Future Parabolic Trough Collector Absorber Coating Development and Service Lifetime Estimation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Preparation
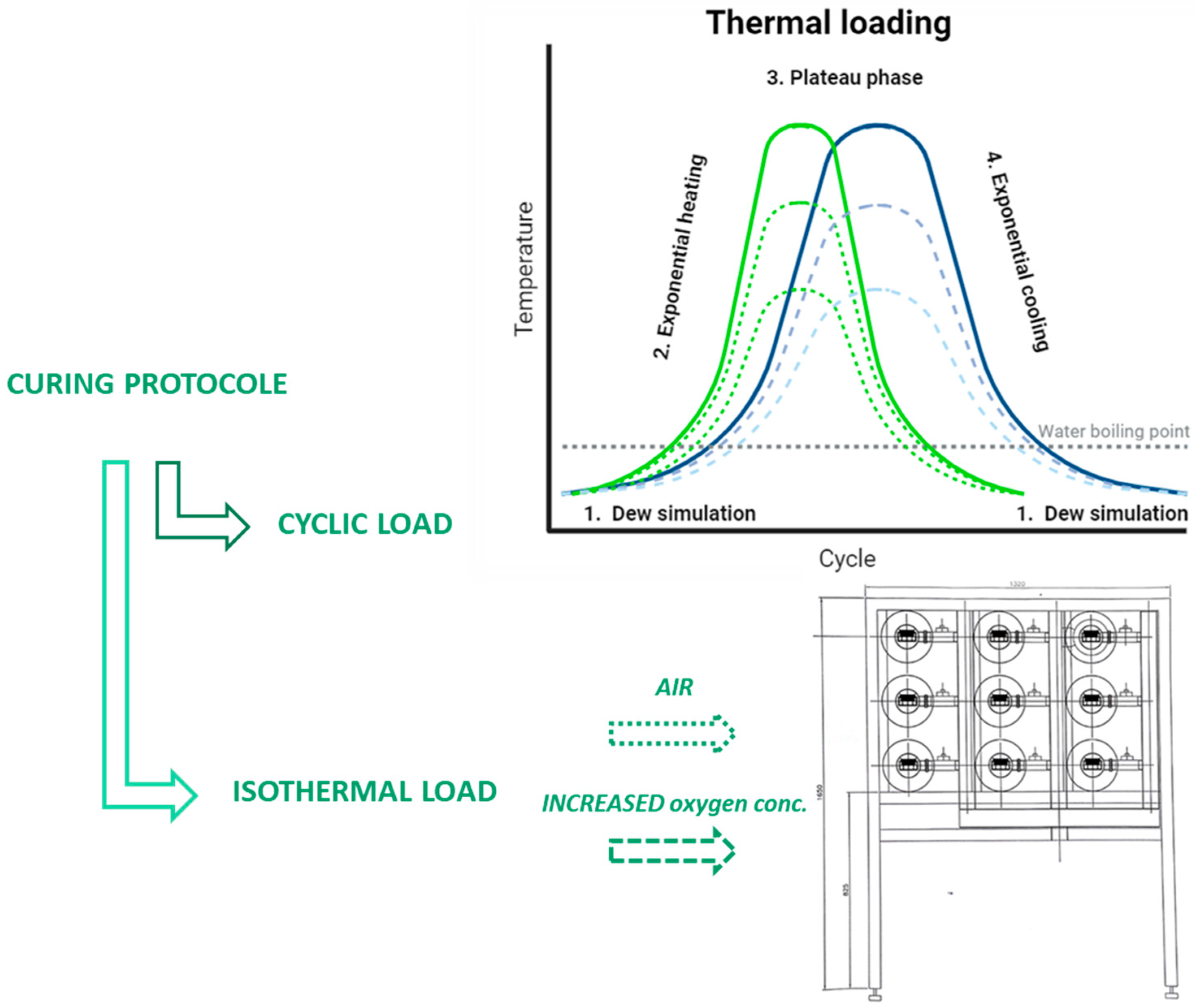
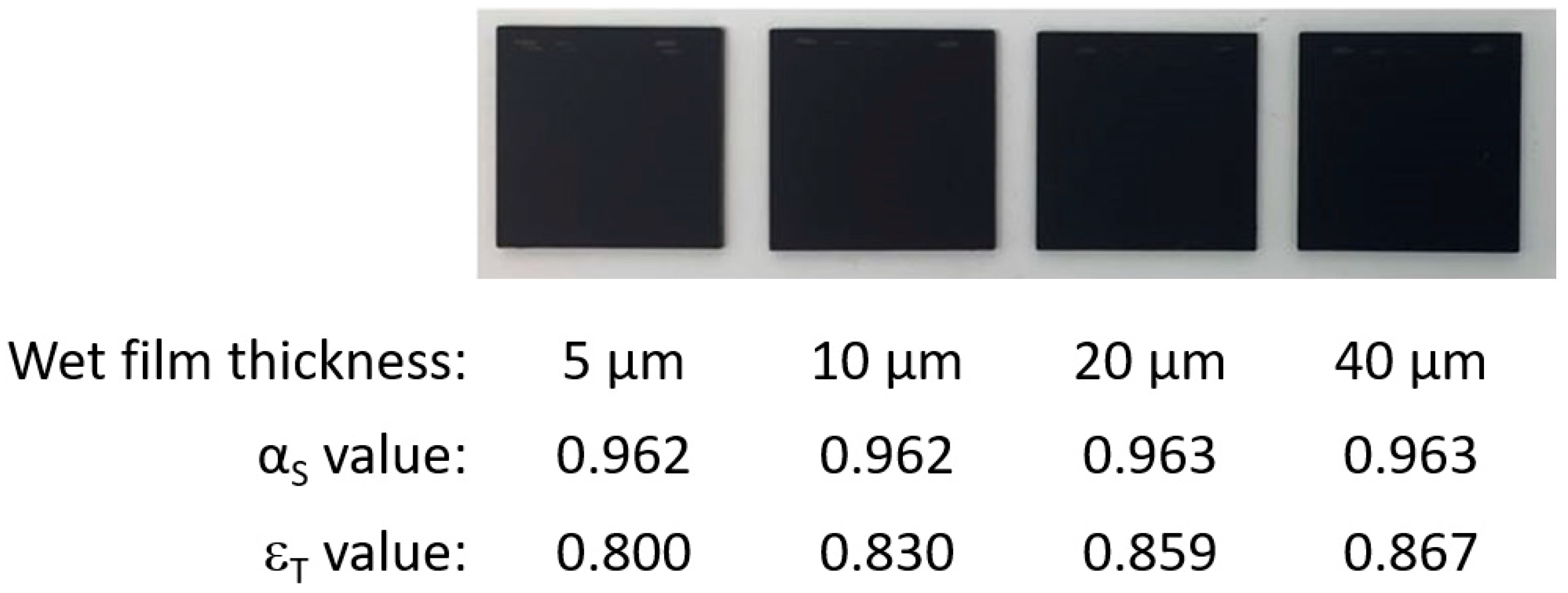
2.2. Coating Load
2.3. Characterization
