Enhancing Flame-Retardant Properties of Polyurethane Composites Using N-β-(Aminoethyl)-γ-aminopropyl Trimethoxysilane and Carbon Black Co-Modified Ammonium Polyphosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
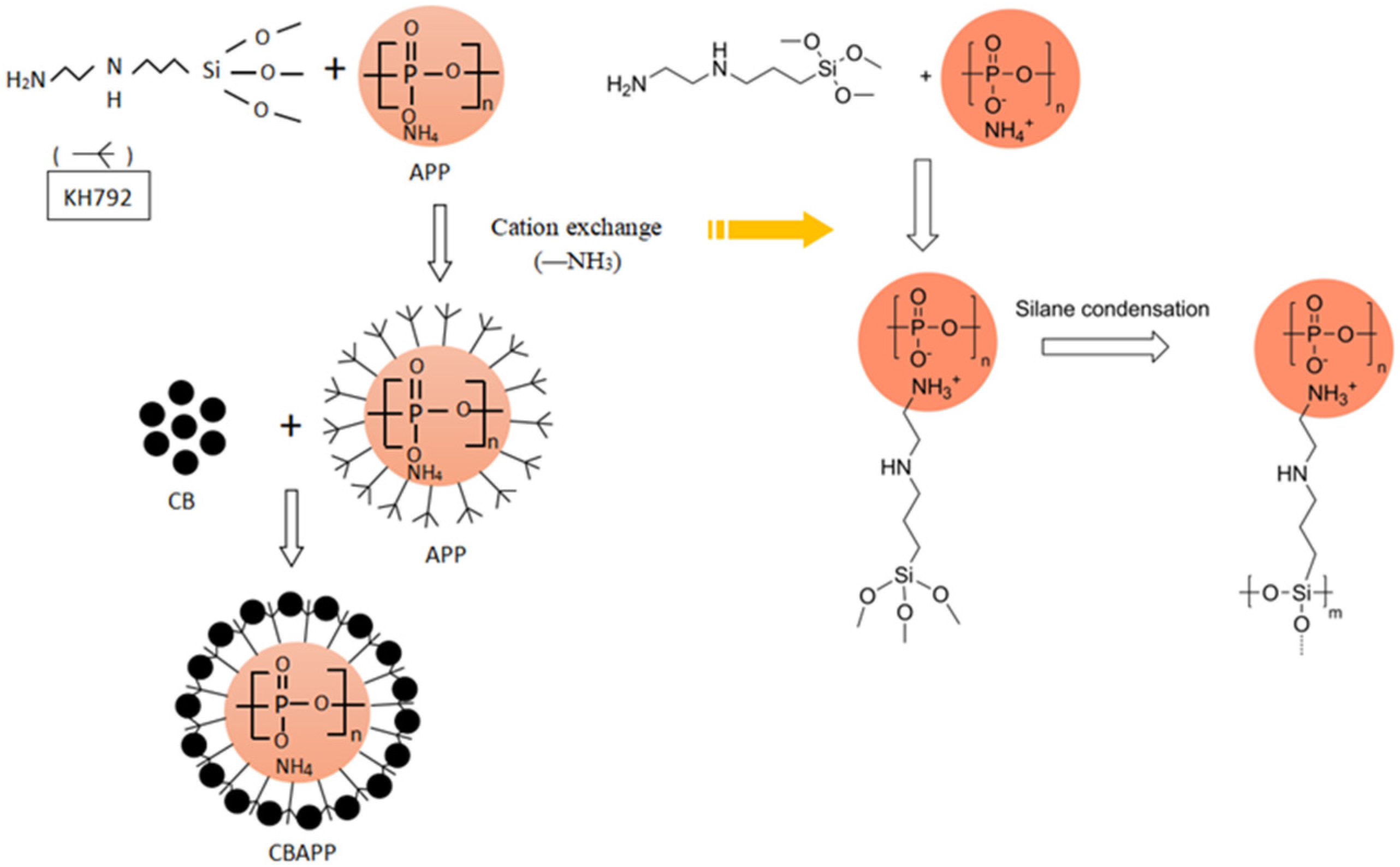
2.2. Preparation of Ammonium Polyphosphate Coated with Carbon Black (CBAPP)
2.3. Preparation of Polyurethane Composites
2.4. Testing and Characterization
3. Results
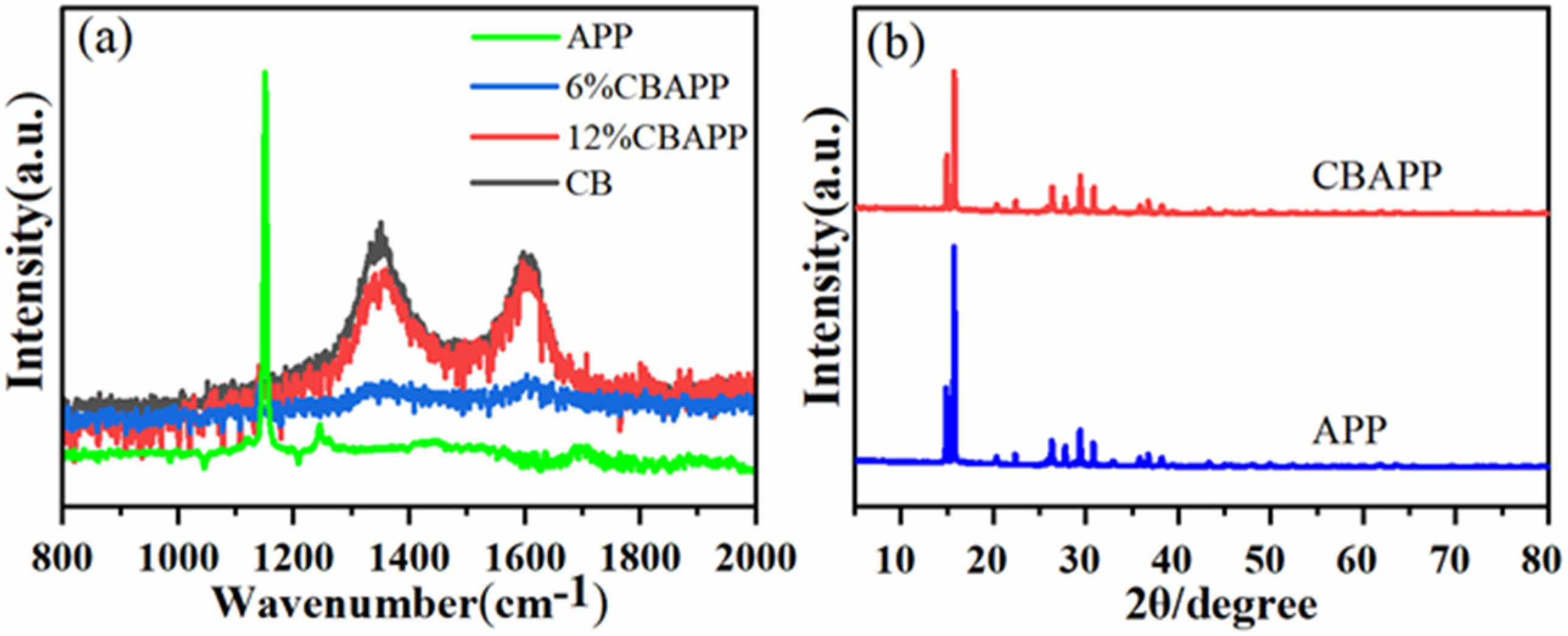
3.1. Characterization of CBAPP Structure and Properties
3.1.1. Raman Analysis
3.1.2. XPS Analysis
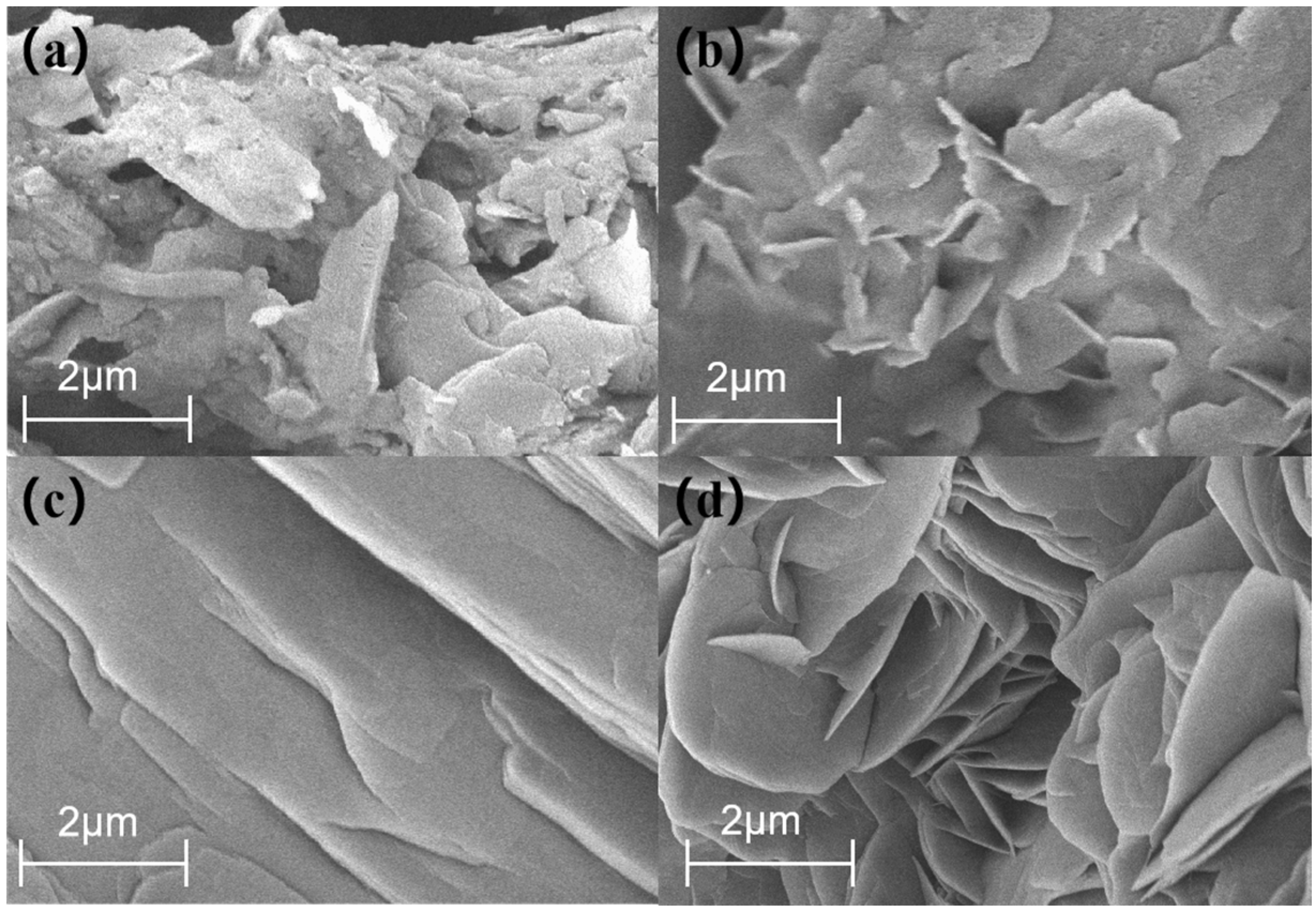
3.1.3. SEM
3.1.4. Thermal Stability of APP and CBAPP
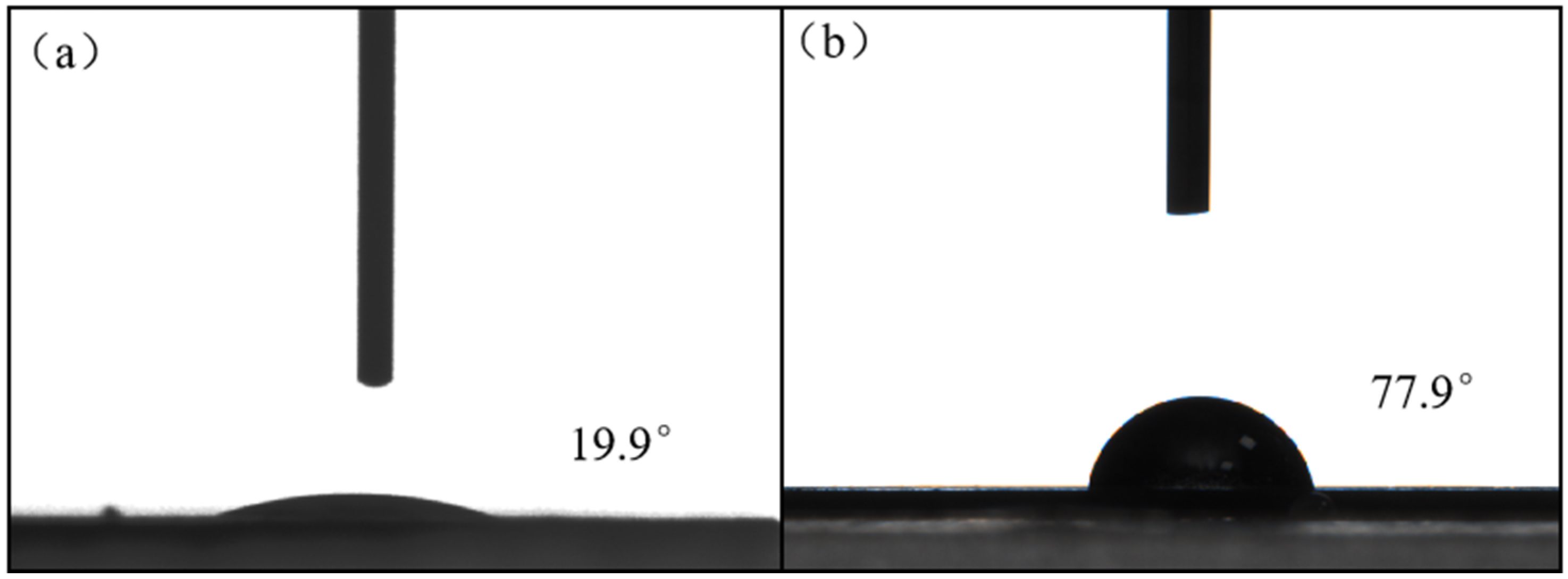
3.1.5. Contact-Angle Measurements
3.2. Performance Analysis of CBAPP/PU Composites
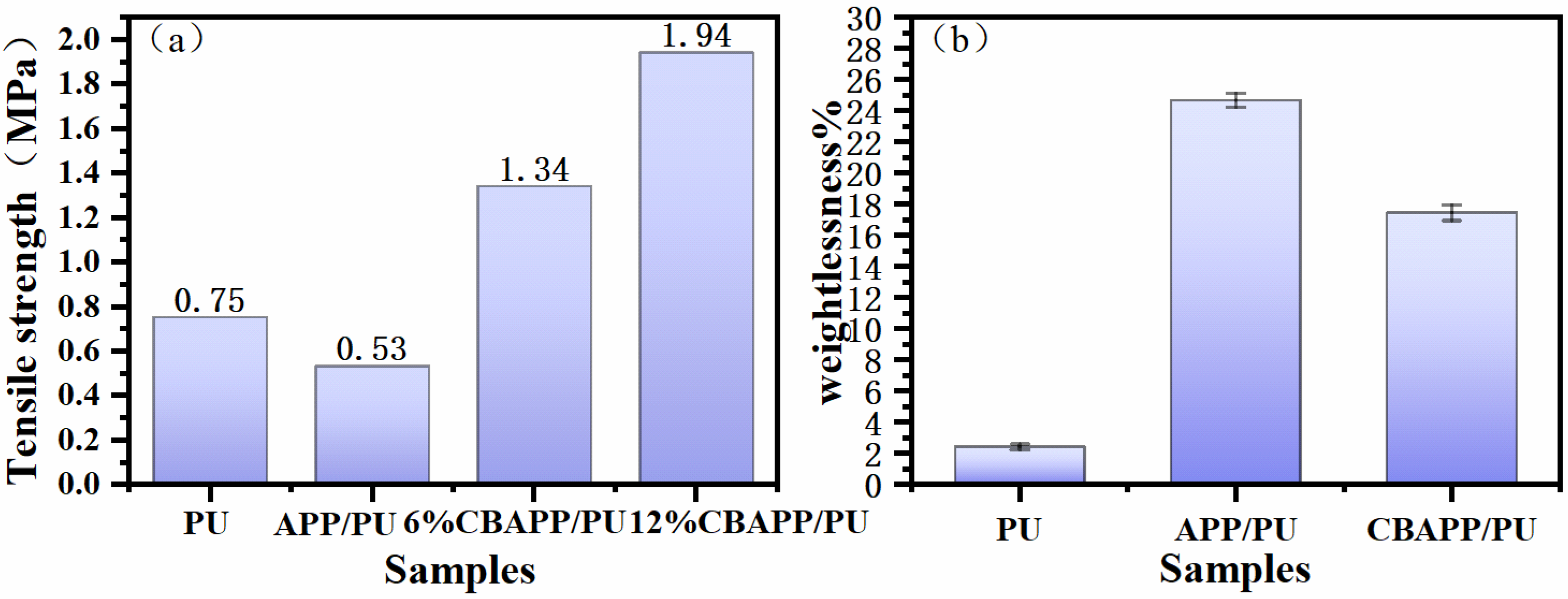
3.2.1. Mechanical Properties and Water Resistance Test
3.2.2. Thermal Stability of PU, APP/PU, and CBAPP/PU
3.2.3. Flame Retardancy
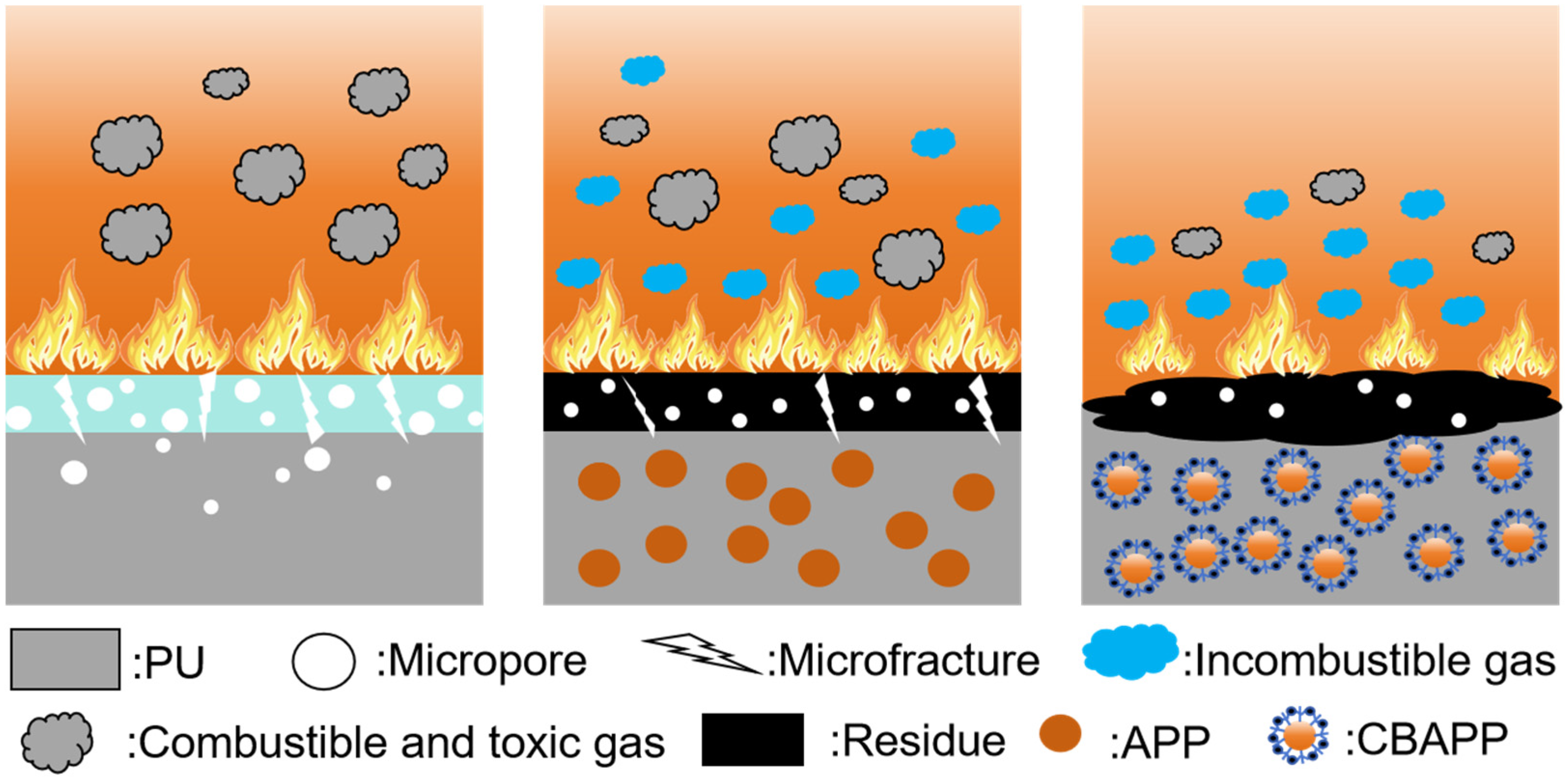
3.2.4. Mechanism of Action of CBAPP/PU Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.-Y.; Huang, S.-C.; Wei, W.-C.; Yang, A.-H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Jia, P.; Zhu, Y.; Lu, J.; Wang, B.; Song, L.; Wang, B.; Hu, Y. Multifunctional fireproof electromagnetic shielding polyurethane films with thermal management performance. Chem. Eng. J. 2022, 439, 135673. