Carbon Steel Corrosion Induced by Sulfate-Reducing Bacteria: A Review of Electrochemical Mechanisms and Pathways in Biofilms
Abstract
:1. Introduction
2. Mechanism of SRB-Induced Metal Corrosion
2.1. Corrosion of Carbon Steel
2.1.1. The Cathodic Depolarization Theory (CDT)
2.1.2. Acid Metabolite Corrosion Theory
2.1.3. The Biocatalytic Cathodic Sulfate Reduction Mechanism (BCSR)
2.2. Corrosion of Cu, Zn
3. Effect of Extracellular Polymers
3.1. EPS Affects MIC
3.1.1. Suppression of MIC
3.1.2. Promotion of MIC
3.2. EPS Affects EET
3.2.1. EPS Influences DET
3.2.2. EPS Influences MET
4. MIC and MFC
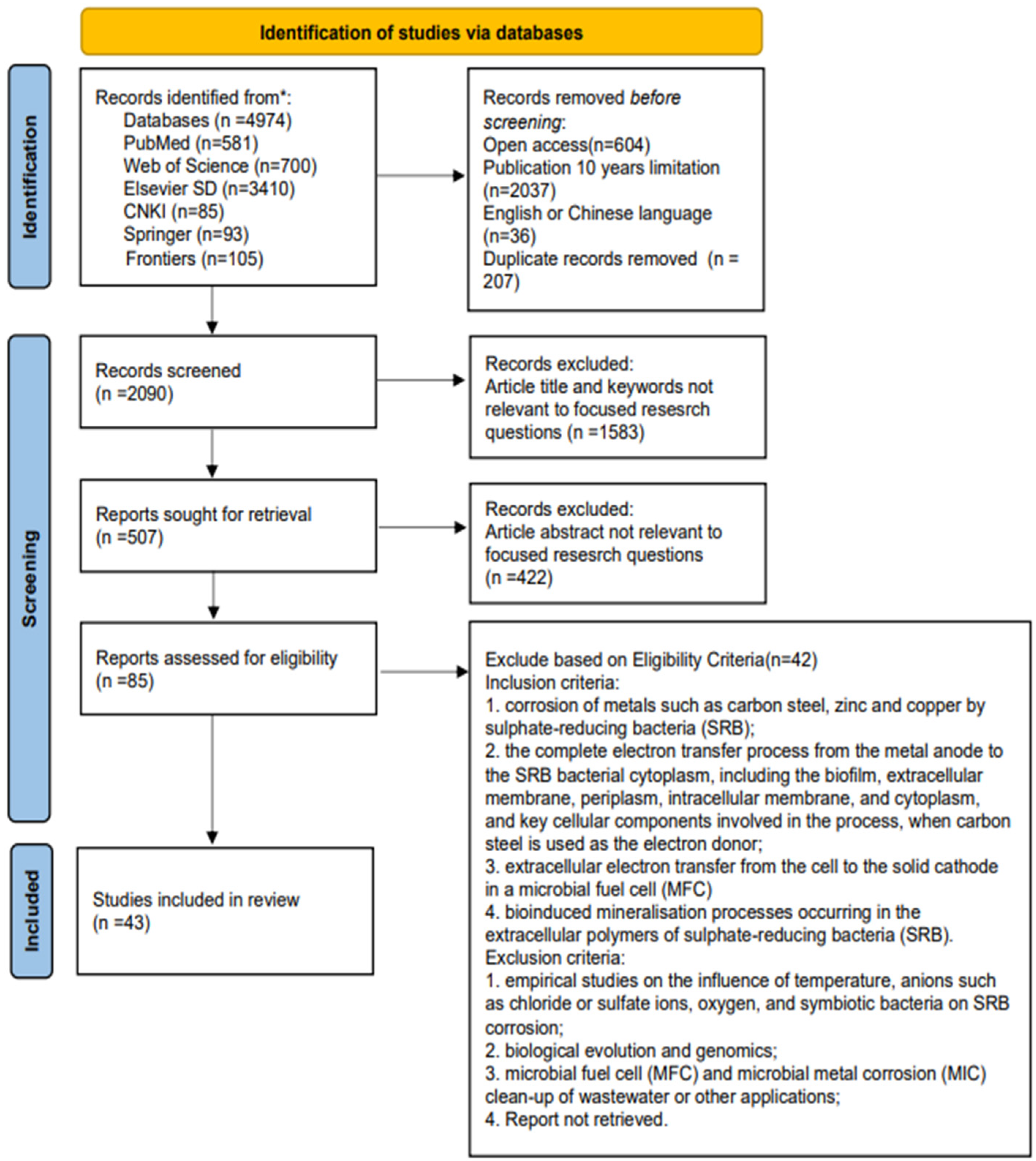
- MIC is now an important branch of metals research. Xu et al. [11] classified MIC into two main categories based on the type of anaerobic metabolism and electron transport: I MIC and II MIC. I MIC refers to the fact that the underlying biofilm close to the metal surface, due to the lack of a carbon source, directly uses the metal (e.g., Fe0) as an electron donor to obtain energy [16]. Since the electrons released from metal anodization cannot “swim” as freely as the ions in solution, the electrons must pass through the biofilm on the surface of the iron [27] and then cross the cell membrane into the cytoplasm, reducing the intracellular electron acceptor and releasing the energy. The SRB metal corrosion mechanism of both the CDT and BCSR involves I MIC. II MIC is caused by corrosive metabolites secreted by microorganisms, including protons and (undissociated) organic acids [11]. These oxidants do not require the involvement of biofilms or biocatalysis, with direct extracellular elimination of electrons from the metal anode. The biofilms can act as a diffusion barrier, maintaining high local metabolite concentrations and causing severe localized metal corrosion. H2S corrosion released by SRB metabolism is II MIC [15].