2.4. Mathematical Modelling
3. Results
3.1. Crack Evolution
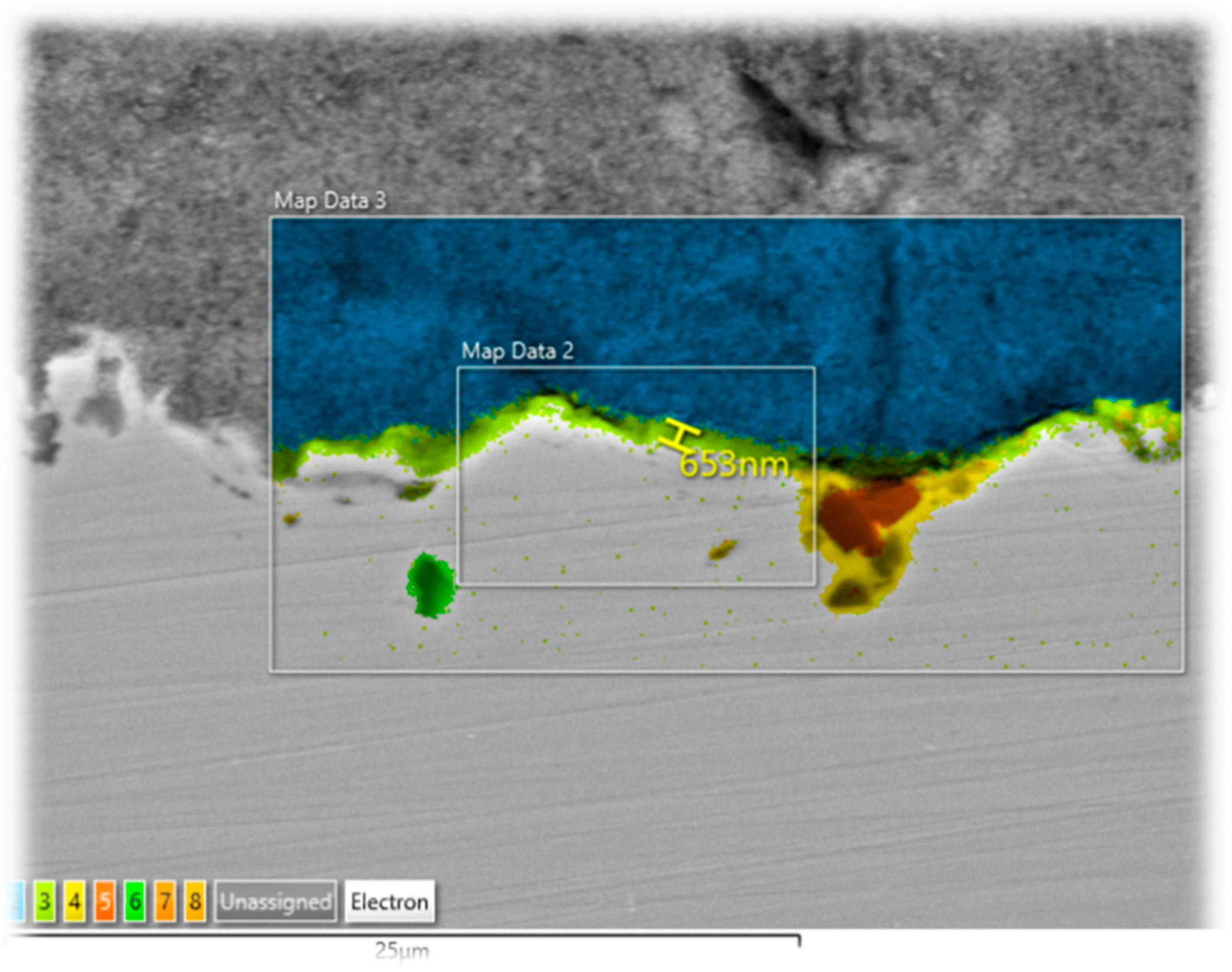
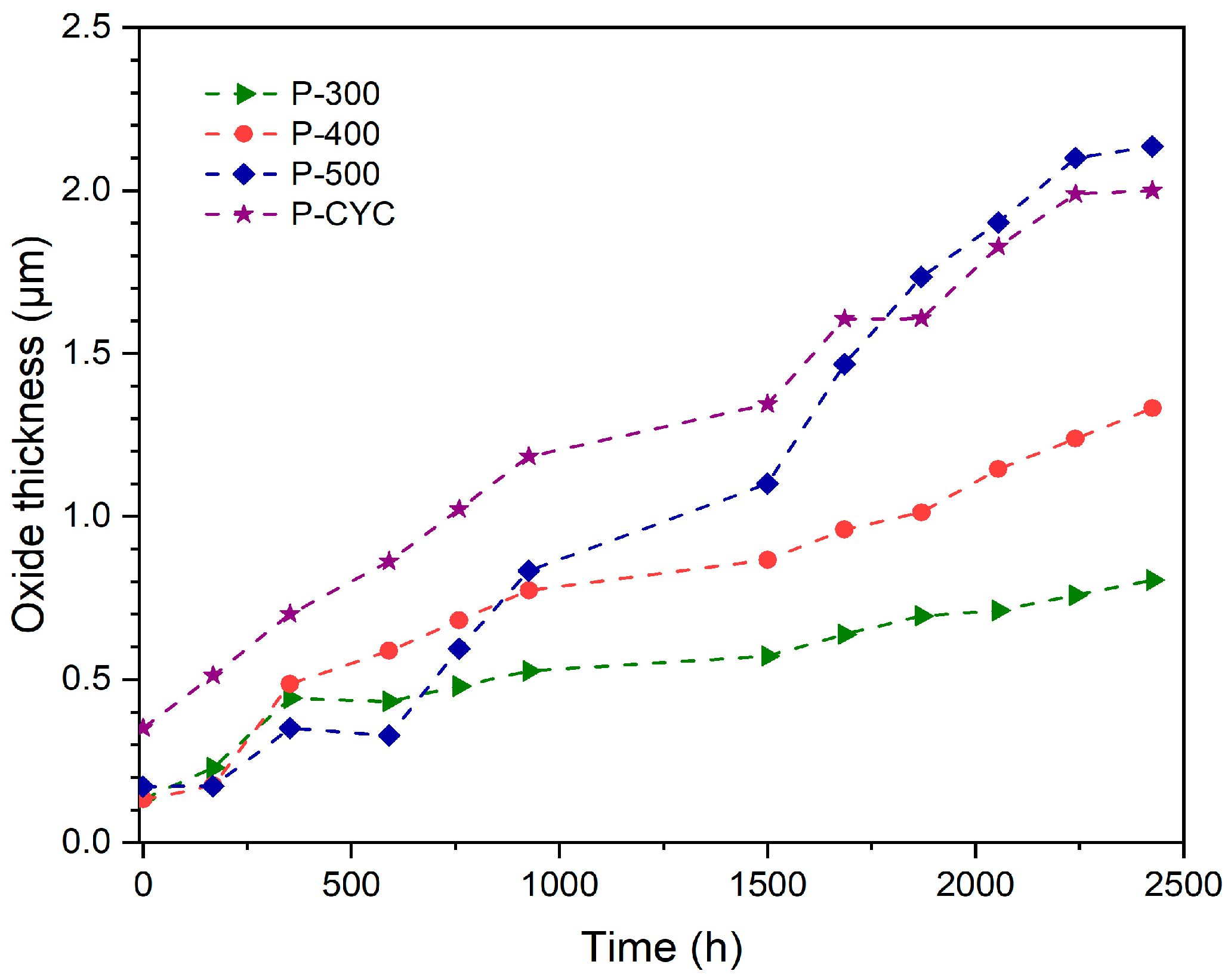
3.2. Oxide Thickness Evaluation
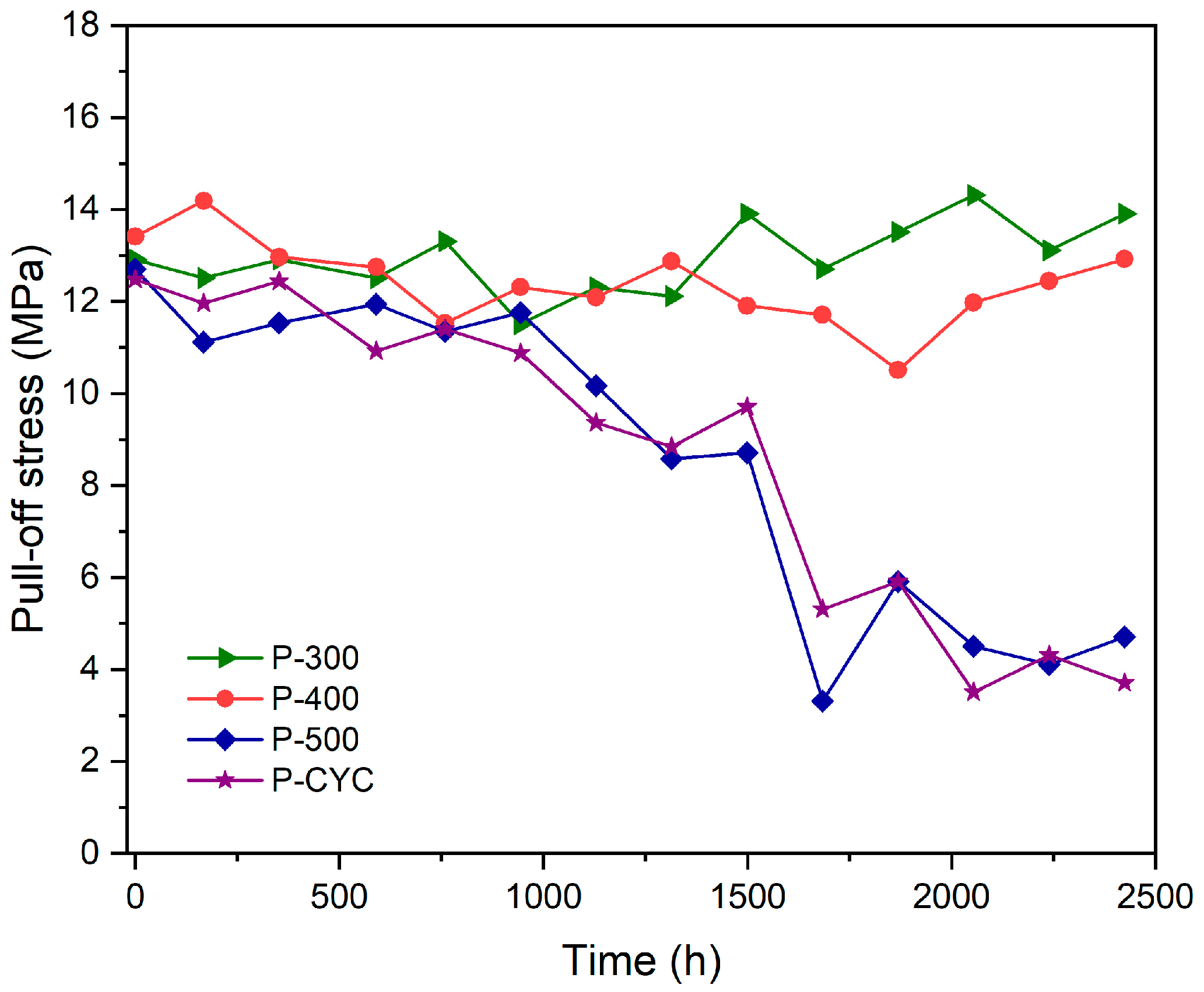
3.3. Adhesion Evaluation
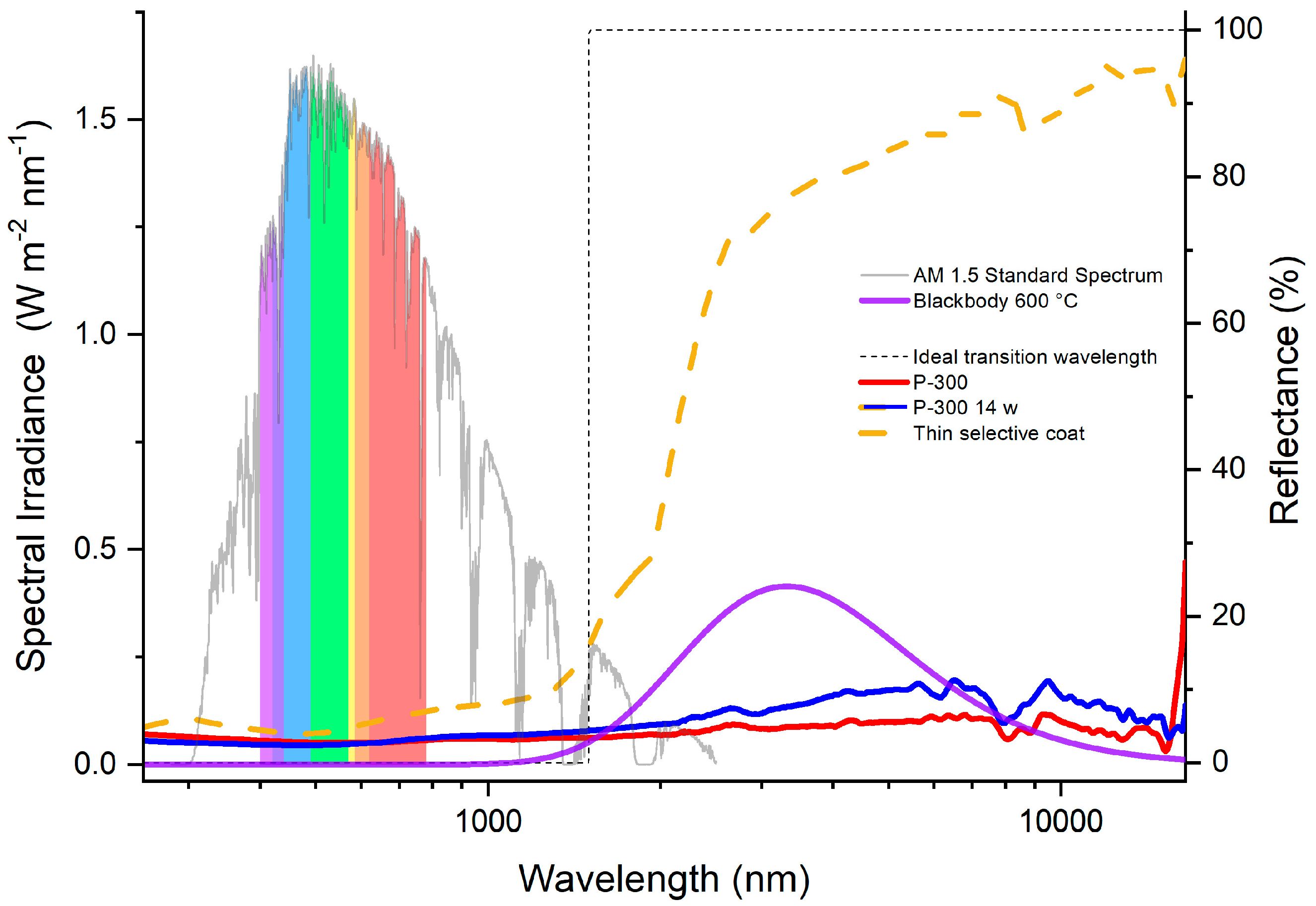