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, J.; Wang, W.; Fan, H.; Chen, Y.; Yan, J.; Xiang, J. Polyurethane covalently modified polydimethylsiloxane (PDMS) coating with increased surface energy and re-coatability. Prog. Org. Coat. 2020, 146, 105744. [Google Scholar] [CrossRef]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, P.; Fan, H.; Sun, Z.; Wen, J. Covalently functionalized graphene towards molecular-level dispersed waterborne polyurethane nanocomposite with balanced comprehensive performance. Appl. Surf. Sci. 2019, 471, 595–606. [Google Scholar] [CrossRef]
- Zhou, Y.; Bu, R.; Gong, J.; Yan, W.; Fan, C. Experimental investigation on downward flame spread over rigid polyurethane and extruded polystyrene foams. Exp. Therm. Fluid Sci. 2018, 92, 346–352. [Google Scholar] [CrossRef]
- Song, S.; Ma, J.; Cao, K.; Chang, G.; Huang, Y.; Yang, J. Synthesis of a novel dicyclic silicon-/phosphorus hybrid and its performance on flame retardancy of epoxy resin. Polym. Degrad. Stab. 2014, 99, 43–52. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, M.; Wang, S.; Fu, H. Superhydrophobic and flame retardant polydimethylsiloxane coatings with layered double hydroxide and ammonium polyphosphate. Prog. Org. Coat. 2022, 172, 107117. [Google Scholar] [CrossRef]