- The concept of EET is adapted from MFC. An MFC refers to the technology that uses biofilm as a catalyst to directly convert the chemical energy generated by oxidizing organic substances into bioelectric energy [54]. When microorganisms with the ability to produce electricity participate in MFC, the direction of EET is directed outward from the cell to the extracellular electrodes (e.g., insoluble Fe (III), Mn (III), Mn (IV), and other oxides [55,56]), which means that electrons released from the oxidation of organic carbon are transported from the cytoplasm to the metal oxide cathode outside the cell. When there is a localized lack of an organic carbon source as an electron donor within the cells of an electroproducing microorganism, this causes EET reversal, resulting in MIC. Xu et al. [57] compared Desulfovibrio vulgaris with different degrees of MIC caused by carbon-source starvation in media with different carbon contents. Bacteria grown on C1018 carbon steel sheets in the ATCC 1249 medium versus the medium with 90% and 99% reduced organic carbon were found to have the most severe metal corrosion caused by biofilm in the case of starved, but not completely starved, carbon sources. Clauwaert et al. [58] and Huang et al. [59] designed the MFC with a biocathode instead of an oxygen cathode. In this case, electrons are released from the anode into the extracellular environment and enter the biofilm covering the surface of the biocathode before being transferred across the cell wall into the cathode biofilm cells, and the direction of electron transfer from the cathode biofilm to the cathode is the same as in MIC. Therefore, electron transfer in biofilm is often reversible, and the electrochemical mechanism and electron transfer mode in MIC is the same as that in the biocathode of MFC, and the mechanism of how extracellular electrons are imported into the intracellular cell through biofilm can be explored based on the microbial export electron pathway [14,15,16].
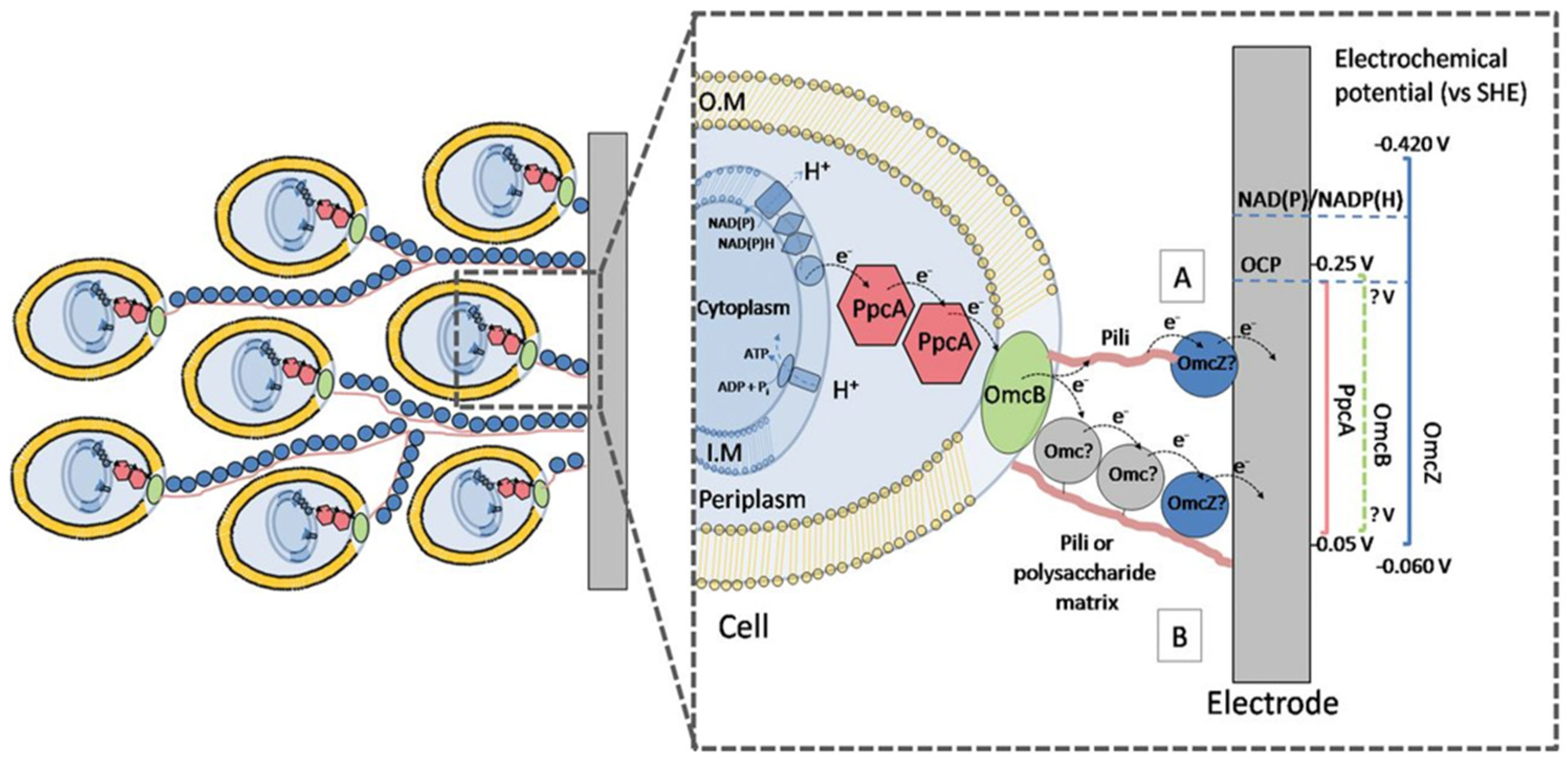
4.1. Electrical Direct Contact Transmission: Shewanella oneidenis MR-1
4.2. Conductive Bacterial Hair Proteins or Nanowires: Geobacter sulfurreducens
4.3. Electron Shuttling Based on Soluble Electron Mediators: Flavins and Phenazines
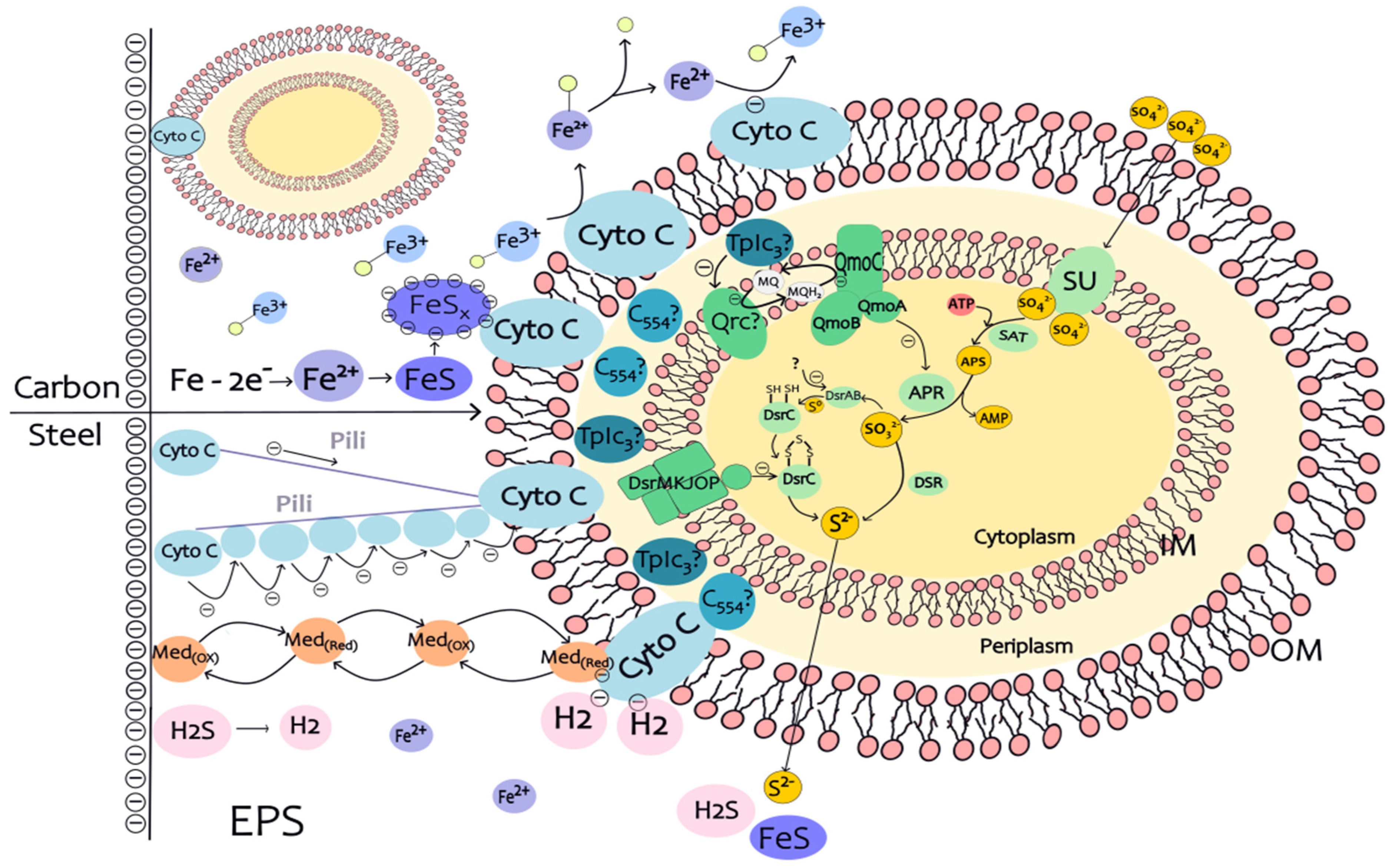