3.4. Coating Optical Property Changes Due to Thermal Load
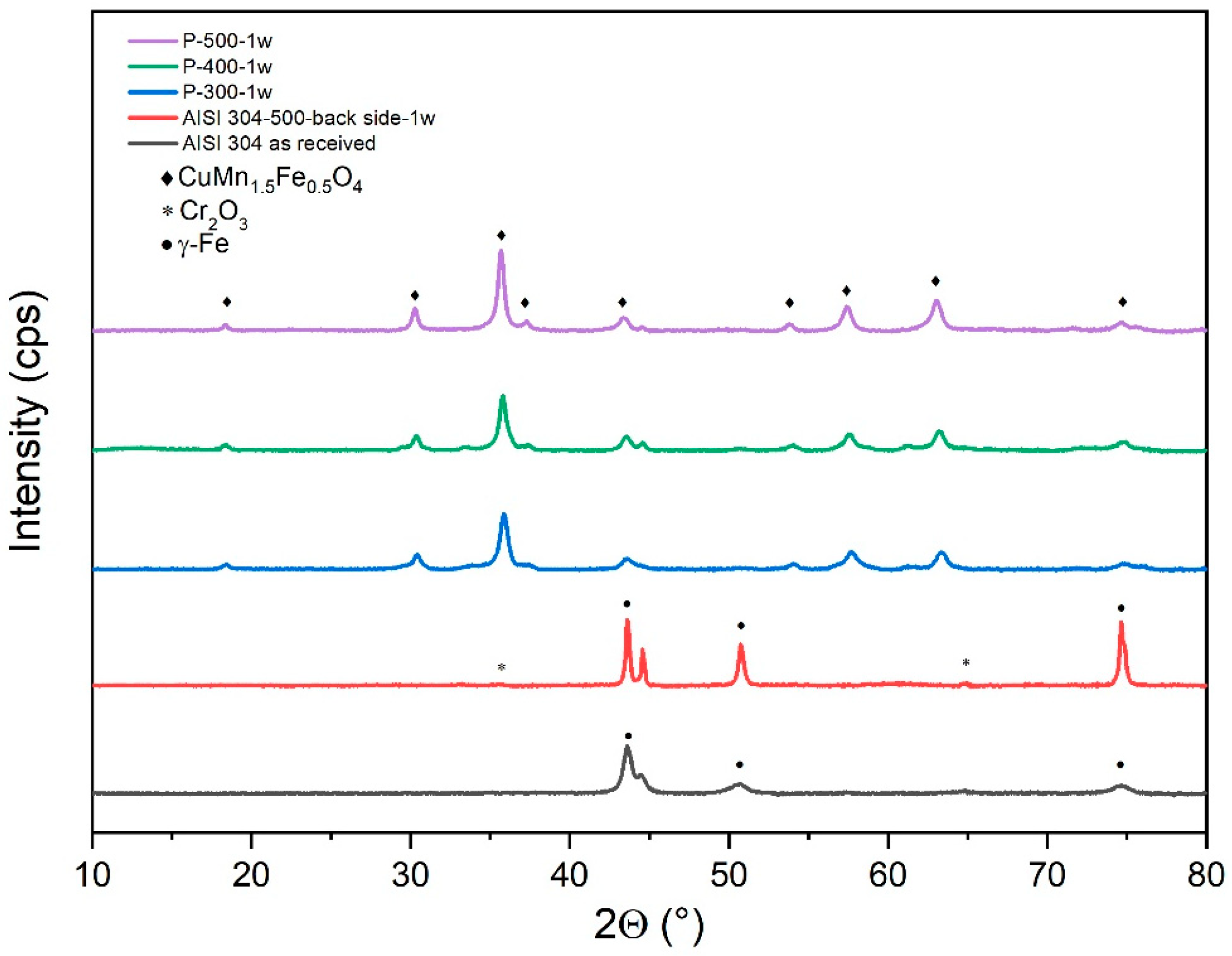
3.5. XRD Spectroscopy Evaluation
3.6. Service Lifetime Prediction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiel, G.P.; Stark, A.K. To Decarbonize Industry, We Must Decarbonize Heat. Joule 2021, 5, 531–550. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.-M.; et al. Net-Zero Emissions Energy Systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef] [PubMed]
- Naegler, T.; Simon, S.; Klein, M.; Gils, H.C. Quantification of the European Industrial Heat Demand by Branch and Temperature Level. Int. J. Energy Res. 2015, 39, 2019–2030. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Communication. Decarbonising Our Energy System to Meet Our Climate Goals; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- EurObserv’ER. Solar Thermal and Concentrated Solar Power Barometer 2023; EurObserv’ER: Paris, France; Available online: https://www.eurobserv-er.org/solar-thermal-and-concentrated-solar-power-barometer-2023/ (accessed on 17 July 2024).