- Li, M.-E.; Wang, S.-X.; Han, L.-X.; Yuan, W.-J.; Cheng, J.-B.; Zhang, A.-N.; Zhao, H.-B.; Wang, Y.-Z. Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef]
- Guo, E.; Tang, B.; Wang, S.; Feng, W.; Wang, X.; Sun, J.; Zhang, S.; Gu, X.; Li, H. A cocktail strategy for enhancing flame retardance and water resistance of butyl acrylate coatings. Mater. Chem. Phys. 2021, 263, 124367. [Google Scholar] [CrossRef]
- Leistner, M.; Haile, M.; Rohmer, S.; Abu-Odeh, A.; Grunlan, J.C. Water-soluble polyelectrolyte complex nanocoating for flame retardant nylon-cotton fabric. Polym. Degrad. Stab. 2015, 122, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Shang, S.; Zhang, H.; Shi, Y.; Yu, B.; Qi, C.; Dong, H.; Chen, X.; Yang, X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. Part B Eng. 2020, 181, 107588. [Google Scholar] [CrossRef]
- Eceiza, I.; Aguirresarobe, R.; Barrio, A.; Fernández-Berridi, M.J.; Irusta, L. Ammonium polyphosphate-melamine synergies in thermal degradation and smoke toxicity of flexible polyurethane foams. Thermochim. Acta 2023, 726, 179554. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, Q.; Zhang, N.; Guo, X.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, C.; Hu, D.; Gui, Y. Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin. Nanomaterials 2018, 811, 951. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; Liu, M.; Li, C.; Meng, L.; Hou, S. Modified Ammonium Polyphosphate and Its Application in Polypropylene Resins. Coatings 2022, 12, 1738. [Google Scholar] [CrossRef]
- Hussain, A.; Landry, V.; Blanchet, P.; Hoang, D.; Dagenais, C. Fire Performance of Intumescent Waterborne Coatings with Encapsulated APP for Wood Constructions. Coatings 2021, 11, 1272. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Jiao, C. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 389, 1357–1387. [Google Scholar] [CrossRef]
- Shi, X.; Pan, Y.; Wang, Y.; Jia, Z.; Chen, T.; Gong, J.; Jiang, J. Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymers 2021, 136, 881. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, D.; Li, W.; Cai, X. Flame retardant efficiency of KH-550 modified urea-formaldehyde resin cooperating with ammonium polyphosphate on polypropylene. Polym. Degrad. Stab. 2018, 151, 160–171. [Google Scholar] [CrossRef]
- Wang, B.; Jia, P.; Zhang, Y.; He, R.; Song, L.; Hu, Y. Multifunctional composite polyvinyl alcohol films prepared with economic conductive carbon black and MXene microcapsuled APP. Mater. Today Phys. 2023, 30, 100928. [Google Scholar] [CrossRef]
- Liu, C.; Yao, A.; Chen, K.; Shi, Y.; Feng, Y.; Zhang, P.; Yang, F.; Liu, M.; Chen, Z. MXene based core-shell flame retardant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part B Eng. 2021, 226, 109363. [Google Scholar] [CrossRef]