5. Electron Transfer in the Corrosion of SRB Microbial Carbon Steel
5.1. Electron Transfer in EPS
5.2. Electron Transfer in the Periplasm from the Outer to the Inner Cell Membrane
5.3. Electron Transfer in SRB Cells
6. Conclusions
- The bioinduced mineralization occurring in EPS can effectively inhibit metal corrosion, providing new ideas for corrosion protection and the development of new, natural green corrosion inhibitors. The biomineralization induced by SRB will form MeS/MeCO3 mineral precipitates on the cell surface, but how the changes in its own physiological and metabolic activities specifically induce the occurrence of extracellular mineralization needs to be further explored experimentally.
- Currently, there are more studies on extracellular electron transfer in MFC, and the transfer mechanism where electrons undergo single-step tunneling in the range of 15.5 Å and undergo long-range jumps driven by redox gradients expands the quantum mechanical perspective for probing electron transfer at the bioenergetics level. The elucidation of the mode, mechanism, and pathway of extracellular electron transfer occurring in MIC, on the other hand, only originates from the inference of reversible processes and lacks substantial and more direct experimental evidence.
- The identification of the types of cytochromes or other electron carriers involved in electron transfer and their roles in the various types of cytochromes present in the EPS layer, extracellular membrane, periplasm, and intracellular membrane during the corrosion of carbon steel induced by SRB still requires further experimental investigation. Molecular tools such as gene mutation, proteomics analysis, and metabolomics studies may play an important role in the identification of key components.
- The actual corrosion environment is often more complex, whether or how temperature, pH, anions, symbiotic bacteria, etc., affect the corrosion process of SRB carbon steel. Further understanding and discussion are needed to determine whether the electrochemical mechanism and pathway of SRB corrosion on other metal materials, such as carbon steel materials of different concentrations, other alloys and so on, occurring in biofilms are consistent, and whether the corrosion mechanism is the same.
