Thermal Corrosion Properties of Composite Ceramic Coating Prepared by Multi-Arc Ion Plating
Abstract
1. Introduction
2. Experiment
3. Results
3.1. Microstructure and Surface Morphology of Coatings
3.2. Thermal Corrosion at 850 °C
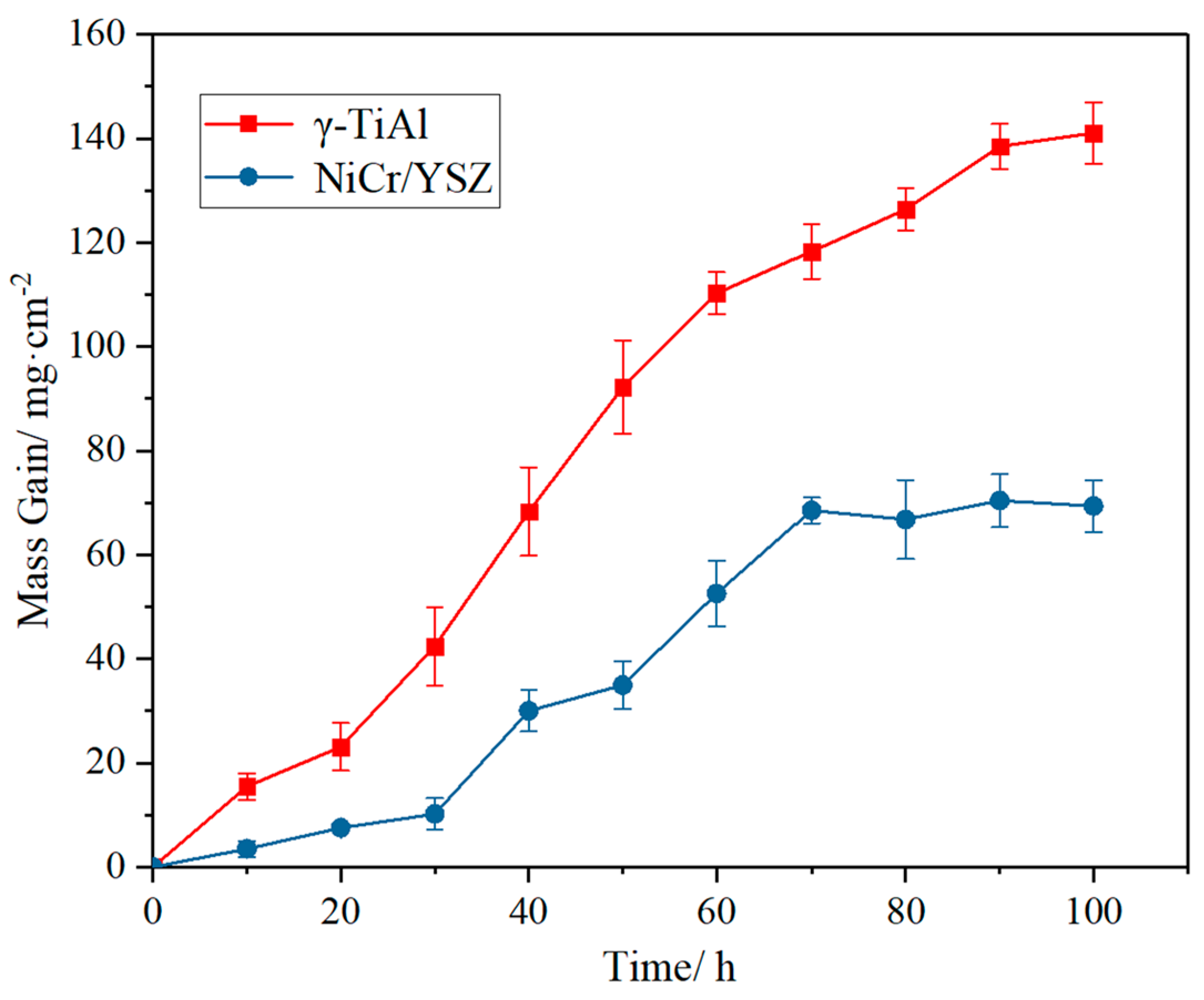