- Yang, H.; Guan, Y.; Ye, L.; Wang, S.; Li, S.; Wen, X.; Chen, X.; Mijowska, E.; Tang, T. Synergistic effect of nanoscale carbon black and ammonium polyphosphate on improving thermal stability and flame retardancy of polypropylene: A reactive network for strengthening carbon layer. Compos. Part B Eng. 2019, 174, 107038. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.; Gao, D.; Wang, Y.; Wen, X.; Tang, T. Striking effect of nanosized carbon black modified by grafting sodium sulfonate on improving the flame retardancy of polycarbonate. Compos. Commun. 2020, 20, 100359. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Benign design of intumescent flame retardant coating incorporated various carbon sources. Constr. Build. Mater. 2020, 236, 117433. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Cui, J.; Li, X.-L.; Díaz Palencia, J.L.; Wang, D.-Y. Chemically inorganic modified ammonium polyphosphate as eco-friendly flame retardant and its high fire safety for epoxy resin. Compos. Commun. 2021, 28, 100959. [Google Scholar] [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Pastuszka, Ł.; Jurecki, L. Flame Resistant Silicone-Containing Coating Materials. Coatings 2020, 105, 479. [Google Scholar] [CrossRef]
- Jiang, B.; Ding, D.; Su, M.; Lu, K.; Yu, C.; Ji, B.; Hong, H. Experimental study on the explosion suppression characteristics of polyethylene dust by ammonium polyphosphate. Powder Technol. 2024, 437, 119491. [Google Scholar] [CrossRef]
- Wan, M.; Shi, C.; Qian, X.; Qin, Y.; Jing, J.; Che, H.; Ren, F.; Li, J.; Yu, B.; Zhou, K. Design of novel double-layer coated ammonium polyphosphate and its application in flame retardant thermoplastic polyurethanes. Chem. Eng. J. 2023, 459, 141448. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Gong, J.; Liu, J.; Tian, N.; Wang, Y.; Jiang, Z.; Qiu, J.; Tang, T. Thermal and flammability properties of polypropylene/carbon black nanocomposites. Polym. Degrad. Stab. 2012, 975, 793–801. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, W.; Huang, J.; He, Y.; Liang, X.; Su, Y.; Wu, W.; Li, R.K.Y. Interface engineering of graphene oxide containing phosphorus/nitrogen towards fire safety enhancement for thermoplastic polyurethane. Compos. Commun. 2021, 27, 100821. [Google Scholar] [CrossRef]
- Gao, D.; Wen, X.; Guan, Y.; Czerwonko, W.; Li, Y.; Gao, Y.; Mijowska, E.; Tang, T. Flame retardant effect and mechanism of nanosized NiO as synergist in PLA/APP/CSi-MCA composites. Compos. Commun. 2020, 17, 170–176. [Google Scholar] [CrossRef]