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Khan, M.S.; Yang, K.; Liu, Z.; Zhou, L.; Liu, W.; Lin, S.; Wang, X.; Shang, C. Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials. Coatings 2023, 13, 1683. [Google Scholar] [CrossRef]
- Rao, P.; Mulky, L. Microbially Influenced Corrosion and Its Control Measures: A Critical Review. J. Bio- Tribo-Corros. 2023, 9, 57. [Google Scholar] [CrossRef]
- Rao, P.; Mulky, L. An Overview of Microbiologically Influenced Corrosion on Stainless Steel. ChemBioEng Rev. 2023, 10, 829–840. [Google Scholar] [CrossRef]
- Kobisy, A.S.; Nassar, H.N.; Tawfik, S.M.; Elshatoury, E.H.; Aiad, I. Mitigation of Eco-unfriendly and Costly Microbial Induced Corrosion Using Novel Synthesized Schiff Base Cationic Surfactants. J. Chem. Technol. Biotechnol. 2021, 96, 941–952. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; Abo-Alkasem, M.I. Environmental Sulfate-Reducing Microorganisms. In Application of Microbes in Environmental and Microbial Biotechnology; Inamuddin, Ahamed, M.I., Prasad, R., Eds.; Springer Nature: Singapore, 2022; pp. 625–654. ISBN 9789811622250. [Google Scholar]
- Kushkevych, I.; Kovářová, A.; Dordevic, D.; Gaine, J.; Kollar, P.; Vítězová, M.; Rittmann, S.K.-M.R. Distribution of Sulfate-Reducing Bacteria in the Environment: Cryopreservation Techniques and Their Potential Storage Application. Processes 2021, 9, 1843. [Google Scholar] [CrossRef]
- Dennis, E.; Julia, G. Corrosion of Iron by Sulfate-Reducing Bacteria: New Views of an Old Problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar]
- Zhou, L.; Wu, J.; Ji, J.-H.; Gao, J.; Liu, Y.-F.; Wang, B.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Characteristics of Microbiota, Core Sulfate-Reducing Taxa and Corrosion Rates in Production Water from Five Petroleum Reservoirs in China. Sci. Total Environ. 2023, 858, 159861. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, W.; Gong, A.; Zheng, S.; Zhao, D.; Zhao, W.; Fan, R. Species of corrosive microbes and corrosion mechanisms. Chin. J. Eng. 2023, 45, 927–940. [Google Scholar] [CrossRef]
- He, J.; Yang, C.; Li, Z. Research Progress of Microbiologically Influenced Corrosion and Protection in Building Industry. J. Chin. Soc. Corros. Prot. 2021, 41, 151–160. [Google Scholar]
- Xu, D.; Li, Y.; Song, F.; Gu, T. Laboratory Investigation of Microbiologically Influenced Corrosion of C1018 Carbon Steel by Nitrate Reducing Bacterium Bacillus licheniformis. Corros. Sci. 2013, 77, 385–390. [Google Scholar] [CrossRef]
- Gu, T.; Zhao, K.; Nesic, S. A New Mechanistic Model for Mic Based on A Biocatalytic Cathodic Sulfate Reduction Theory. In Proceedings of the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009. [Google Scholar]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Gu, T.; Wang, D.; Lekbach, Y.; Xu, D. Extracellular Electron Transfer in Microbial Biocorrosion. Curr. Opin. Electrochem. 2021, 29, 100763. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron Mediators Accelerate the Microbiologically Influenced Corrosion of 304 Stainless Steel by the Desulfovibrio vulgaris Biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Xu, D.; Nan, L.; Liu, H.; Li, Q.; Yang, K. Study on Mechanisms of Microbiologically Influenced Corrision of Metal from the Perspective of Bioelectrochemistry and Bio-energetics. Chin. J. Mater. Res. 2016, 30, 161–170. [Google Scholar]
- Vengatesh, G.; Ganapathi Sundaram, R.; Punitha, N. Menadione Sodium Bisulfite as an Efficient Anti-Corrosion Additive for Mild Steel in Acid Corrosion: Electrochemical, Surface Morphological and Theoretical Studies. J. Mol. Liq. 2024, 410, 125653. [Google Scholar] [CrossRef]
- Jin, J.; Guan, Y. The Mutual Co-Regulation of Extracellular Polymeric Substances and Iron Ions in Biocorrosion of Cast Iron Pipes. Bioresour. Technol. 2014, 169, 387–394. [Google Scholar] [CrossRef]
- Huang, H.; Liu, S.; Jiang, C. Microbiologically influenced corrosion and mechanisms. Microbiol. China 2017, 44, 1699–1713. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically Influenced Corrosion and Current Mitigation Strategies: A State of the Art Review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Dong, X.; Guan, F.; Xu, L.; Duan, J.; Hou, B. A review: Progress on the Corrosion Mechanism of Sulfate-reducing Bacteria in Marine Environment on Metal Materials. J. Chin. Soc. Corros. Prot. 2021, 41, 1–12. [Google Scholar]
- Blackwood, D.J. An Electrochemist Perspective of Microbiologically Influenced Corrosion. Corros. Mater. Degrad. 2020, 1, 59–76. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Hu, Y.; Zhou, L.; Zheng, B. Growth Characteristics of Thermophile Sulfate-Reducing Bacteria and Its Effect on Carbon Steel. Mater. Corros. 2009, 60, 218–224. [Google Scholar] [CrossRef]