3.2.1. Thermal Corrosion Kinetics Curve
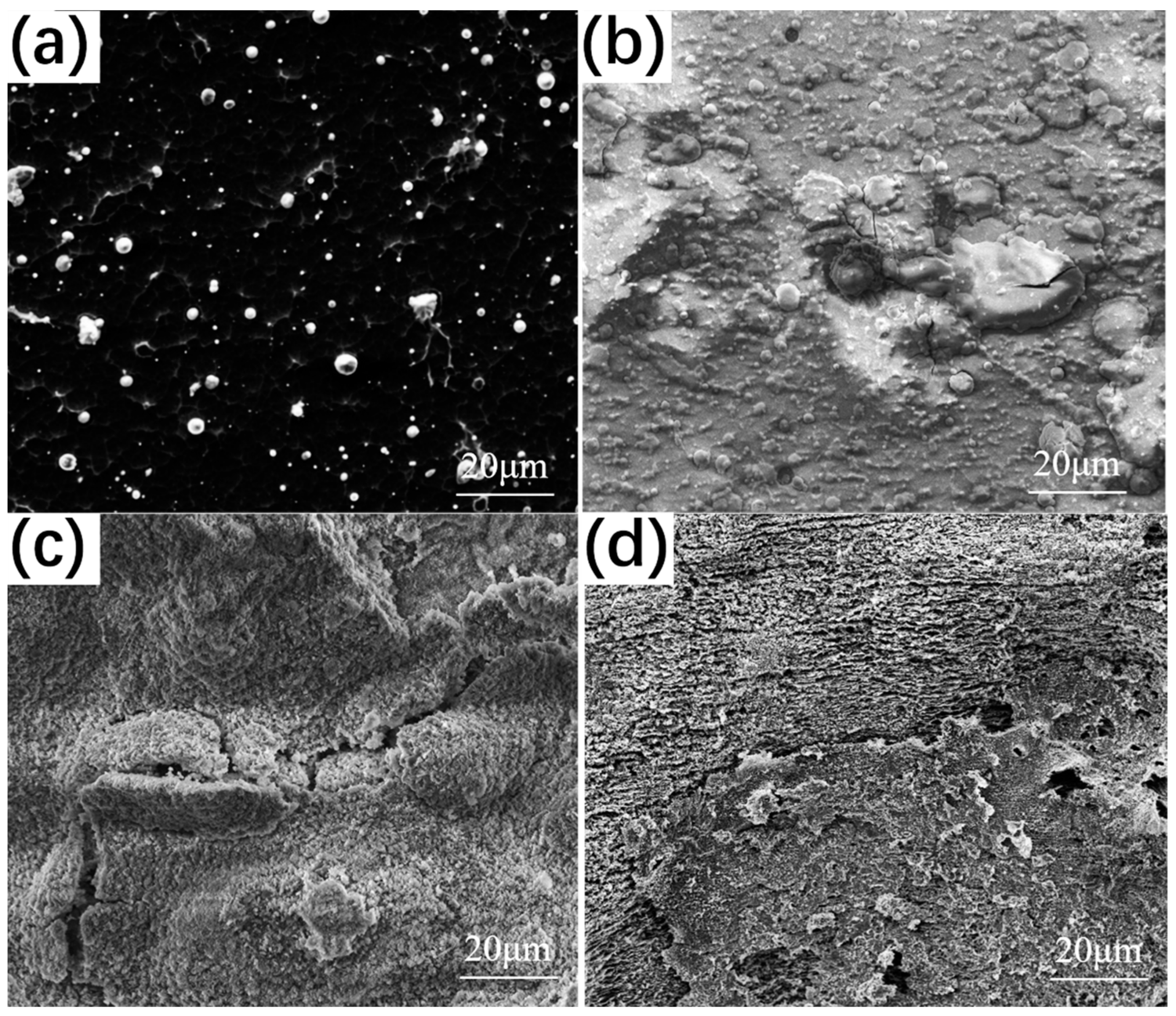
3.2.2. Surface Morphology after Thermal Corrosion
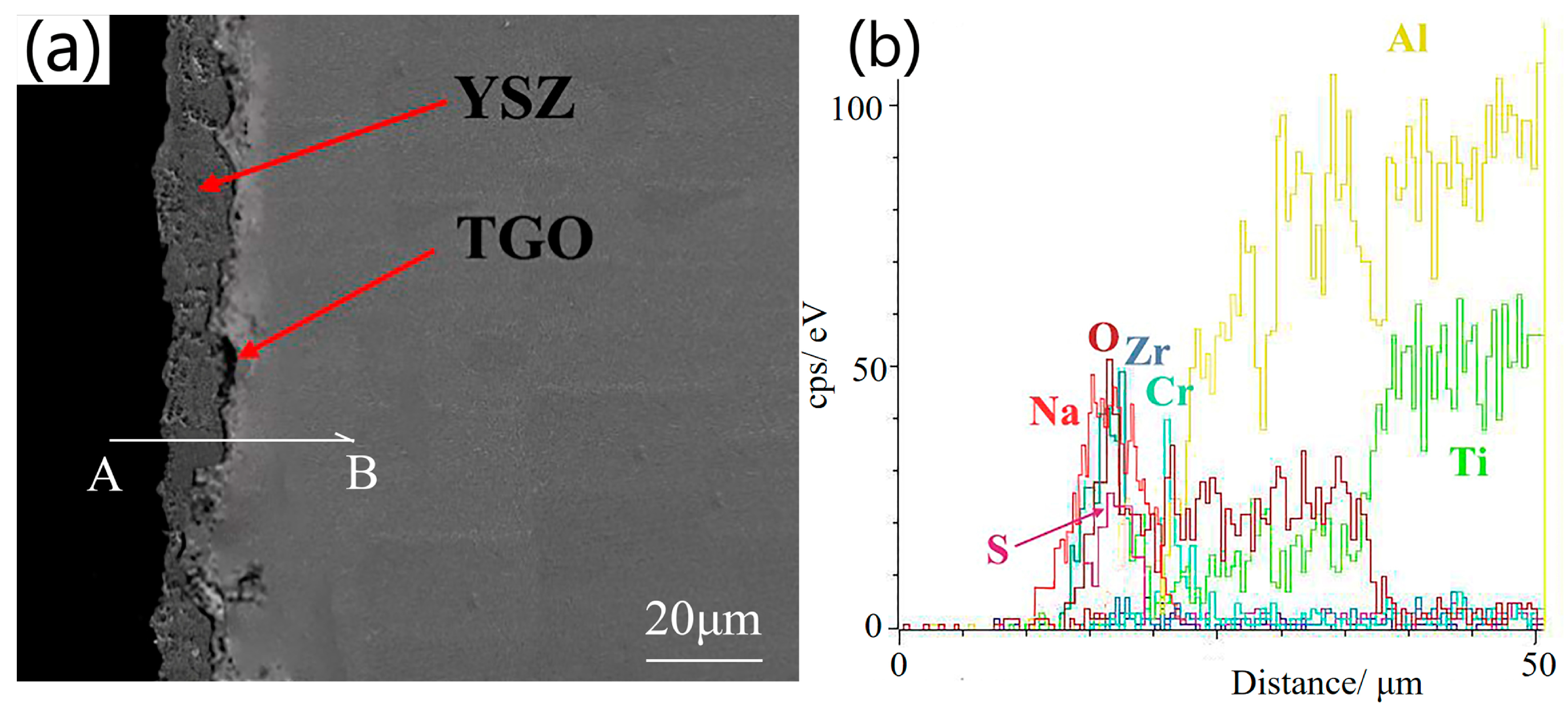
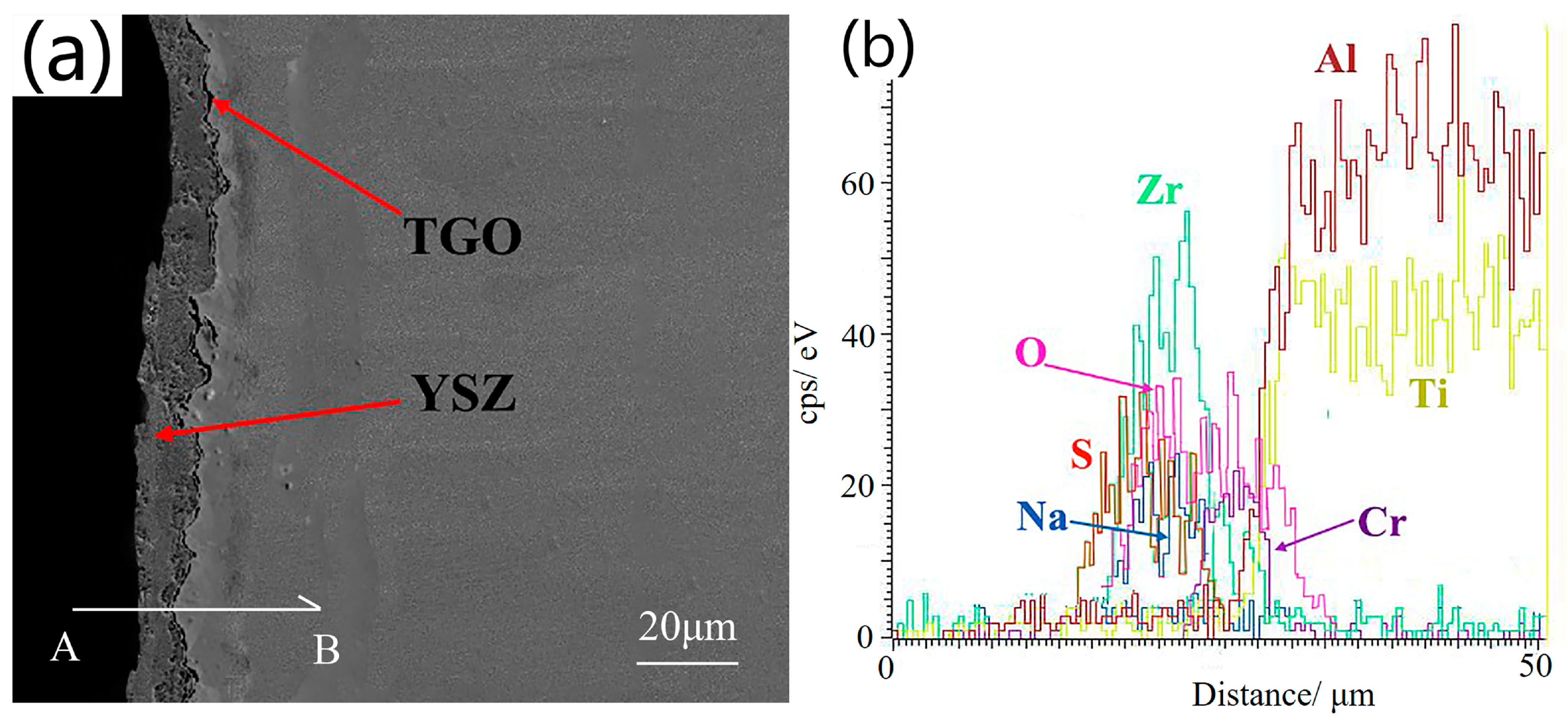
3.2.3. Cross-Sectional Microstructure after Thermal Corrosion