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar Energy: Potential and Future Prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Noc, L.; Jerman, I. Review of the Spectrally Selective (CSP) Absorber Coatings, Suitable for Use in SHIP. Sol. Energy Mater. Sol. Cells 2022, 238, 111625. [Google Scholar] [CrossRef]
- Cao, F.; McEnaney, K.; Chen, G.; Ren, Z. A Review of Cermet-Based Spectrally Selective Solar Absorbers. Energy Environ. Sci. 2014, 7, 1615–1627. [Google Scholar] [CrossRef]
- Roberts, D.E. A Figure of Merit for Selective Absorbers in Flat Plate Solar Water Heaters. Sol. Energy 2013, 98, 503–510. [Google Scholar] [CrossRef]
- Kozelj, M.; Orel, B.; Steinbuecher, M.; Jerman, I.; Vodlan, M. Aminosilane Modified Pigments for Spectrally Selective Coatings, Method for Preparation and Their Use in Coatings. EP2261288B1, 16 January 2019. [Google Scholar]
- Kuckelkorn, T.D.; Silmy, K.D.; Dreyer, S.D. Radiation-Selective Absorber Coating and Absorber Tube with Radiation-Selective Absorber Coating. EP2312234A2, 20 April 2011. [Google Scholar]
- Asaad Yasseen, A.-R.; Istvan, S.; Istvan, F. Selective Absorber Coatings and Technological Advancements in Performance Enhancement for Parabolic Trough Solar Collector. J. Therm. Sci. 2022, 31, 1990–2008. [Google Scholar] [CrossRef]
- Jerman, I.; Zepeda, F.; Noc, L. Merging of Oxide Species with Black Spinel Structure by CSP Operating Temperature. AIP Conf. Proc. 2018, 2033, 220002. [Google Scholar] [CrossRef]
- Jerman, I.; Kovac, J.; Colovic, M.; Zepeda, F.; Noc, L. POSS-Modified Black Pigment for CSP Deployment. AIP Conf. Proc. 2018, 2033, 220003. [Google Scholar] [CrossRef]
- Noc, L.; Sest, E.; Kapun, G.; Ruiz-Zepeda, F.; Binyamin, Y.; Merzel, F.; Jerman, I. High-Solar-Absorptance CSP Coating Characterization and Reliability Testing with Isothermal and Cyclic Loads for Service-Life Prediction. Energy Environ. Sci. 2019, 12, 1679–1694. [Google Scholar] [CrossRef]
- Noc, L.; Ruiz-Zepeda, F.; Merzel, F.; Jerman, I. High-Temperature “Ion Baseball” for Enhancing Concentrated Solar Power Efficiency. Sol. Energy Mater. Sol. Cells 2019, 200, 109974. [Google Scholar] [CrossRef]
- Hosseini, S.; Torres, J.F.; Taheri, M.; Tsuda, K.; Tricoli, A.; Lipiński, W.; Coventry, J. Aging Mechanisms for High-Temperature Solar Absorber Coatings under Extensive Thermal Cycling. Sol. Energy Mater. Sol. Cells 2024, 271, 112856. [Google Scholar] [CrossRef]
- Ambrosini, A.; Lambert, T.N.; Boubault, A.; Hunt, A.; Davis, D.J.; Adams, D.; Hall, A.C. Thermal Stability of Oxide-Based Solar Selective Coatings for CSP Central Receivers; American Society of Mechanical Engineers Digital Collection. Energy Sustain. 2015, 56840, V001T05A022. [Google Scholar] [CrossRef]
- Wang, X.J.; Jiang, L.J.; Du, M.; Hao, L.; Liu, X.P.; Yu, Q.H. The Degradation of Solar Thermal Absorption Coatings. Energy Procedia 2014, 49, 1747–1755. [Google Scholar] [CrossRef]
- Reoyo-Prats, R.; Carling Plaza, A.; Faugeroux, O.; Claudet, B.; Soum-Glaude, A.; Hildebrandt, C.; Binyamin, Y.; Agüero, A.; Meißner, T. Accelerated Aging of Absorber Coatings for CSP Receivers under Real High Solar Flux—Evolution of Their Optical Properties. Sol. Energy Mater. Sol. Cells 2019, 193, 92–100. [Google Scholar] [CrossRef]
- Torres, J.F.; Ellis, I.; Coventry, J. Degradation Mechanisms and Non-Linear Thermal Cycling Effects in a High-Temperature Light-Absorber Coating. Sol. Energy Mater. Sol. Cells 2020, 218, 110719. [Google Scholar] [CrossRef]