- Bao, Q. The Study of Corrosion Mechanism Induced by Sulphate Reducing Bacteria and Composite Antibacterial Materials. Ph.D. Thesis, Graduate School of the Chinese Academy of Sciences (Institute of Oceanography), Qingdao, China, 2013. [Google Scholar]
- Dall’Agnol, L.T.; Cordas, C.M.; Moura, J.J.G. Influence of Respiratory Substrate in Carbon Steel Corrosion by a Sulphate Reducing Prokaryote Model Organism. Bioelectrochemistry 2014, 97, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Characklis, W.G. Corrosion of Mild Steel Under Anaerobic Biofilm. Corrosion 1993, 49, 186–199. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic Microbiologically Influenced Corrosion Mechanisms Interpreted Using Bioenergetics and Bioelectrochemistry: A Review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Dou, W.; Pu, Y.; Han, X.; Song, Y.; Chen, S.; Gu, T. Corrosion of Cu by a Sulfate Reducing Bacterium in Anaerobic Vials with Different Headspace Volumes. Bioelectrochemistry 2020, 133, 107478. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Jia, R.; Jin, P.; Liu, J.; Chen, S.; Gu, T. Investigation of the Mechanism and Characteristics of Copper Corrosion by Sulfate Reducing Bacteria. Corros. Sci. 2018, 144, 237–248. [Google Scholar] [CrossRef]
- Wang, D.; Unsal, T.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.; Saleh, M.A.; Gu, T. Sulfate Reducing Bacterium Desulfovibrio vulgaris Caused Severe Microbiologically Influenced Corrosion of Zinc and Galvanized Steel. Int. Biodeterior. Biodegrad. 2021, 157, 105160. [Google Scholar] [CrossRef]
- Lv, M.; Li, Z.; Du, M.; Xiao, F.; Chen, X. Formation, Function and Evolution of Biofilm in Microbiologically Influenced Corrosion. Surf. Technol. 2019, 48, 59–68+139. [Google Scholar] [CrossRef]
- Ke, N.; Ni, Y.; He, J.; Liu, H.; Jin, Z.; Liu, H. Research Progress of Metal Corrosion Caused by Extracellular Polymeric Substances of Microorganisms. J. Chin. Soc. Corros. Prot. 2024, 44, 278–294. [Google Scholar]
- Pal, M.K.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio- Tribo-Corros. 2022, 8, 76. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Li, J. Review of the research on biomineralization. Geol. Resour. 2009, 18, 317–320+297. [Google Scholar]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-Inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef]
- Wen, Q.; Qin, Y.; Zheng, J.; Wei, Q.; Zhang, Y.; Jiang, Y. Research advances in the fixation of heavy metals in acid mine wastewater by sulfate reducing bacteria. Chem. Ind. Eng. Prog. 2022, 41, 5578–5587. [Google Scholar] [CrossRef]
- Huang, M. Research Progress and Application of Biomineralization. Adv. Microbiol. 2021, 10, 175–181. [Google Scholar] [CrossRef]
- Hao, X.; Bai, Y.; Lou, Y.; Zhang, D. Research Progress of Microbial Mineralization Impact on Inhibits Metal Corrosion Behavior. Surf. Technol. 2021, 50, 18–29. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Mckenzie, J.A.; Warthmann, R.J.; Vasconcelos, C. Microbially Influenced Formation of Mg-Calcite and Ca-Dolomite in the Presence of Exopolymeric Substances Produced by Sulphate-Reducing Bacteria. Terra Nova 2014, 26, 72–77. [Google Scholar] [CrossRef]
- Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; Ueshima, M.; González, L.A.; Roberts, J.A. Ordered Low-Temperature Dolomite Mediated by Carboxyl-Group Density of Microbial Cell Walls. AAPG Bull. 2013, 97, 2113–2125. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, J.; Zhang, W.; Wang, S.; Liu, W. Research Status Review of Microbial Induced Carbonate Precipitation Technology in Reinforcing Sand. Subgrade Eng. 2018, 5, 6–11. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A.D. Review on Microbial Induced Calcite Precipitation Mechanisms Leading to Bacterial Selection for Microbial Concrete. Constr. Build. Mater. 2019, 225, 67–75. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, Minerals and Microbes: Geomicrobiology and Bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the Alkalinity Engine: The Role of Electron Donors in the Organomineralization Potential of Sulfate-reducing Bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, B.; Chen, J.; Jing, C.; Zhong, M.; Shan, Q. Prospect Research on the Diversity of Extracellular Mineralization Process Induced by Mineralizing Microorganisms and Its Use as a Treatment for Soil Pollutants. Sustainability 2023, 15, 4858. [Google Scholar] [CrossRef]
- Marques, M.J.F.; Jaume, J.; Mercier, D.; Seyeux, A.; Zanna, S.; Basseguy, R.; Marcus, P. The Positive Impact of Biomineralization for Marine Corrosion Protection of AA5083 Alloy. Corros. Sci. 2024, 233, 112053. [Google Scholar] [CrossRef]
- Shan, X.; Wang, J.; Du, M.; Tian, Z. Inhibitory Effect of Marine Bacillus Sp. and Its Biomineralization on the Corrosion of X65 Steel in Offshore Oilfield Produced Water. Bioelectrochemistry 2024, 157, 108659. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, F. Electrochemical Roles of Extracellular Polymeric Substances in Biofilms. Curr. Opin. Electrochem. 2017, 4, 206–211. [Google Scholar] [CrossRef]