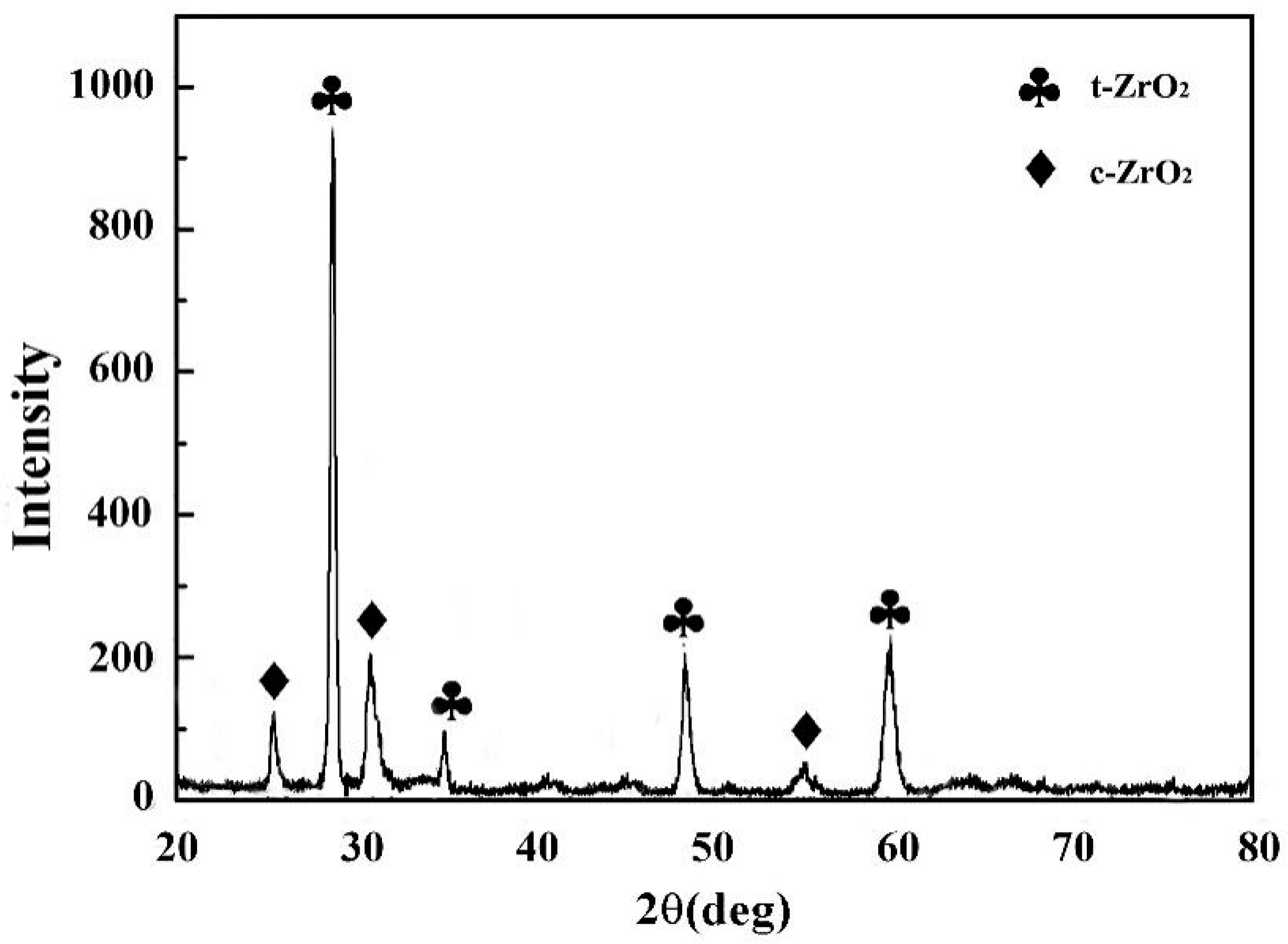
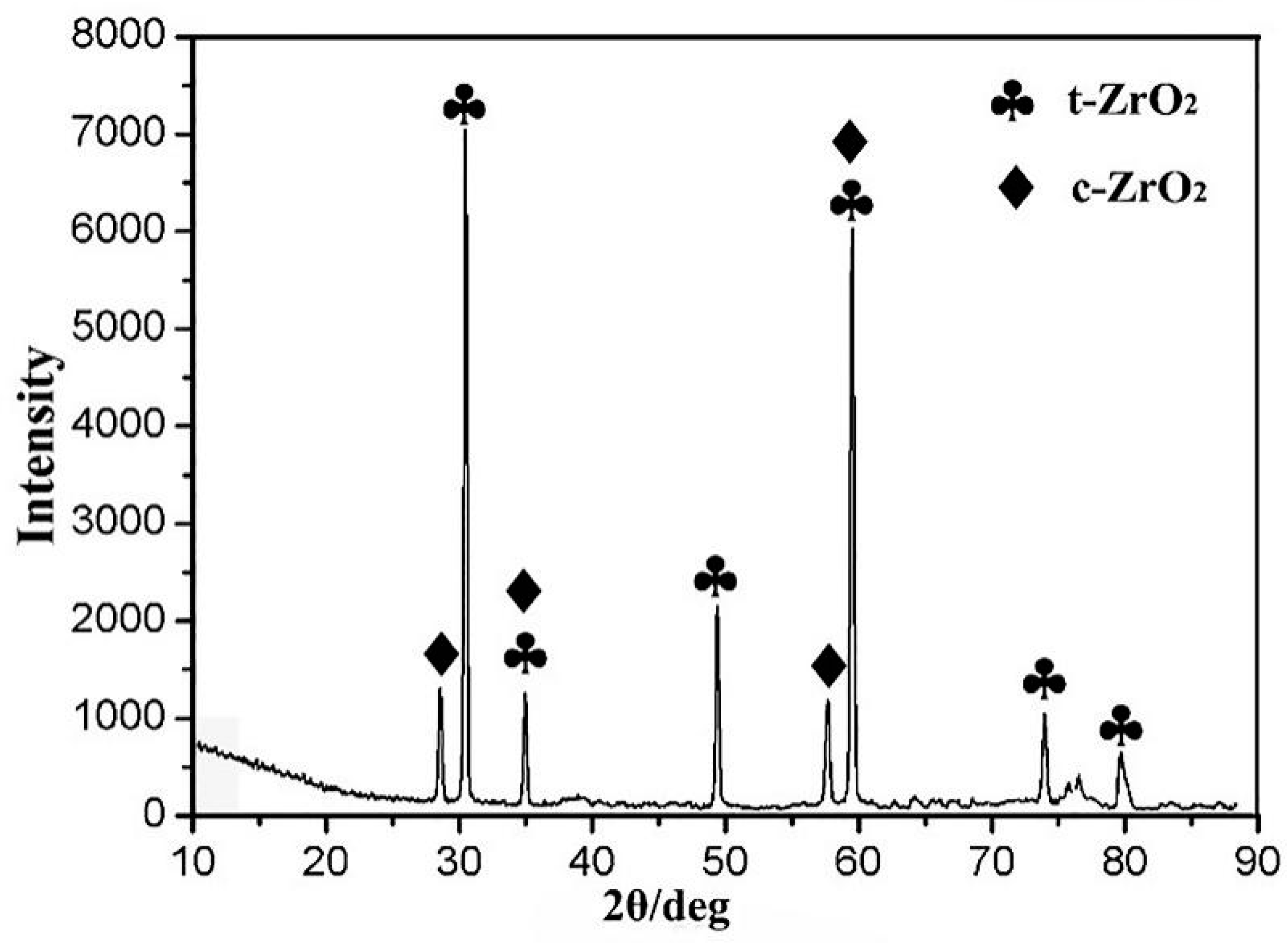
3.2.4. Thermal Corrosion Products
3.3. Thermal Corrosion at 950 °C
3.3.1. Thermal Corrosion Kinetics Curve
3.3.2. Surface Morphology after Thermal Corrosion
3.3.3. Cross-Sectional Microstructure after Thermal Corrosion
