Superhydrophilic Surface Creation and Its Temporal Transition to Hydrophobicity on Copper via Femtosecond Laser Texturing
Abstract
:1. Introduction
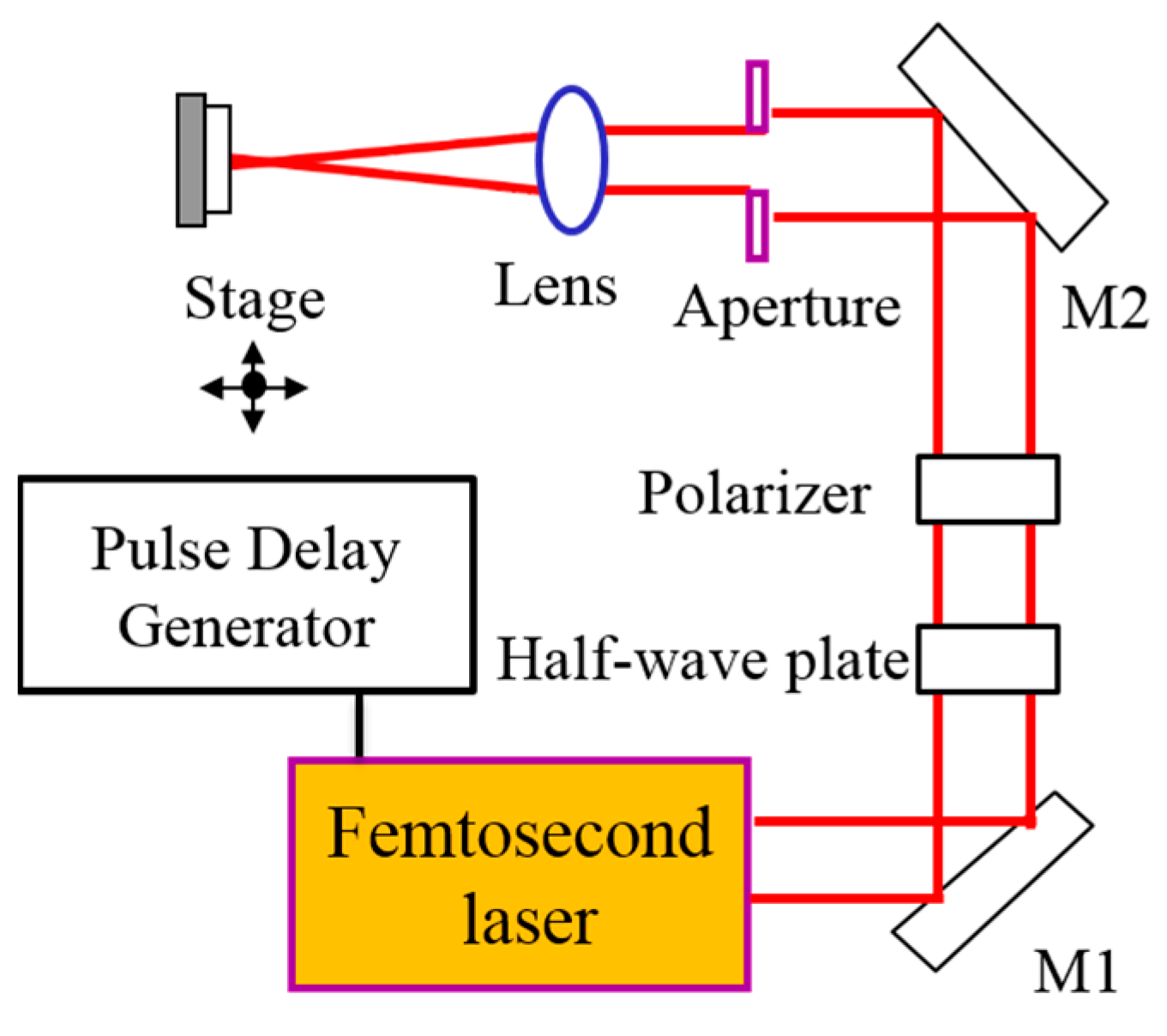
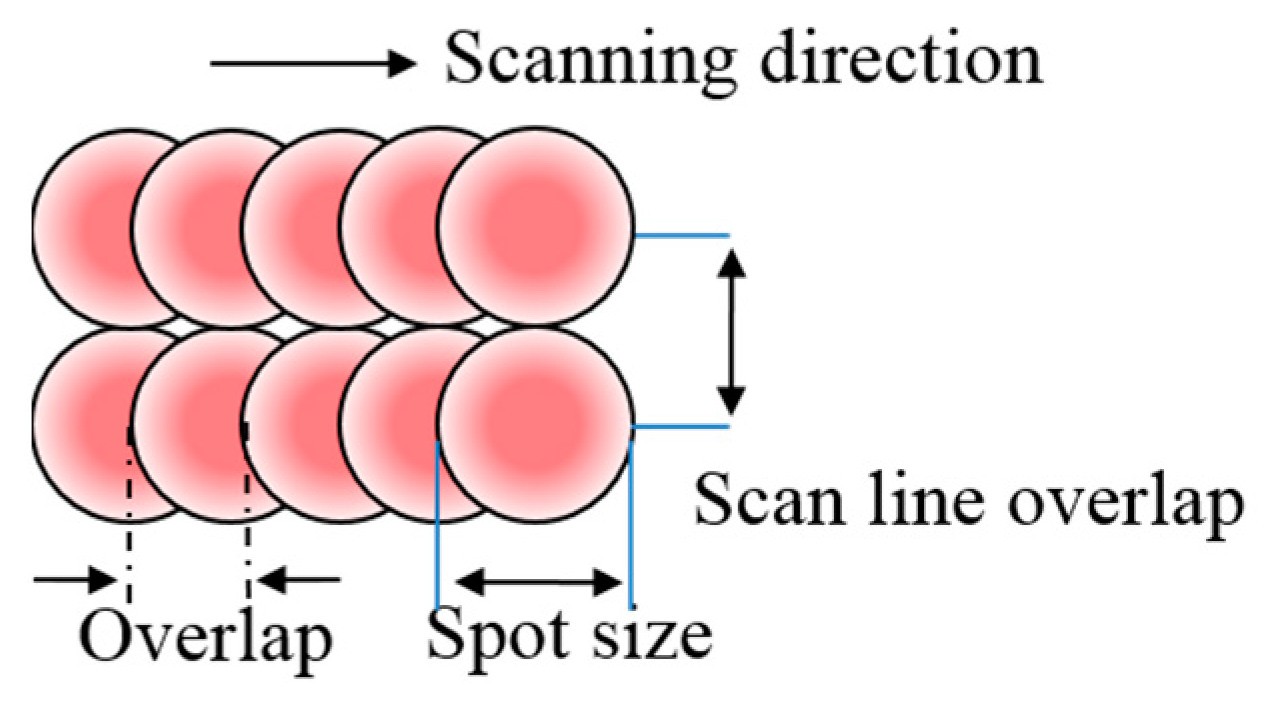
2. Materials and Methods
3. Results and Discussion
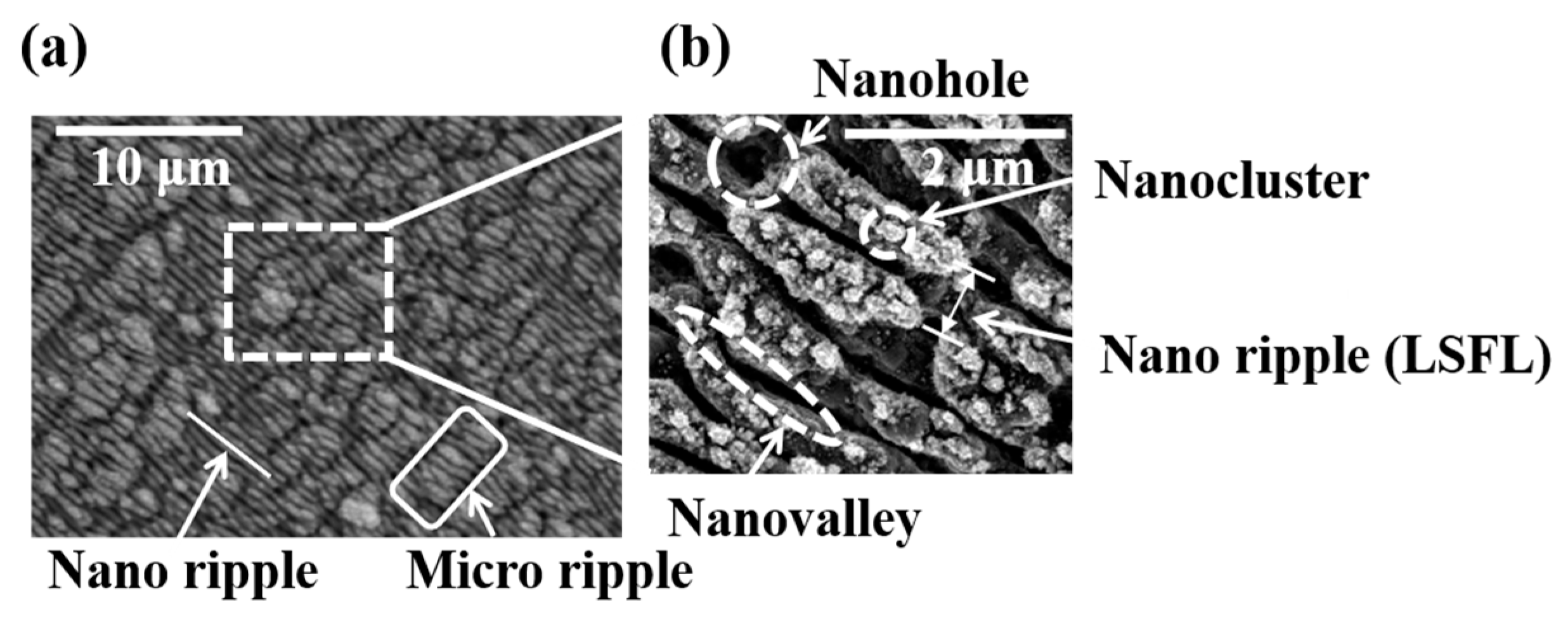
3.1. LIPSS Varying with Laser Parameters
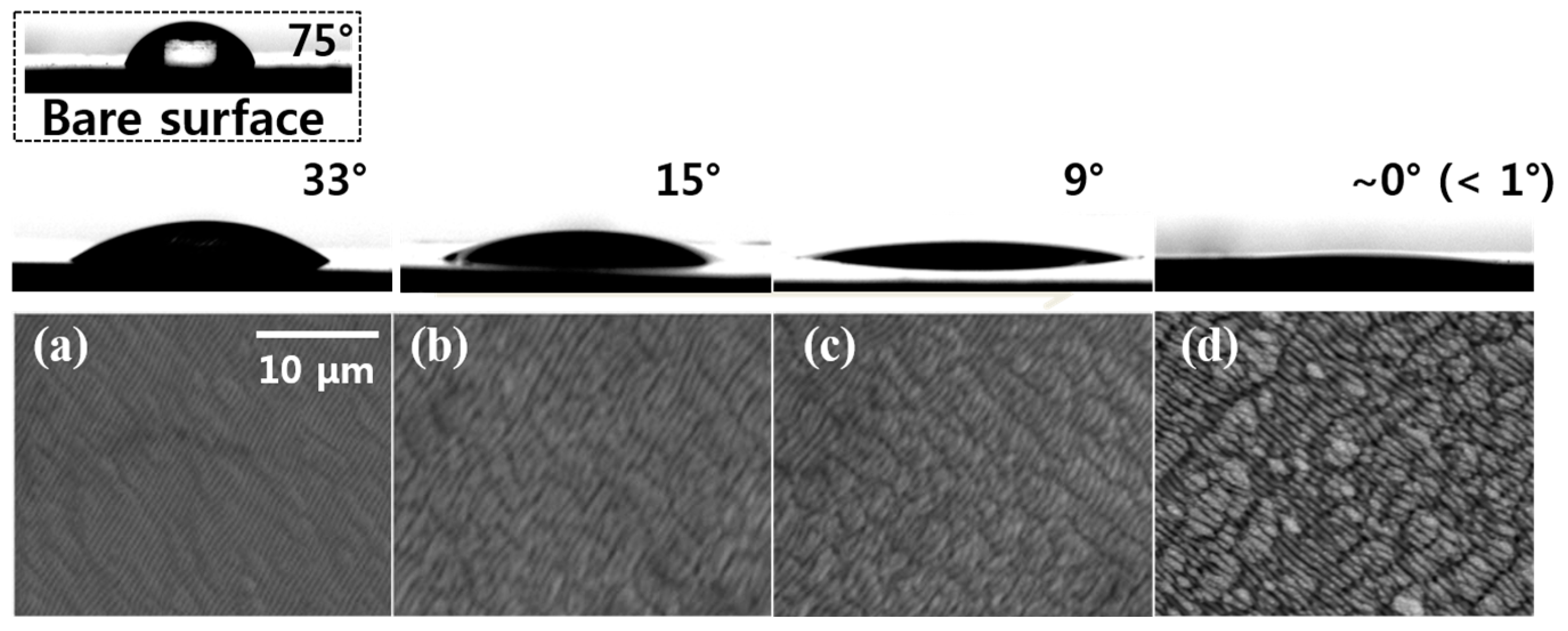
3.2. Wettability of the Laser-Modified Surfaces
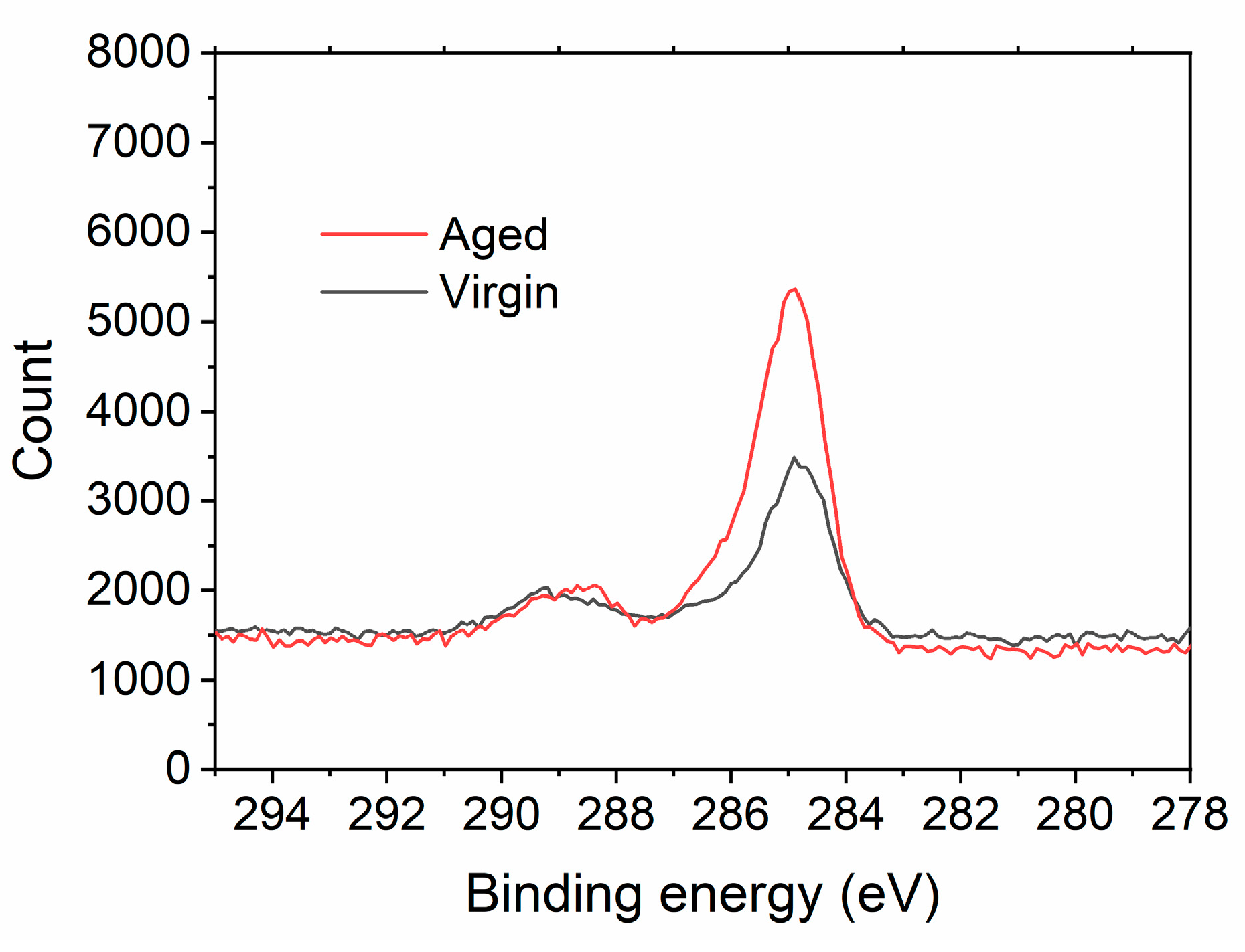
3.3. Aging Effect: Transition from Superhydrophilic to Hydrophobic Surface
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.J.; Jiang, L. Design and Creation of Superwetting/Antiwetting Surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Zhai, W.; Zhou, W.; Nai, S.M.L. Effect of Interface Wettability on Additively Manufactured Metal Matrix Composites: A Case Study of 316L-Y2O3 Oxide Dispersion-Strengthened Steel. Metals 2024, 14, 170. [Google Scholar] [CrossRef]
- Paul, D.; Zaplotnik, R.; Primc, G.; Vesel, A.; Mozetič, M. Evolution of the Surface Wettability of Vertically Oriented Multilayer Graphene Sheets Deposited by Plasma Technology. Nanomaterials 2024, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhao, D.; Zhang, J.; Shen, D.; Lu, Y.; Dong, L.; Xiao, Z.; Liu, Y.; Fan, X. Wettability conversion on ZnO nanowire arrays surface modified by oxygen plasma treatment and annealing. Chem. Phys. Lett. 2005, 413, 450–453. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.H.; Kim, J. Wettability of dual-scaled surfaces fabricated by the combination of a conventional silicon wet-etching and a ZnO solution method. J. Micromech. Microeng. 2009, 19, 095002. [Google Scholar] [CrossRef]