- Rabaey, K. Bioelectrochemical Systems: From Extracellular Electron Tranfer to Biotechnological Application; Science Press: Beijing, China, 2012. [Google Scholar]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular Polymeric Substances Are Transient Media for Microbial Extracellular Electron Transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef]
- Li, S.-W.; Sheng, G.-P.; Cheng, Y.-Y.; Yu, H.-Q. Redox Properties of Extracellular Polymeric Substances (EPS) from Electroactive Bacteria. Sci. Rep. 2016, 6, 39098. [Google Scholar] [CrossRef]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular Polymeric Substances from Shewanella Sp. HRCR-1 Biofilms: Characterization by Infrared Spectroscopy and Proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef]
- Saunders, S.H.; Tse, E.C.M.; Yates, M.D.; Otero, F.J.; Trammell, S.A.; Stemp, E.D.A.; Barton, J.K.; Tender, L.M.; Newman, D.K. Extracellular DNA Promotes Efficient Extracellular Electron Transfer by Pyocyanin in Pseudomonas aeruginosa Biofilms. Cell 2020, 182, 919–932.e19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent Advances in Microbial Fuel Cells (MFCs) and Microbial Electrolysis Cells (MECs) for Wastewater Treatment, Bioenergy and Bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518. [Google Scholar] [CrossRef]
- Myers, C.R.; Nealson, K.H. Bacterial Manganese Reduction and Growth with Manganese Oxide as the Sole Electron Acceptor. Science 1988, 240, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Saffarini, D. Iron and Manganese in Anaerobic Respiration: Environmental Significance, Physiology, and Regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gu, T. Carbon Source Starvation Triggered More Aggressive Corrosion against Carbon Steel by the Desulfovibrio vulgaris Biofilm. Int. Biodeterior. Biodegrad. 2014, 91, 74–81. [Google Scholar] [CrossRef]
- Clauwaert, P.; van de Ha, D.; Boon, N.; Verbeken, K.; Verstraete, M.; Rabaey, K.; Verstraete, W. Open Air Biocathode Enables Effective Electricity Generation with Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Regan, J.M.; Quan, X. Electron Transfer Mechanisms, New Applications, and Performance of Biocathode Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Cavallaro, G.; Rosato, A. Cytochrome c: Occurrence and Functions. Chem. Rev. 2006, 106, 90–115. [Google Scholar] [CrossRef]
- Breuer, M.; Rosso, K.M.; Blumberger, J.; Butt, J.N. Multi-Haem Cytochromes in Shewanella oneidensis MR-1: Structures, Functions and Opportunities. J. R. Soc. Interface 2015, 12, 20141117. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.K.; Barua, S.; Reed, S.B.; Romine, M.F.; Nealson, K.H.; Fredrickson, J.K.; Tiedje, J.M.; Zhou, J. Reduction of Nitrate in Shewanella oneidensis Depends on Atypical NAP and NRF Systems with NapB as a Preferred Electron Transport Protein from CymA to NapA. ISME J. 2009, 3, 966–976. [Google Scholar] [CrossRef]
- Piper, S.E.H.; Edwards, M.J.; van Wonderen, J.H.; Casadevall, C.; Martel, A.; Jeuken, L.J.C.; Reisner, E.; Clarke, T.A.; Butt, J.N. Bespoke Biomolecular Wires for Transmembrane Electron Transfer: Spontaneous Assembly of a Functionalized Multiheme Electron Conduit. Front. Microbiol. 2021, 12, 714508. [Google Scholar] [CrossRef]
- Shi, L.; Squier, T.C.; Zachara, J.M.; Fredrickson, J.K. Respiration of Metal (Hydr)Oxides by Shewanella and Geobacter: A Key Role for Multihaem c-Type Cytochromes. Mol. Microbiol. 2007, 65, 12–20. [Google Scholar] [CrossRef]
- Zacharoff, L.A.; El-Naggar, M.Y. Redox Conduction in Biofilms: From Respiration to Living Electronics. Curr. Opin. Electrochem. 2017, 4, 182–189. [Google Scholar] [CrossRef]
- Smith, L.J.; Kahraman, A.; Thornton, J.M. Heme Proteins—Diversity in Structural Characteristics, Function, and Folding. Proteins Struct. Funct. Bioinform. 2010, 78, 2349–2368. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Long-Range Electron Transfer. Proc. Natl. Acad. Sci. USA 2005, 102, 3534–3539. [Google Scholar] [CrossRef]
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry, 5th ed.; National Center Biotechnology Informationõs Bookshelf; Palgrave MacMillan: London, UK, 2002; pp. 93–126. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level; Wiley: Hoboken, NJ, USA, 2016; p. 484. [Google Scholar]
- Page, C.C.; Moser, C.C.; Chen, X.; Dutton, P.L. Natural Engineering Principles of Electron Tunnelling in Biological Oxidation–Reduction. Nature 1999, 402, 47–52. [Google Scholar] [CrossRef]
- Xu, S.; Barrozo, A.; Tender, L.M.; Krylov, A.I.; El-Naggar, M.Y. Multiheme Cytochrome Mediated Redox Conduction through Shewanella oneidensis MR-1 Cells. J. Am. Chem. Soc. 2018, 140, 10085–10089. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Walker, D.J.F. Geobacter Protein Nanowires. Front. Microbiol. 2019, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.S.; Schrott, G.D.; Busalmen, J.P. A Long Way to the Electrode: How Do Geobacter Cells Transport Their Electrons? Biochem. Soc. Trans. 2012, 40, 1274–1279. [Google Scholar] [CrossRef]