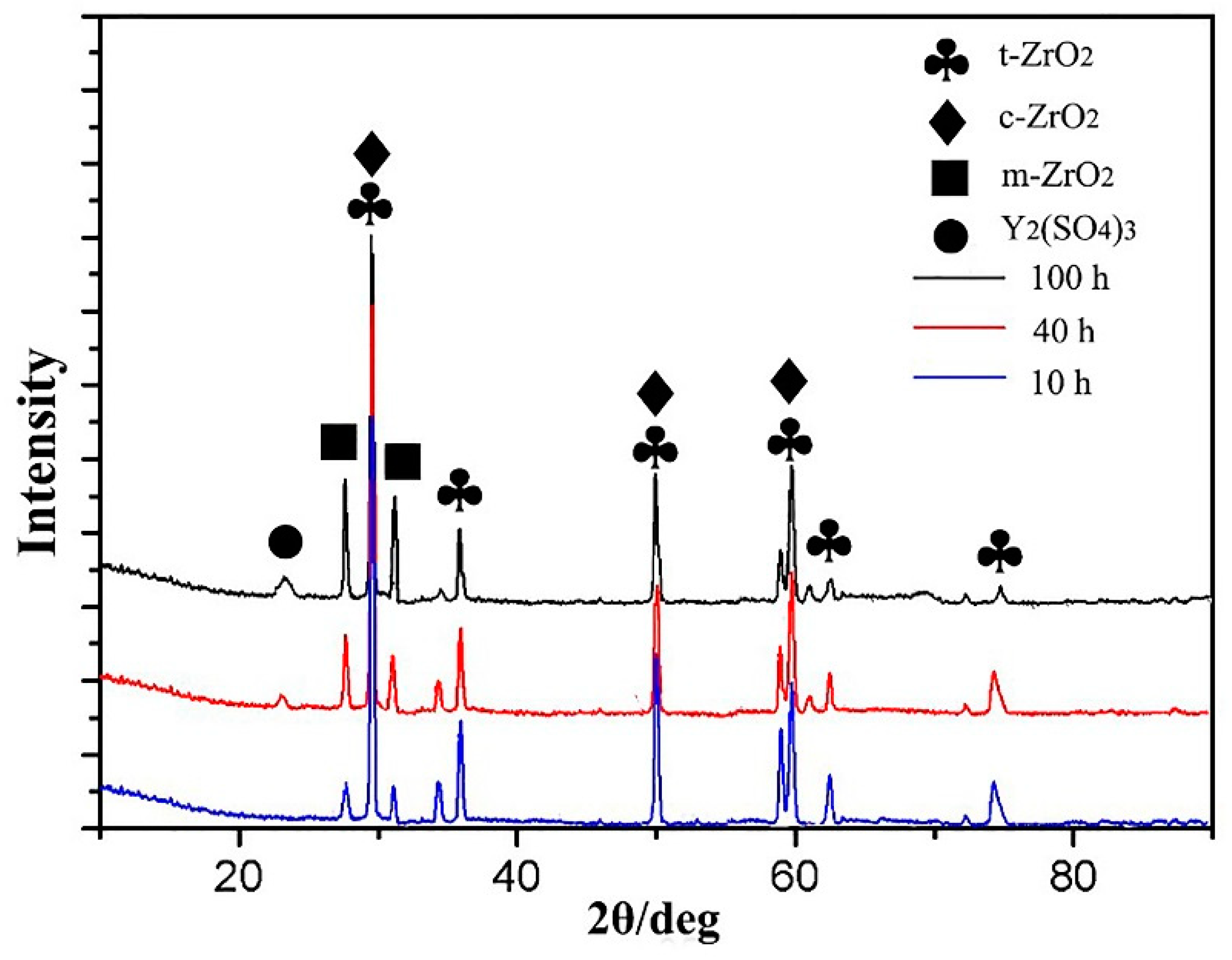
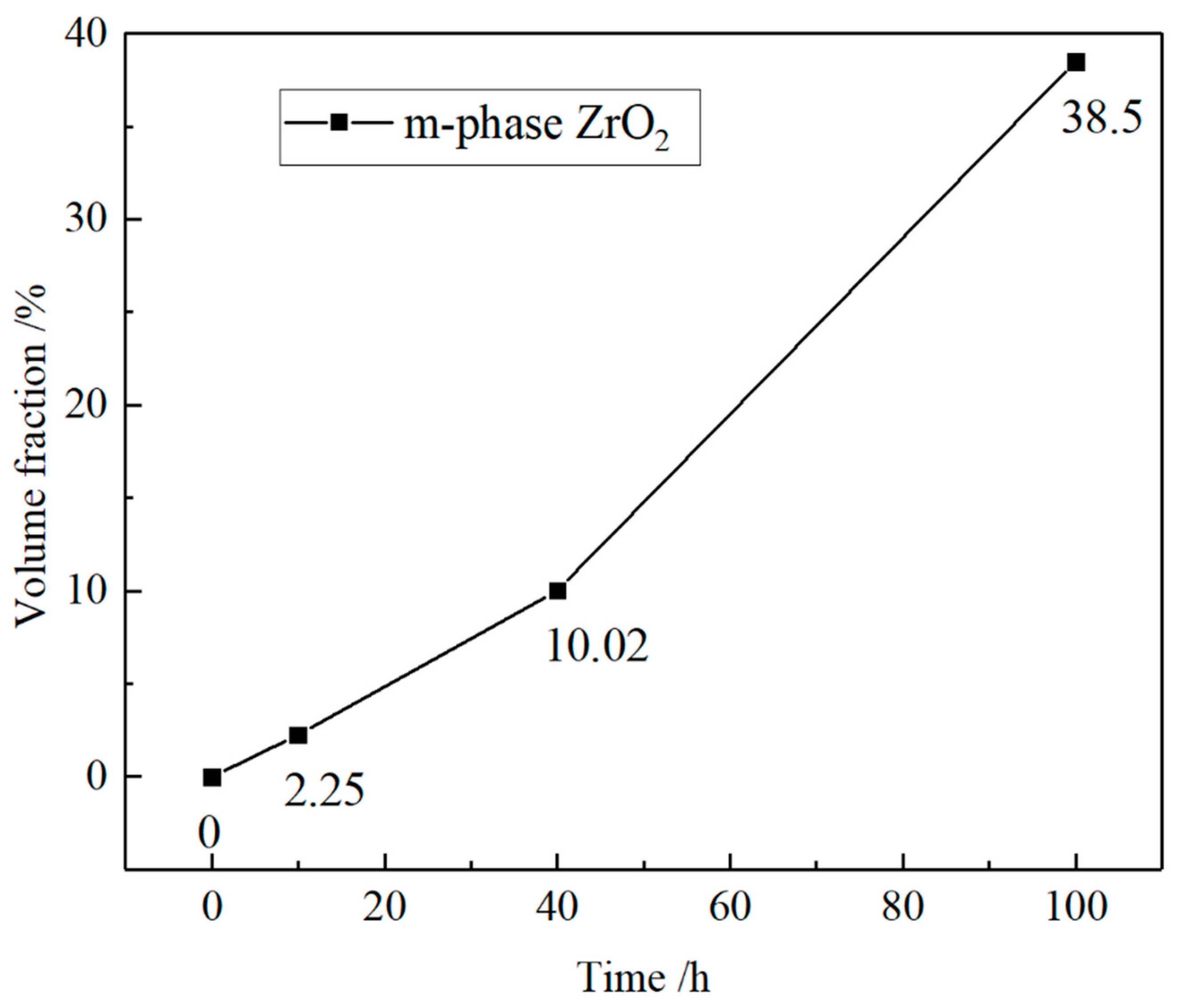
3.3.4. Thermal Corrosion Products
4. Discussion
- Initial stage: As the temperature rises, molten salt forms a film on the surface of the NiCr/YSZ coating. The molten salt infiltrates the coating through cracks and pores, increasing the contact area between the molten salt and the coating.
- Intermediate stage: The molten salt reacts with Y2O3, generating corrosion products and gradually consuming Y2O3. This leads to the transformation of some t-ZrO2 into m-ZrO2. During the t → m transformation, the volume expands, leading to the formation and propagation of cracks. This allows the thermal corrosion reaction to penetrate further into the coating, thereby accelerating the thermal corrosion process.