- Martínez, N.; Lopez-Herraiz, M.; Rico, A.; Múnez, C.J.; Poza, P. Influence of Different Thermal Degradation Processes on the Optical Property of Pyromark-2500. Sol. Energy 2023, 253, 58–72. [Google Scholar] [CrossRef]
- Carlsson, B.; Frei, U.; Köhl, M.; Möller, K. Accelerated Life Testing of Solar Energy Materials—Case Study of Some Selective Solar Absorber Coatings for DHW Systems; International Energy Agency, Solar Heating and Cooling Programme Task X: Solar Materials Research and Development, SP-Report 1994:13; SP Sveriges Tekniska Forskningsinstitut: Lund, Sweden, 1994; Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:lnu:diva-3496 (accessed on 26 April 2010).
- Brunold, S.; Frei, U.; Carlsson, B.; Möller, K.; Köhl, M. Accelerated Life Testing of Solar Absorber Coatings: Testing Procedure and Results. Sol. Energy 2000, 68, 313–323. [Google Scholar] [CrossRef]
- EN 12975-3-1; Solar Collectors—General Requirements. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2022.
- ISO 22975-3:2014; Solar Energy—Collector Components and Materials—Part 3: Absorber Surface Durability. International Organization for Standardization: Geneva, Switzerland, 2014.
- Cai, N.; Zhou, G.; Müller, K.; Starr, D.E. Temperature and Pressure Dependent Mott Potentials and Their Influence on Self-Limiting Oxide Film Growth. Appl. Phys. Lett. 2012, 101, 171605. [Google Scholar] [CrossRef]
- ISO 9845-1; Solar Energy—Reference Solar Spectral Irradiance at the Ground at Different Receiving Conditions—Part 1: Direct Normal and Hemispherical Solar Irradiance for Air Mass 1,5. International Organization for Standardization: Geneva, Switzerland, 1992.
- Manson, S.S. Behavior of Materials under Conditions of Thermal Stress. 1954. Available online: https://ntrs.nasa.gov/citations/19930092197 (accessed on 19 July 2024).
- Torres, J.F.; Tsuda, K.; Murakami, Y.; Guo, Y.; Hosseini, S.; Asselineau, C.-A.; Taheri, M.; Drewes, K.; Tricoli, A.; Lipiński, W.; et al. Highly Efficient and Durable Solar Thermal Energy Harvesting via Scalable Hierarchical Coatings Inspired by Stony Corals. Energy Environ. Sci. 2022, 15, 1893–1906. [Google Scholar] [CrossRef]
- Hosseini, S.; Torres, J.F.; Taheri, M.; Tricoli, A.; Lipiński, W.; Coventry, J. Stability and Characterisation of Pyromark 2500 Cured at Different Temperatures. Sol. Energy 2023, 265, 112102. [Google Scholar] [CrossRef]
- Issartel, C.; Buscail, H.; Caudron, E.; Cueff, R.; Riffard, F.; Perrier, S.; Jacquet, P.; Lambertin, M. Influence of Nitridation on the Oxidation of a 304 Steel at 800 °C. Corros. Sci. 2004, 46, 2191–2201. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drinčić, A.; Noč, L.; Merzel, F.; Jerman, I. Future Parabolic Trough Collector Absorber Coating Development and Service Lifetime Estimation. Coatings 2024, 14, 1111. https://doi.org/10.3390/coatings14091111
Drinčić A, Noč L, Merzel F, Jerman I. Future Parabolic Trough Collector Absorber Coating Development and Service Lifetime Estimation. Coatings. 2024; 14(9):1111. https://doi.org/10.3390/coatings14091111
Chicago/Turabian StyleDrinčić, Ana, Luka Noč, Franci Merzel, and Ivan Jerman. 2024. "Future Parabolic Trough Collector Absorber Coating Development and Service Lifetime Estimation" Coatings 14, no. 9: 1111. https://doi.org/10.3390/coatings14091111