- Yan, J.; Xu, P.; Zhang, P.; Fan, H. Surface-modified ammonium polyphosphate for flame-retardant and reinforced polyurethane composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127092. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Chen, X.; Hong, W.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. A halogen-free, flame retardant, waterborne polyurethane coating based on the synergistic effect of phosphorus and silicon. Prog. Org. Coat. 2021, 158, 106359. [Google Scholar] [CrossRef]
- Piao, J.; Lai, Y.; Ren, J.; Wang, Y.; Feng, T.; Wang, Y.; Liu, W.; Dong, H.; Chen, W.; Jiao, C.; et al. Zn-doped carbon microspheres as synergist in intumescent flame-retardant thermoplastic polyurethane composites: Mechanism of char residues layer regulation. Compos. Commun. 2022, 32, 101173. [Google Scholar] [CrossRef]





| Sample | PU | APP | 3% CBAPP | 6% CBAPP | 9% CBAPP | 12% CBAPP |
|---|---|---|---|---|---|---|
| PU | 100 | |||||
| PU1 | 80 | 20 | ||||
| PU2 | 80 | 20 | ||||
| PU3 | 80 | 20 | ||||
| PU4 | 80 | 20 | ||||
| PU5 | 80 | 20 |
| Samples | T-5%/°C | Tmax1/°C | Tmax2/°C | Residual Amount at 800 °C/wt% |
|---|---|---|---|---|
| PU | 390.9 | 423.6 | 459.1 | 44.2 |
| APP/PU | 344.3 | 353.0 | 432.1 | 58.1 |
| 12% CBAPP/PU | 350.4 | 355.2 | 429.9 | 60.7 |
| Samples | T-5%/°C | Tmax1/°C | Tmax2/°C | Tmax3/°C | Residual Amount at 800 °C/wt% |