- Final stage: The internal stress from the t → m transformation, combined with the stresses from TGO and mismatched thermal expansion, can result in the formation of cracks between the metal matrix, bonding layer, and ceramic layer. These stresses eventually lead to coating failure and exfoliation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voice, W.E.; Henderson, M.; Shelton, E.F.J.; Wu, X. Gamma titanium aluminide, TNB. Intermetallics 2005, 13, 959–964. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Salehnasab, B.; Poursaeidi, E.; Mortazavi, S.A.; Farokhian, G.H. Hot corrosion failure in the first stage nozzle of a gas turbine engine. Eng. Fail. Anal. 2016, 60, 316–325. [Google Scholar] [CrossRef]
- Garip, Y.; Ozdemir, O. Comparative study of the oxidation and hot corrosion behaviors of TiAl-Cr intermetallic alloy produced by electric current activated sintering. J. Alloys Compd. 2019, 780, 364–377. [Google Scholar] [CrossRef]
- Jude, S.A.A.; Jappes Jtwinowlin Adamkhan, M. Thermal barrier coatings for high-temperature application on superalloy substrates—A review. Mater. Today Proc. 2022, 60, 1670–1675. [Google Scholar] [CrossRef]
- Anirudh, S.; Satheesh, K.; Balasubramanian, K.; Praveen, K.B. Probing into atomically thin layered nano-materials protective coating for aerospace and strategic defence application—A review. J. Alloys Compd. 2023, 968, 172203. [Google Scholar]
- Tian, S.; Zhang, Y.; Jiang, H.; Zhang, S.; Zeng, S.; Song, D.; Li Chong Liao, Z.; Chen, Y.; Zhang, Y. Effect of micron/nano Nb particles on high-temperature oxidation behavior of TiAl alloy/thermal barrier coating system. Surf. Coat. Technol. 2024, 492, 131206. [Google Scholar] [CrossRef]
- Taylor, R.; Brandon, J.R.; Morrell, P. Microstructure, composition and property relationships of plasma-sprayed thermal barrier coatings. Surf. Coat. Technol. 1992, 50, 141–149. [Google Scholar] [CrossRef]
- Voyer, J.; Gitzhofer, F.; Boulos, M.I. Study of the Performance of TBC under Thermal Cycling Conditions using an Acoustic Emission Rig. J. Therm. Spray Technol. 1998, 7, 181–190. [Google Scholar] [CrossRef]
- Troczynski, T.; Yang, Q.; John, G. Post-deposition treatment of zirconia thermal barrier coatings using sol-gel alumina. J. Therm. Spray Technol. 1999, 8, 229–234. [Google Scholar] [CrossRef]
- Schilbe, J.E. Substrate alloy element diffusion in thermal barrier coatings. Surf. Coat. Technol. 2000, 133–134, 35–39. [Google Scholar] [CrossRef]
- Zhou, S.; Xiong, Z.; Dai, X.; Liu, J.; Zhang, T.; Wang, C. Microstructure and oxidation resistance of cryomilled NiCrAlY coating by laser induction hybrid rapid cladding. Surf. Coat. Technol. 2014, 258, 943–949. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhang, G.; Feng, Z.C.; Zhang, B.C.; Liu, F.J. Oxidation behavior of laser remelted plasma sprayed NiCrAlY and NiCrAlY-Al2O3 coatings. Surf. Coat. Technol. 2001, 138, 56–60. [Google Scholar] [CrossRef]
- Ansari, M.; Shoja-Razavi, R.; Barekat, M.; Man, H.C. High-temperature oxidation behavior of laser-aided additively manufactured NiCrAlY coating. Corros. Sci. 2017, 118, 168–177. [Google Scholar] [CrossRef]
- Peng Bin Xu, Y.X.; Wang, Q. High-temperature structural evolution and mechanical properties of oxygen-containing NiCrAlY coatings. Appl. Surf. Sci. 2024, 671, 160745. [Google Scholar] [CrossRef]
- Gavahian, J.M.; Shoja, R.R.; Valefi, Z.; Hamed, N.; Taghi-Ramezani, S. Evaluating laser surface melting of NiCrAlY-APS coating and its effect on high-temperature oxidation behavior of NiCrAlY/YSZ thermal barrier coating before and after surface melting. Heliyon 2024, 12, 23094. [Google Scholar] [CrossRef]
- Senturk, B.S.; Garces, H.F.; Ortiz, A.L.; Dwivedi, G.; Sampath, S.; Padture, N.P. CMAS-resistant plasma sprayed thermal barrier coatings based on Y2O3-Stabilized ZrO2 with Al3+ and Ti4+ solute additions. J. Therm. Spray Technol. 2014, 23, 708–715. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, J.; Huang, J.; Zhao, X. Microstructures and properties of double-ceramic-layer thermal barrier coatings of La2(Zr0.7Ce 0.3)2O7/8YSZ made by atmospheric plasma spraying. Appl. Surf. Sci. 2015, 340, 173–181. [Google Scholar] [CrossRef]
- Bengtsson, P.; Johannesson, T. Characterization of microstructural defects in plasma-sprayed thermal barrier coatings. J. Therm. Spray Technol. 1995, 4, 245–251. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Blasi, C.; Brentari, A.; Schioppa, M. Microstructural, mechanical and thermal characteristics of zirconia-based thermal barrier coatings deposited by plasma spraying. Ceram. Int. 2015, 41, 11776–11785. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and Economical Aspects of Current Thermal Barrier Coating Systems for Gas Turbine Engines by Thermal Spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Tadeusz, B.; Tadeusz, W. Surface Engineering of Metals; Taylor and Francis, CRC Press: Warsaw, Poland, 2010. [Google Scholar]
- Khono, M.; Ikenaga, M.; Ichimura, H. Properties and Industrial Application of Arc Ion Plating Films. J. Surf. Finish. Soc. Jpn. 2011, 44, 708–714. [Google Scholar] [CrossRef]
