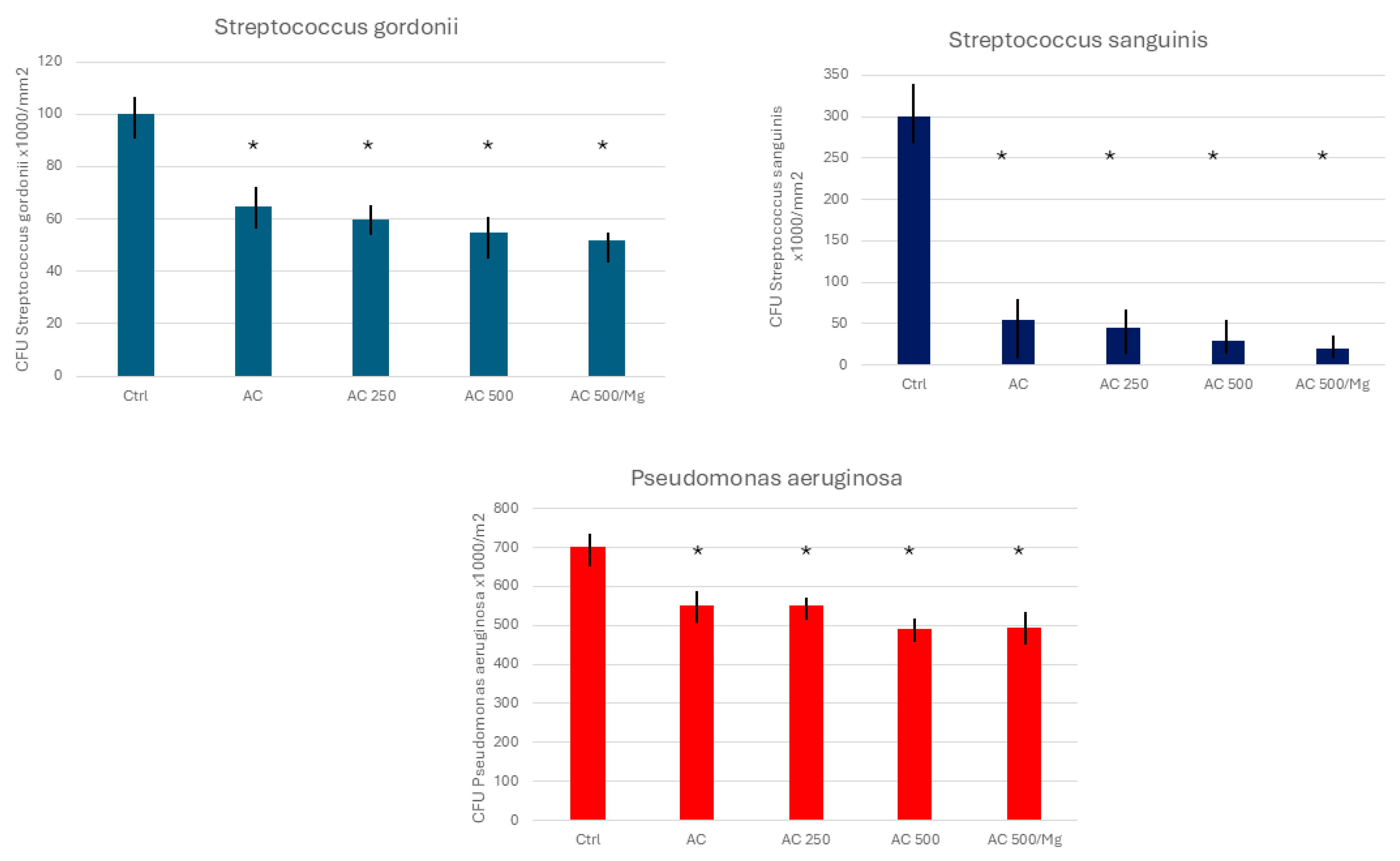
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 199–214. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontology 2000 2019, 81, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 305–315. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant Dent. 2015, 1, 22. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 840–852. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implant. Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Englezos, E.; Cosyn, J.; Koole, S.; Jacquet, W.; De Bruyn, H. Resective Treatment of Peri-implantitis: Clinical and Radiographic Outcomes After 2 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 729–735. [Google Scholar] [CrossRef]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 340–356. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2022, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin. Oral Implant. Res. 2014, 25, 132–136. [Google Scholar] [CrossRef]
- Monje, A.; Schwarz, F. Principles of Combined Surgical Therapy for the Management of Peri-Implantitis. Clin. Adv. Periodontics 2014 2022, 12, 57–63. [Google Scholar] [CrossRef]
- Monje, A.; Amerio, E.; Cha, J.K.; Kotsakis, G.; Pons, R.; Renvert, S.; Sanz-Martin, I.; Schwarz, F.; Sculean, A.; Stavropoulos, A.; et al. LStrategies for implant surface decontamination in peri-implantitis therapy. Int. J. Oral Implantol. 2022, 15, 213–248. [Google Scholar]
- Dostie, S.; Alkadi, L.T.; Owen, G.; Bi, J.; Shen, Y.; Haapasalo, M.; Larjava, H.S. Chemotherapeutic decontamination of dental implants colonized by mature multispecies oral biofilm. J. Clin. Periodontol. 2017, 44, 403–409. [Google Scholar] [CrossRef]
- Gosau, M.; Hahnel, S.; Schwarz, F.; Gerlach, T.; Reichert, T.E.; Bürgers, R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin. Oral Implant. Res. 2010, 21, 866–872. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implant. Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef]
- de Tapia, B.; Valles, C.; Ribeiro-Amaral, T.; Mor, C.; Herrera, D.; Sanz, M.; Nart, J. The adjunctive effect of a titanium brush in implant surface decontamination at peri-implantitis surgical regenerative interventions: A randomized controlled clinical trial. J. Clin. Periodontol. 2019, 46, 586–596. [Google Scholar] [CrossRef]
- Francis, S.; Iaculli, F.; Perrotti, V.; Piattelli, A.; Quaranta, A. Titanium Surface Decontamination: A Systematic Review of In Vitro Comparative Studies. Int. J. Oral Maxillofac. Implant. 2022, 37, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Azzola, F.; Ionescu, A.C.; Ottobelli, M.; Cavalli, N.; Brambilla, E.; Corbella, S.; Francetti, L. Biofilm formation on dental implant surface treated by implantoplasty: An in-situ study. Dent. J. 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Yen Nee, W.; Raja Awang, R.A.; Hassan, A. Effects on the Titanium Implant Surface by Different Hygiene Instrumentations: A Narrative Review. Cureus 2022, 14, e30884. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ghaffar, H.; Taib, H.; Hassan, A. A Review of Bacterial Colonization on Dental Implants with Various Hygiene Instruments. Cureus 2023, 15, e47483. [Google Scholar] [CrossRef]