- McDonald, B.T.; Cui, T. Superhydrophilic surface modification of copper surfaces by layer-by-layer self-assembly and liquid phase deposition of TiO2 thin film. J. Colloid Interface Sci. 2011, 354, 1–6. [Google Scholar] [CrossRef]
- Ngo, C.-V.; Liu, Y.; Li, W.; Yang, J.; Guo, C. Scalable Wettability Modification of Aluminum Surface through Single-Shot Nanosecond Laser Processing. Nanomaterials 2023, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, P. The theory of the surface wettability angle in the formation of an oil film in internal combustion piston engines. Materials 2023, 16, 4092. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Barberoglou, M.; Zorba, V.; Manousaki, A.; Pagkozidis, A.; Stratakis, E.; Fotakis, C. Reversible photoinduced wettability transition of hierarchical ZnO structures. J. Phys. Chem. C 2009, 113, 2891–2895. [Google Scholar] [CrossRef]
- Eiamchai, P.; Chindaudom, P.; Horprathum, M.; Patthanasettakul, V.; Limsuwan, P. Design and investigation of photo-induced super-hydrophilic materials for car mirrors. Mater. Des. 2009, 30, 3428–3435. [Google Scholar] [CrossRef]
- Shi, J.J.; Yang, E.L. Non-UV driven self-cleaning and anti-fogging glasses prepared by ultrasonic nebulization of TiO2 hydrosol. Adv. Mater. Res. 2012, 549, 674–678. [Google Scholar] [CrossRef]
- Betz, A.R.; Jenkins, J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.D.; Kim, H.; Ahn, H.S.; Jo, H.; Kim, J.; Kim, M.H. Effects of nano-fluid and surfaces with nano structure on the increase of CHF. Exp. Therm. Fluid Sci. 2010, 34, 487–495. [Google Scholar] [CrossRef]