|---|---|---|---|---|---|
| APP | 334.1 | 342.9 | 611.3 | 748.1 | 15.4 |
| 12% CBAPP | 329.0 | 332.7 | 619.1 | — | 38.3 |
| Samples | APP/wt% | CBAPP/wt% | PU/wt% | LOI/vol% | UL-94 (Grade) |
|---|---|---|---|---|---|
| PU | 0 | 0 | 100 | 23.5 | NR |
| APP/PU | 20 | 0 | 80 | 34.4 | V-0 |
| CBAPP/PU | 0 | 20 | 80 | 41.5 | V-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Hao, W.; Xu, B.; Zhang, K.; Bi, J.; Wu, J.; Wang, Z. Enhancing Flame-Retardant Properties of Polyurethane Composites Using N-β-(Aminoethyl)-γ-aminopropyl Trimethoxysilane and Carbon Black Co-Modified Ammonium Polyphosphate. Coatings 2024, 14, 1126. https://doi.org/10.3390/coatings14091126
Fu L, Hao W, Xu B, Zhang K, Bi J, Wu J, Wang Z. Enhancing Flame-Retardant Properties of Polyurethane Composites Using N-β-(Aminoethyl)-γ-aminopropyl Trimethoxysilane and Carbon Black Co-Modified Ammonium Polyphosphate. Coatings. 2024; 14(9):1126. https://doi.org/10.3390/coatings14091126
Chicago/Turabian StyleFu, Lisha, Wanjun Hao, Baoluo Xu, Kexi Zhang, Jianhua Bi, Jingxing Wu, and Zhong Wang. 2024. "Enhancing Flame-Retardant Properties of Polyurethane Composites Using N-β-(Aminoethyl)-γ-aminopropyl Trimethoxysilane and Carbon Black Co-Modified Ammonium Polyphosphate" Coatings 14, no. 9: 1126. https://doi.org/10.3390/coatings14091126