- Gil, F.J.; Rodriguez, A.; Espinar, E.; Llamas, J.M.; Padullés, E.; Juárez, A. Effect of oral bacteria on the mechanical behavior of titanium dental implants. Int. J. Oral Maxillofac. Implant. 2012, 27, 64–68. [Google Scholar]
- Wu, X.; Cai, C.; Gil, J.; Jantz, E.; Al Sakka, Y.; Padial-Molina, M.; Suárez-López del Amo, F. Characteristics of Particles and Debris Released after Implantoplasty: A Comparative Study. Materials 2022, 15, 602. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty. An in vivo study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Korniienko, V.; Husak, Y.; Diedkova, K.; Varava, Y.; Grebnevs, V.; Pogorielova, O.; Bērtiņš, M.; Korniienko, V.; Zandersone, B.; Ramanaviciene, A.; et al. Antibacterial Potential and Biocompatibility of Chitosan/Polycaprolactone Nanofibrous Membranes Incorporated with Silver Nanoparticles. Polymers 2024, 16, 1729. [Google Scholar] [CrossRef]
- Punset, M.; Vilarrasa, J.; Nart, J.; Manero, J.M.; Bosch, B.; Padrós, R.; Perez, R.A.; Gil, J. Citric acid passivation of titanium dental implants for minimizing bacterial colonization impact. Coatings 2021, 11, 214. [Google Scholar] [CrossRef]
- Cruz, N.; Gil, J.; Punset, M.; Manero, J.M.; Tondela, J.P.; Verdeguer, P.; Aparicio, C.; Rúperez, E. Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes. Coatings 2022, 12, 154. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; Garcia-Garcia, M.; Sanchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellon, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Pena, J.; Engel, E.; Mendoza, A.; Planell, J.A. Microstructural, mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys. J. Mater. Sci. Mater. Med. 2004, 15, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, J.; Àlvarez, G.; Soler-Ollé, A.; Gil, J.; Nart, J.; Blanc, V. Bacterial Adhesion of TESPSA and Citric Acid on Different Titanium Surfaces Substrate Roughness: An In Vitro Study with a Multispecies Oral Biofilm Model. Materials 2023, 16, 4592. [Google Scholar] [CrossRef]
- Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. [Google Scholar] [CrossRef]
- Guillem, J.; Delgado, L.; Godoy-Galardo, M.; Pegueroles, M.; Herrero, M.; Gil, F.J. Fibroblast adhesion and activation onto micro-machined titanium surfaces. Clin. Oral Implant. Res. 2013, 24, 770–780. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhou, L.Y.; Qian, S.J.; Gu, Y.X.; Shi, J.Y.; Lai, H.C. Improved response of human gingival fibroblasts to titanium coated with micro-/nano-structured tantalum. Int. J. Implant. Dent. 2021, 7, 36. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef]
- Eftekhar Ashtiani, R.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. In Evidence-Based Complementary and Alternative Medicine; Hindawi Limited: London, UK, 2021; Volume 2021. [Google Scholar]