- Ahn, H.S.; Lee, C.; Kim, H.; Jo, H.; Kang, S.; Kim, J.; Shin, J.; Kim, M.H. Pool boiling CHF enhancement by micro/nanoscale modification of zircaloy-4 surface. Nucl. Eng. Des. 2010, 240, 3350–3360. [Google Scholar] [CrossRef]
- Ye, J.; Yin, Q.; Zhou, Y. Superhydrophilicity of anodic aluminum oxide films: From “honeycomb” to “bird’s nest”. Thin Solid Film. 2009, 517, 6012–6015. [Google Scholar] [CrossRef]
- Lawrence, J.; Li, L. Wettability characteristics of a modified mild steel with CO2, Nd: YAG, excimer and high power diode lasers. J. Phys. D Appl. Phys. 1999, 32, 2311. [Google Scholar] [CrossRef]
- Tseng, S.-F.; Hsiao, W.-T.; Chen, M.-F.; Huang, K.-C.; Hsiao, S.-Y.; Lin, Y.-S.; Chou, C.-P. Surface wettability of silicon substrates enhanced by laser ablation. Appl. Phys. A 2010, 101, 303–308. [Google Scholar] [CrossRef]
- Chang, T.-L.; Tsai, T.-K.; Yang, H.-P.; Huang, J.-Z. Effect of ultra-fast laser texturing on surface wettability of microfluidic channels. Microelectron. Eng. 2012, 98, 684–688. [Google Scholar] [CrossRef]
- Kam, D.; Bhattacharya, S.; Mazumder, J. Control of the wetting properties of an AISI 316L stainless steel surface by femtosecond laser-induced surface modification. J. Micromech. Microeng. 2012, 22, 105019. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Caputo, G.; Nobile, C.; Kipp, T.; Blasi, L.; Grillo, V.; Carlino, E.; Manna, L.; Cingolani, R.; Cozzoli, P.D.; Athanassiou, A. Reversible wettability changes in colloidal TiO2 nanorod thin-film coatings under selective UV laser irradiation. J. Phys. Chem. C 2008, 112, 701–714. [Google Scholar] [CrossRef]
- Zhang, L.; Shoji, M. Nucleation site interaction in pool boiling on the artificial surface. Int. J. Heat Mass Transf. 2003, 46, 513–522. [Google Scholar] [CrossRef]