- Liu, X.; Walker, D.J.F.; Nonnenmann, S.S.; Sun, D.; Lovley, D.R. Direct Observation of Electrically Conductive Pili Emanating from Geobacter Sulfurreducens. mBio 2021, 12, e0220921. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; O’Brien, J.P.; Yi, S.M.; Yalcin, S.E.; Srikanth, V.; Shen, C.; Vu, D.; Ing, N.L.; Hochbaum, A.I.; et al. Structure of Microbial Nanowires Reveals Stacked Hemes That Transport Electrons over Micrometers. Cell 2019, 177, 361. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.R.; Schultz, Z.D. SERS Speciation of the Electrochemical Oxidation-Reduction of Riboflavin. The Analyst 2016, 141, 5078–5087. [Google Scholar] [CrossRef]
- Tan, S.L.J.; Webster, R.D. Electrochemically Induced Chemically Reversible Proton-Coupled Electron Transfer Reactions of Riboflavin (Vitamin B2). J. Am. Chem. Soc. 2012, 134, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella Secretes Flavins That Mediate Extracellular Electron Transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Zheng, P.; Zhang, M. Progress in research of electron transfer mediator(ETM). J. Zhejiang Univ. Life Sci. 2016, 42, 573–581. [Google Scholar]
- Chen, J.-J.; Chen, W.; He, H.; Li, D.-B.; Li, W.-W.; Xiong, L.; Yu, H.-Q. Manipulation of Microbial Extracellular Electron Transfer by Changing Molecular Structure of Phenazine-Type Redox Mediators. Environ. Sci. Technol. 2013, 47, 1033–1039. [Google Scholar] [CrossRef]
- Feng, J.; Qian, Y.; Wang, Z.; Wang, X.; Xu, S.; Chen, K.; Ouyang, P. Enhancing the Performance of Escherichia Coli-Inoculated Microbial Fuel Cells by Introduction of the Phenazine-1-Carboxylic Acid Pathway. J. Biotechnol. 2018, 275, 1–6. [Google Scholar] [CrossRef]
- Wei, B.; Xv, J.; Gao, L.; Qin, Q.; Fu, Q.; Yu, C.; Sun, C.; Wang, Z. Research Progress on Sulfate Reducing Bacteria Induced Corrosion of Pipeline Steel in Soil Environment. Surf. Technol. 2021, 50, 30–44. [Google Scholar] [CrossRef]
- Ding, L. Study on Corrosion Resistance of Anti-Microbial Tubing and Casing Steel. Bachelor’s Thesis, Xi’an Shiyou University, Xi’an, China, 2024. [Google Scholar]
- Wang, D.; Yang, C.; Saleh, M.A.; Alotaibi, M.D.; Mohamed, M.E.; Xu, D.; Gu, T. Conductive Magnetite Nanoparticles Considerably Accelerated Carbon Steel Corrosion by Electroactive Desulfovibrio Vulgaris Biofilm. Corros. Sci. 2022, 205, 110440. [Google Scholar] [CrossRef]
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the Effect of Carbon Steel Exposure in Sulfide Containing Solutions to Microbially Induced Corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Alrammah, F.; Xu, L.; Patel, N.; Kontis, N.; Rosado, A.; Gu, T. Conductive Magnetic Nanowires Accelerated Electron Transfer between C1020 Carbon Steel and Desulfovibrio vulgaris Biofilm. Sci. Total Environ. 2024, 925, 171763. [Google Scholar] [CrossRef]
- Li, H.; Xu, D.; Li, Y.; Feng, H.; Liu, Z.; Li, X.; Gu, T.; Yang, K. Extracellular Electron Transfer Is a Bottleneck in the Microbiologically Influenced Corrosion of C1018 Carbon Steel by the Biofilm of Sulfate-Reducing Bacterium Desulfovibrio vulgaris. PLoS ONE 2015, 10, e0136183. [Google Scholar] [CrossRef]
- Tan, Y.; Adhikari, R.Y.; Malvankar, N.S.; Pi, S.; Ward, J.E.; Woodard, T.L.; Nevin, K.P.; Xia, Q.; Tuominen, M.T.; Lovley, D.R. Synthetic Biological Protein Nanowires with High Conductivity. Small 2016, 12, 4481–4485. [Google Scholar] [CrossRef] [PubMed]
- Zhuravel, R.; Huang, H.; Polycarpou, G.; Polydorides, S.; Motamarri, P.; Katrivas, L.; Rotem, D.; Sperling, J.; Zotti, L.A.; Kotlyar, A.B.; et al. Backbone Charge Transport in Double-Stranded DNA. Nat. Nanotechnol. 2020, 15, 836–840. [Google Scholar] [CrossRef]
- Finkenstadt, V.L. Natural Polysaccharides as Electroactive Polymers. Appl. Microbiol. Biotechnol. 2005, 67, 735–745. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Plage, B.; Schnitzer, M. A Chemical Structure for Humic Substances. Sci. Nat. 1991, 78, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Newman, D.K. Extracellular Electron Transfer. Cell. Mol. Life Sci. CMLS 2001, 58, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, D.; Stuchebrukhov, A.A. DNA Repair Mechanism by Photolyase: Electron Transfer Path from the Photolyase Catalytic Cofactor FADH(-) to DNA Thymine Dimer. J. Theor. Biol. 2001, 210, 237–248. [Google Scholar] [CrossRef]
- Keller, K.L.; Rapp-Giles, B.J.; Semkiw, E.S.; Porat, I.; Brown, S.D.; Wall, J.D. New Model for Electron Flow for Sulfate Reduction in Desulfovibrio Alaskensis G20. Appl. Environ. Microbiol. 2014, 80, 855–868. [Google Scholar] [CrossRef]
- Venceslau, S.S.; Lino, R.R.; Pereira, I.A.C. The Qrc Membrane Complex, Related to the Alternative Complex III, Is a Menaquinone Reductase Involved in Sulfate Respiration. J. Biol. Chem. 2010, 285, 22774–22783. [Google Scholar] [CrossRef]