- Sartori, M.; Giavaresi, G.; Parrilli, A.; Ferrari, A.; Aldini, N.N.; Morra, M.; Cassinelli, C.; Bollati, D.; Fini, M. Collagen type I coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat. Int. Orthop. 2015, 39, 2041–2052. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanimoto, Y. A review of improved fixation methods for dental implants. Part I: Surface optimization for rapid osseointegration. J. Prosthodont. Res. 2015, 59, 20–33. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Neumann, A.; Ng, H.P.; Lapovok, R.; Kasper, C.; Lowe, T.C.; Anumalasetty, V.N.; Estrin, Y. Combined effect of grain refinement and surface modification of pure titanium on the attachment of mesenchymal stem cells and osteoblast-like SaOS-2 cells. Mater. Sci. Eng. C 2017, 71, 483–497. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 524–532. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.B.; Laing, P.G.; Hodge, E.S. The ionization of metal implants in living tissues. J. Bone Jt. Surg. 1960, 42, 77–90. [Google Scholar] [CrossRef]
- Ferguson, A.B.; Akahoshi, Y.; Laing, P.G.; Hodge, E.S. Characteristics of trace ion release from embedded metal implants in the rabbit. J. Bone Jt. Surg. 1962, 44, 317–336. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.; Proubasta, I.; Vazquez, J. Fundamentos de Biomecánica y Biomateriales; Ergon: Barcelona, Spain, 1997; pp. 125–132. [Google Scholar]
- Gil, F.J.; Planell, J.A. Aplicaciones biomédicas del titanio v sus aleaciones. Biomecánica 1993, 1, 34–43. [Google Scholar] [CrossRef]
- Lukaszewska-Kuska, M.; Idzior-Haufa, M.; Dorocka-Bobkowska, B. Evaluation of human osteoblast metabolic activity in modified titanium-conditioned medium. Proc. Inst. Mech. Eng. H 2020, 234, 603–611. [Google Scholar] [CrossRef]
- Lukaszewska-Kuska, M.; Wirstlein, P.; Majchrowski, R.; Dorocka-Bobkowska, B. The effects of titanium topography and chemical composition on human osteoblast cell. Physiol. Res. 2021, 70, 413–423. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Martini, D.; Belletti, S.; Tinti, A.; Raspanti, M.; Taddei, P.; Ruggeri, A.; Scandroglio, R. Different titanium surface treatment influences human mandibular osteoblast response. J. Periodontol. 2004, 75, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Sader, M.S.; Balduino, A.; Soares Gde, A.; Borojevic, R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin. Oral Implant. Res. 2005, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Yurttutan, M.E.; Keskin, A. Evaluation of the effects of different sand particles that used in dental implant roughened for osseointegration. BMC Oral Health 2018, 18, 47. [Google Scholar] [CrossRef]
- Wang, Q.G.; Zhou, P.; Liu, S.F.; Attarilar, S.; Ma, R.L.W.; Zhong, Y.S.; Wang, L.Q. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Ayad, N.B.; Hyzy, S.L.; Boyan, B.D.; Olivares-Navarrete, R. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin. Oral Implant. Res. 2017, 28, 414–423. [Google Scholar] [CrossRef]