- Chatpun, S.; Watanabe, M.; Shoji, M. Experimental study on characteristics of nucleate pool boiling by the effects of cavity arrangement. Exp. Therm. Fluid Sci. 2004, 29, 33–40. [Google Scholar] [CrossRef]
- Borowiec, A.; Haugen, H. Subwavelength ripple formation on the surfaces of compound semiconductors irradiated with femtosecond laser pulses. Appl. Phys. Lett. 2003, 82, 4462–4464. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C. Ultrafast dynamics of femtosecond laser-induced periodic surface pattern formation on metals. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Wagner, R.; Gottmann, J.; Horn, A.; Kreutz, E.W. Subwavelength ripple formation induced by tightly focused femtosecond laser radiation. Appl. Surf. Sci. 2006, 252, 8576–8579. [Google Scholar] [CrossRef]
- Vorobyev, A.; Makin, V.; Guo, C. Periodic ordering of random surface nanostructures induced by femtosecond laser pulses on metals. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Das, S.K.; Dasari, K.; Rosenfeld, A.; Grunwald, R. Extended-area nanostructuring of TiO2 with femtosecond laser pulses at 400 nm using a line focus. Nanotechnology 2010, 21, 155302. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Negar Mirvakili, M.; Kamal, S.; Englezos, P.; Hatzikiriakos, S.G. Laser-patterned super-hydrophobic pure metallic substrates: Cassie to Wenzel wetting transitions. J. Adhes. Sci. Technol. 2011, 25, 2789–2809. [Google Scholar] [CrossRef]
- Allahyari, E.; Nivas, J.J.; Oscurato, S.L.; Salvatore, M.; Ausanio, G.; Vecchione, A.; Fittipaldi, R.; Maddalena, P.; Bruzzese, R.; Amoruso, S. Laser surface texturing of copper and variation of the wetting response with the laser pulse fluence. Appl. Surf. Sci. 2019, 470, 817–824. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, W.; Xu, C.; Ren, L. Superhydrophobic copper surface textured by laser for delayed icing phenomenon. Langmuir 2020, 36, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Može, M.; Zupančič, M.; Steinbücher, M.; Golobič, I.; Gjerkeš, H. Nanosecond laser-textured copper surfaces hydrophobized with self-assembled monolayers for enhanced pool boiling heat transfer. Nanomaterials 2022, 12, 4032. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Xiong, D. Direct laser texturing technique for metal surfaces to achieve superhydrophobicity. Mater. Today Phys. 2022, 23, 100651. [Google Scholar] [CrossRef]
- Ta, D.V.; Dunn, A.; Wasley, T.J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Connaughton, C.; Shephard, J.D. Nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications. Appl. Surf. Sci. 2015, 357, 248–254. [Google Scholar] [CrossRef]
- Long, J.; He, Z.; Zhou, C.; Xie, X.; Cao, Z.; Zhou, P.; Zhu, Y.; Hong, W.; Zhou, Z. Hierarchical micro-and nanostructures induced by nanosecond laser on copper for superhydrophobicity, ultralow water adhesion and frost resistance. Mater. Des. 2018, 155, 185–193. [Google Scholar] [CrossRef]
- Bonse, J.; Rosenfeld, A.; Krüger, J. On the role of surface plasmon polaritons in the formation of laser-induced periodic surface structures upon irradiation of silicon by femtosecond-laser pulses. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Q.; Jiang, X.; Zhou, X.; Liu, Y.; Hu, X.; Zhang, J.; Yao, J.; Xu, J. Formation mechanism of high spatial frequency laser-induced periodic surface structures and experimental support. Appl. Surf. Sci. 2022, 580, 152107. [Google Scholar] [CrossRef]