- Jiang, X.F.; Liu, Q.C.; Wang, H.B. Technology and Application of Multi-Arc Ion Plating. J. Chongqing Univ. 2006, 29, 52–55. [Google Scholar]
- Yan, D.; Xu, W.; Li, J.; Ji, L.; Liu, X.; Shi, L.; Sun, C.; Li, H. Effect of nitrogen/argon ratios on structures and properties of high-entropy (ZrTiNbV)N nitride films by multi-arc ion plating. Vacuum 2024, 225, 113173. [Google Scholar] [CrossRef]
- Shi, C.M.; Wang, T.G.; Pei, Z.L.; Gong, J.; Sun, C. Effects of the Thickness Ratio of CrN vs Cr2O3 Layer on the Properties of Double-layered CrN/Cr2O3 Coatings Deposited by Arc Ion Plating. J. Mater. Sci. Technol. 2014, 30, 473–479. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Luo, C.; Yu, C.; Li, M.; Han, W. Effect of pre-oxidation and sea salt on the hot corrosion behavior of MCrAlY coatings and AlSi coatings. Surf. Coat. Technol. 2024, 477, 130354. [Google Scholar] [CrossRef]
- Tian, C.; Yang, B.; He, J.; Wang, H.; Wang, Z.; Wang, G.; Fu, D. Structure and tribological properties of CrTiAlN coatings deposited by multi-arc ion plating. Plasma Sci. Technol. 2011, 13, 55–60. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ-TiAl-based alloys—A review. Intermetallics 2014, 56, 1–14. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Xu, Z.; Wang, H.; Zhu, C. Hot corrosion behaviour of Al-Si coating in mixed sulphate at 1150 °C. Corros. Sci. 2019, 147, 313–320. [Google Scholar] [CrossRef]
- Cai, J.; Gao, C.; Lv, P.; Zhang, C.; Guan, Q.; Lu, J.; Xu, X. Hot corrosion behaviour of thermally sprayed CoCrAlY coating irradiated by high-current pulsed electron beam. J. Alloys Compd. 2019, 784, 1221–1233. [Google Scholar] [CrossRef]
- Habibi, M.H.; Yang, S.; Guo, S.M. Phase stability and hot corrosion behavior of ZrO2-Ta2O5 compound in Na2SO4-V2O5 mixtures at elevated temperatures. Ceram. Int. 2014, 40, 4077–4083. [Google Scholar] [CrossRef]
- Habibi, M.H.; Wang, L.; Liang, J.; Guo, S.M. An investigation on hot corrosion behavior of YSZ-Ta2O5 in Na2SO4+V2O5 salt at 1100 °C. Corros. Sci. 2013, 75, 409–414. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, K.; Dong, X.; Wang, R.; Duan, W.; Mei, X.; Wang, W.; Cui, J.; Zhang, S.; Xu, C. The role of the surface morphology and segmented cracks on the damage forms of laser re-melted thermal barrier coatings in presence of a molten salt (Na2SO4 + V2O5). Corros. Sci. 2017, 115, 56–67. [Google Scholar] [CrossRef]
- Habibi, M.H.; Guo, S.M. The hot corrosion behavior of plasma sprayed zirconia coatings stabilized with yttria, ceria, and titania in sodium sulfate and vanadium oxide. Mater. Corros. 2015, 66, 270–277. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhang, Z.Y.; Zhang, Q.M.; Li, Y. Comparison of the hot corrosion of nanostructured and microstructured thermal barrier coatings. Mater. Corros. 2014, 65, 613–618. [Google Scholar] [CrossRef]
- Loghman-Estarki, M.R.; Nejati, M.; Edris, H.; Shoja Razavi, R.; Jamali, H.; Pakseresht, A.H. Evaluation of hot corrosion behavior of plasma sprayed scandia and yttria co-stabilized nanostructured thermal barrier coatings in the presence of molten sulfate and vanadate salt. J. Eur. Ceram. Soc. 2015, 35, 693–702. [Google Scholar] [CrossRef]
- Sun, Y.; Miao, Q.; Sun, S.; Liang, W.; Ding, Z.; Niu, J.; Jia, F.; Xu, J.; Gao, J. High-Temperature Hot Corrosion Resistance of CrAl/NiCoCrAlY/AlSiY Gradient Composite Coating on TiAl Alloy. Coatings 2024, 14, 1067. [Google Scholar] [CrossRef]
- Keyvani, A.; Saremi, M.; Sohi, M.H. An investigation on oxidation, hot corrosion and mechanical properties of plasma-sprayed conventional and nanostructured YSZ coatings. Surf. Coat. Technol. 2011, 206, 208–216. [Google Scholar] [CrossRef]
- Tsai, P.C.; Lee, J.H.; Chang, C.L. Improving the erosion resistance of plasma-sprayed zirconia thermal barrier coatings by laser glazing. Surf. Coat. Technol. 2007, 202, 719–724. [Google Scholar] [CrossRef]




| Ti | Al | V | Nb | Cr | N | C | O |
|---|---|---|---|---|---|---|---|
| 50.64 | 46.50 | 1.50 | 1.00 | 0.20 | 0.05 | 0.10 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Wei, X.; Cao, J.; Ma, Y.; Su, H.; Zhao, T.; You, J.; Lv, Y. Thermal Corrosion Properties of Composite Ceramic Coating Prepared by Multi-Arc Ion Plating. Coatings 2024, 14, 1150. https://doi.org/10.3390/coatings14091150
Ding F, Wei X, Cao J, Ma Y, Su H, Zhao T, You J, Lv Y. Thermal Corrosion Properties of Composite Ceramic Coating Prepared by Multi-Arc Ion Plating. Coatings. 2024; 14(9):1150. https://doi.org/10.3390/coatings14091150
Chicago/Turabian StyleDing, Feng, Xiaoxin Wei, Jiangdong Cao, Yujie Ma, Hongbin Su, Ting Zhao, Jiahan You, and Yazhong Lv. 2024. "Thermal Corrosion Properties of Composite Ceramic Coating Prepared by Multi-Arc Ion Plating" Coatings 14, no. 9: 1150. https://doi.org/10.3390/coatings14091150
APA StyleDing, F., Wei, X., Cao, J., Ma, Y., Su, H., Zhao, T., You, J., & Lv, Y. (2024). Thermal Corrosion Properties of Composite Ceramic Coating Prepared by Multi-Arc Ion Plating. Coatings, 14(9), 1150. https://doi.org/10.3390/coatings14091150