- Canabarro, A.; Diniz, M.G.; Paciornik, S.; Carvalho, L.; Sampaio, E.M.; Beloti, M.M.; Rosa, A.L.; Fischer, R.G. High concentration of residual aluminum oxide on titanium surface inhibits extracellular matrix mineralization. J. Biomed. Mater. Res. A 2008, 87, 588–597. [Google Scholar] [CrossRef]
- Al-asbahi, M.G.S.S.; Al-Ofiry, B.A.; Saad, F.A.A.; Al-Gunaid, M.Q.A. Silver nanoparticles biosynthesis using mixture of Lactobacillus sp. and Bacillus sp. growth and their antibacterial activity. Sci. Rep. 2024, 14, 10224. [Google Scholar] [CrossRef]
- Mishra, M.; Ballal, A.; Rath, D.; Rath, A. Novel silver nanoparticle-antibiotic combinations as promising antibacterial and anti-biofilm candidates against multiple-antibiotic resistant ESKAPE microorganisms. Colloids Surf. B Biointerfaces 2024, 236, 113826. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Garcia, I.; Nuñez-Márquez, E.; Moreno-Muñoz, J.; Gil, J.; Delgado, L.M.; Rondón-Romero, J.L.; Monsalve-Guil, L. Silver coating on dental implantabutment connection screws as potential strategy to prevent loosening and minimizing bacteria adhesion. Front. Bioeng. Biotechnol. 2024, 11, 1293582. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lee, S.; Lee, H.G.; Choi, D.; Lim, K.M. Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM. Toxics 2022, 10, 558. [Google Scholar] [CrossRef]

| Nitrogen | Carbon | Hydrogen | Iron | Oxygen | Titanium |
|---|---|---|---|---|---|
| 0.05 | 0.10 | 0.12 | 0.30 | 0.35 | Balance |
| Dissolution | Chemical Composition |
|---|---|
| 25% Citric acid (AC) | Citric acid 25% in volume (v). |
| 25% Citric acid + Collagen 250 (AC 250) | Citric acid 25% (v) with 0.25 g Collagen/L. |
| 25% Citric acid + Collagen 500 (AC 500) | Citric acid 25% (v) with 0.50 g Collagen/L. |
| 25% Citric acid + Collagen 500 + 1% Mg (AC 500/Mg) | Citric acid 25% (v) with 0.50 g Collagen/L. and 10% of Mg(NO3)2·6H2O |
| Treatment | Roughness Sa (μm) |
|---|---|
| As-received | 0.15 ± 0.09 |
| Implantoplasty (Ctrl) | 0.25 ± 0.15 |
| AC | 0.33 ± 0.13 * |
| AC 250 | 0.30 ± 0.10 * |
| AC 500 | 0.25 ± 0.11 * |
| AC 500/Mg | 0.27 ± 0.10 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Garrido, P.; Bosch, B.M.; Gil, J.; Fernández-Domínguez, M. Surface Decontamination of Titanium Dental Implants Subjected to Implantoplasty by Treatment with Citric Acid Solutions. Coatings 2024, 14, 1093. https://doi.org/10.3390/coatings14091093
Fernández-Garrido P, Bosch BM, Gil J, Fernández-Domínguez M. Surface Decontamination of Titanium Dental Implants Subjected to Implantoplasty by Treatment with Citric Acid Solutions. Coatings. 2024; 14(9):1093. https://doi.org/10.3390/coatings14091093
Chicago/Turabian StyleFernández-Garrido, Pilar, Begoña M. Bosch, Javier Gil, and Manuel Fernández-Domínguez. 2024. "Surface Decontamination of Titanium Dental Implants Subjected to Implantoplasty by Treatment with Citric Acid Solutions" Coatings 14, no. 9: 1093. https://doi.org/10.3390/coatings14091093