- Murali, R.; Yu, H.; Speth, D.R.; Wu, F.; Metcalfe, K.S.; Crémière, A.; Laso-Pèrez, R.; Malmstrom, R.R.; Goudeau, D.; Woyke, T.; et al. Physiological Potential and Evolutionary Trajectories of Syntrophic Sulfate-Reducing Bacterial Partners of Anaerobic Methanotrophic Archaea. PLoS Biol. 2023, 21, e3002292. [Google Scholar] [CrossRef]
- Diao, M.; Dyksma, S.; Koeksoy, E.; Ngugi, D.K.; Anantharaman, K.; Loy, A.; Pester, M. Global Diversity and Inferred Ecophysiology of Microorganisms with the Potential for Dissimilatory Sulfate/Sulfite Reduction. FEMS Microbiol. Rev. 2023, 47, fuad058. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Wang, W.; Wang, L. Research progress of the mircobial sulfur-cycling network. Acta Microbiol. Sin. 2021, 61, 1567–1581. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Keller, K.L.; Wall, J.D.; Pereira, I.A.C. The Membrane QmoABC Complex Interacts Directly with the Dissimilatory Adenosine 5´-Phosphosulfate Reductase in Sulfate Reducing Bacteria. Front. Microbiol. 2012, 3, 137. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.H.; Lourenço, A.I.; Morais, F.; Teixeira, M.; Xavier, A.V.; Saraiva, L.M.; Pereira, I.A.C. A Novel Membrane-Bound Respiratory Complex from Desulfovibrio Desulfuricans ATCC 27774. Biochim. Biophys. Acta (BBA)—Bioenerg. 2003, 1605, 67–82. [Google Scholar] [CrossRef]
- Mander, G.J.; Duin, E.C.; Linder, D.; Stetter, K.O.; Hedderich, R. Purification and Characterization of a Membrane-bound Enzyme Complex from the Sulfate-reducing Archaeon Archaeoglobus fulgidus Related to Heterodisulfide Reductase from Methanogenic Archaea. Eur. J. Biochem. 2002, 269, 1895–1904. [Google Scholar] [CrossRef]
- Duarte, A.G.; Santos, A.A.; Pereira, I.A.C. Electron Transfer between the QmoABC Membrane Complex and Adenosine 5′-Phosphosulfate Reductase. Biochim. Biophys. Acta (BBA)—Bioenerg. 2016, 1857, 380–386. [Google Scholar] [CrossRef]
- Pereira, I.A.C.; Ramos, A.R.; Grein, F.; Marques, M.C.; Da Silva, S.M.; Venceslau, S.S. A Comparative Genomic Analysis of Energy Metabolism in Sulfate Reducing Bacteria and Archaea. Front. Microbiol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Pereira, I.A.C. Respiratory Membrane Complexes of Desulfovibrio. In Proceedings of the Microbial Sulfur Metabolism; Dahl, C., Friedrich, C.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 24–35. [Google Scholar]
- Ferreira, D.; Barbosa, A.C.C.; Oliveira, G.P.; Catarino, T.; Venceslau, S.S.; Pereira, I.A.C. The DsrD Functional Marker Protein Is an Allosteric Activator of the DsrAB Dissimilatory Sulfite Reductase. Proc. Natl. Acad. Sci. USA 2022, 119, e2118880119. [Google Scholar] [CrossRef]
- Santos, A.A.; Venceslau, S.S.; Grein, F.; Leavitt, W.D.; Dahl, C.; Johnston, D.T.; Pereira, I.A.C. A Protein Trisulfide Couples Dissimilatory Sulfate Reduction to Energy Conservation. Science 2015, 350, 1541–1545. [Google Scholar] [CrossRef]
- Barbosa, A.C.C.; Venceslau, S.S.; Pereira, I.A.C. DsrMKJOP Is the Terminal Reductase Complex in Anaerobic Sulfate Respiration. Proc. Natl. Acad. Sci. USA 2024, 121, e2313650121. [Google Scholar] [CrossRef]
- Venceslau, S.S.; Stockdreher, Y.; Dahl, C.; Pereira, I.A.C. The “Bacterial Heterodisulfide” DsrC Is a Key Protein in Dissimilatory Sulfur Metabolism. Biochim. Biophys. Acta BBA—Bioenerg. 2014, 1837, 1148–1164. [Google Scholar] [CrossRef] [PubMed]
- Parey, K.; Warkentin, E.; Kroneck, P.M.H.; Ermler, U. Reaction Cycle of the Dissimilatory Sulfite Reductase from Archaeoglobus fulgidus. Biochemistry 2010, 49, 8912–8921. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.F.; Vonrhein, C.; Matias, P.M.; Venceslau, S.S.; Pereira, I.A.C.; Archer, M. The Crystal Structure of Desulfovibrio vulgaris Dissimilatory Sulfite Reductase Bound to DsrC Provides Novel Insights into the Mechanism of Sulfate Respiration. J. Biol. Chem. 2008, 283, 34141. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Qiu, L.; Qiu, L. Carbon Steel Corrosion Induced by Sulfate-Reducing Bacteria: A Review of Electrochemical Mechanisms and Pathways in Biofilms. Coatings 2024, 14, 1105. https://doi.org/10.3390/coatings14091105
Liu N, Qiu L, Qiu L. Carbon Steel Corrosion Induced by Sulfate-Reducing Bacteria: A Review of Electrochemical Mechanisms and Pathways in Biofilms. Coatings. 2024; 14(9):1105. https://doi.org/10.3390/coatings14091105
Chicago/Turabian StyleLiu, Na, Lina Qiu, and Lijuan Qiu. 2024. "Carbon Steel Corrosion Induced by Sulfate-Reducing Bacteria: A Review of Electrochemical Mechanisms and Pathways in Biofilms" Coatings 14, no. 9: 1105. https://doi.org/10.3390/coatings14091105
APA StyleLiu, N., Qiu, L., & Qiu, L. (2024). Carbon Steel Corrosion Induced by Sulfate-Reducing Bacteria: A Review of Electrochemical Mechanisms and Pathways in Biofilms. Coatings, 14(9), 1105. https://doi.org/10.3390/coatings14091105