- Bonse, J.; Krüger, J.; Höhm, S.; Rosenfeld, A. Femtosecond laser-induced periodic surface structures. J. Laser Appl. 2012, 24. [Google Scholar] [CrossRef]
- Bonse, J.; Krüger, J. Pulse number dependence of laser-induced periodic surface structures for femtosecond laser irradiation of silicon. J. Appl. Phys. 2010, 108. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, F.; Cheng, Y.; Xu, N.; Xu, Z. Origin of laser-induced near-subwavelength ripples: Interference between surface plasmons and incident laser. ACS Nano 2009, 3, 4062–4070. [Google Scholar] [CrossRef]
- Razi, S.; Asghari, M.; Mollabashi, M. Angle-and polarization-dependent reflection and backscattering from FS-LIPSS covered stainless steel surfaces: Experimental study. Eur. Phys. J. Plus 2020, 135, 1–19. [Google Scholar] [CrossRef]
- Xiao, C.; Shi, P.; Yan, W.; Chen, L.; Qian, L.; Kim, S.H. Thickness and structure of adsorbed water layer and effects on adhesion and friction at nanoasperity contact. Colloids Interfaces 2019, 3, 55. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Micro-, nano-and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1631–1672. [Google Scholar] [CrossRef]
- Su, Y.; Ji, B.; Zhang, K.; Gao, H.; Huang, Y.; Hwang, K. Nano to micro structural hierarchy is crucial for stable superhydrophobic and water-repellent surfaces. Langmuir 2010, 26, 4984–4989. [Google Scholar] [CrossRef]
- Nagy, N. Capillary bridges on hydrophobic surfaces: Analytical contact angle determination. Langmuir 2022, 38, 6201–6208. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Hashida, M.; Tokita, S.; Namba, S.; Okamuro, K. Mechanism for self-formation of periodic grating structures on a metal surface by a femtosecond laser pulse. Phys. Rev. B—Condens. Matter Mater. Phys. 2009, 79, 033409. [Google Scholar] [CrossRef]
- Sipe, J.; Young, J.F.; Preston, J.; Van Driel, H. Laser-induced periodic surface structure. I. Theory. Phys. Rev. B 1983, 27, 1141. [Google Scholar] [CrossRef]
- Bizi-Bandoki, P.; Valette, S.; Audouard, E.; Benayoun, S. Effect of stationary femtosecond laser irradiation on substructures’ formation on a mold stainless steel surface. Appl. Surf. Sci. 2013, 270, 197–204. [Google Scholar] [CrossRef]
- Kim, S.H.; Sohn, I.-B.; Jeong, S. Parallel ripple formation during femtosecond laser grooving of ceramic. Appl. Phys. A 2011, 103, 1053–1057. [Google Scholar] [CrossRef]
- Chang, F.-M.; Cheng, S.-L.; Hong, S.-J.; Sheng, Y.-J.; Tsao, H.-K. Superhydrophilicity to superhydrophobicity transition of CuO nanowire films. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Pei, M.-D.; Wang, B.; Li, E.; Zhang, X.-h.; Song, X.-m.; Yan, H. The fabrication of superhydrophobic copper films by a low-pressure-oxidation method. Appl. Surf. Sci. 2010, 256, 5824–5827. [Google Scholar] [CrossRef]
- Zeng, D.; Yung, K.C.; Xie, C. UV Nd: YAG laser ablation of copper: Chemical states in both crater and halo studied by XPS. Appl. Surf. Sci. 2003, 217, 170–180. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of Cu (OH) 2 into CuO, revisited. Solid State Sci. 2003, 5, 1471–1474. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.-Y. Oxygen adsorption induced superhydrophilic-to-superhydrophobic transition on hierarchical nanostructured CuO surface. J. Colloid Interface Sci. 2012, 377, 438–441. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of polycrystalline copper thin films at ambient conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Shirazy, M.R.; Blais, S.; Fréchette, L.G. Mechanism of wettability transition in copper metal foams: From superhydrophilic to hydrophobic. Appl. Surf. Sci. 2012, 258, 6416–6424. [Google Scholar] [CrossRef]
- Conradi, M.; Sever, T.; Gregorčič, P.; Kocijan, A. Short-and long-term wettability evolution and corrosion resistance of uncoated and polymer-coated laser-textured steel surface. Coatings 2019, 9, 592. [Google Scholar] [CrossRef]


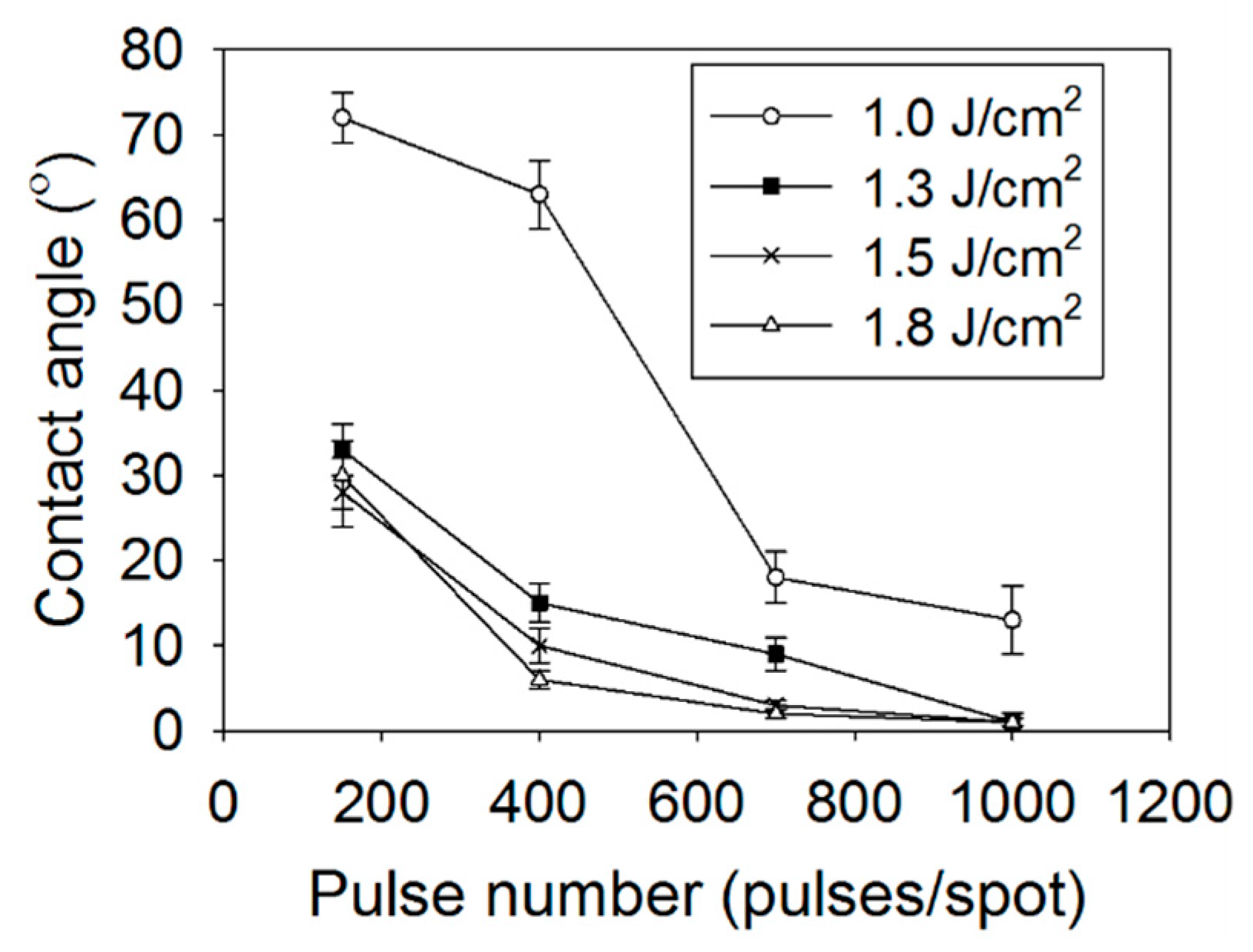





| Scanning Speed [μm/s] | Overlapped Pulse Number [Pulses/Spot] |
|---|---|
| 500 | 150 |
| 187.5 | 400 |
| 107.1 | 700 |
| 75 | 1000 |
| Cu/Cu2O | CuO | Cu(OH)2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak (eV) | Area (%) | FWHM (eV) | Peak (eV) | Area (%) | FWHM (eV) | Peak (eV) | Area (%) | FWHM (eV) | |
| Virgin | 932.7 | 76.76 | 0.97 | 933.8 | 8.31 | 2.00 | 935.1 | 14.93 | 2.40 |
| Aged | 932.7 | 72.52 | 1.10 | 933.8 | 19.85 | 2.00 | 935.1 | 7.63 | 2.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J. Superhydrophilic Surface Creation and Its Temporal Transition to Hydrophobicity on Copper via Femtosecond Laser Texturing. Coatings 2024, 14, 1107. https://doi.org/10.3390/coatings14091107
Ha J. Superhydrophilic Surface Creation and Its Temporal Transition to Hydrophobicity on Copper via Femtosecond Laser Texturing. Coatings. 2024; 14(9):1107. https://doi.org/10.3390/coatings14091107
Chicago/Turabian StyleHa, Jeonghong. 2024. "Superhydrophilic Surface Creation and Its Temporal Transition to Hydrophobicity on Copper via Femtosecond Laser Texturing" Coatings 14, no. 9: 1107. https://doi.org/10.3390/coatings14091107





