Electrosprayed Chitosan Nanospheres-Based Films: Evaluating the Effect of Molecular Weight on Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
Chitosan
2.2. Development of Electrosprayed Chitosan-Based Films
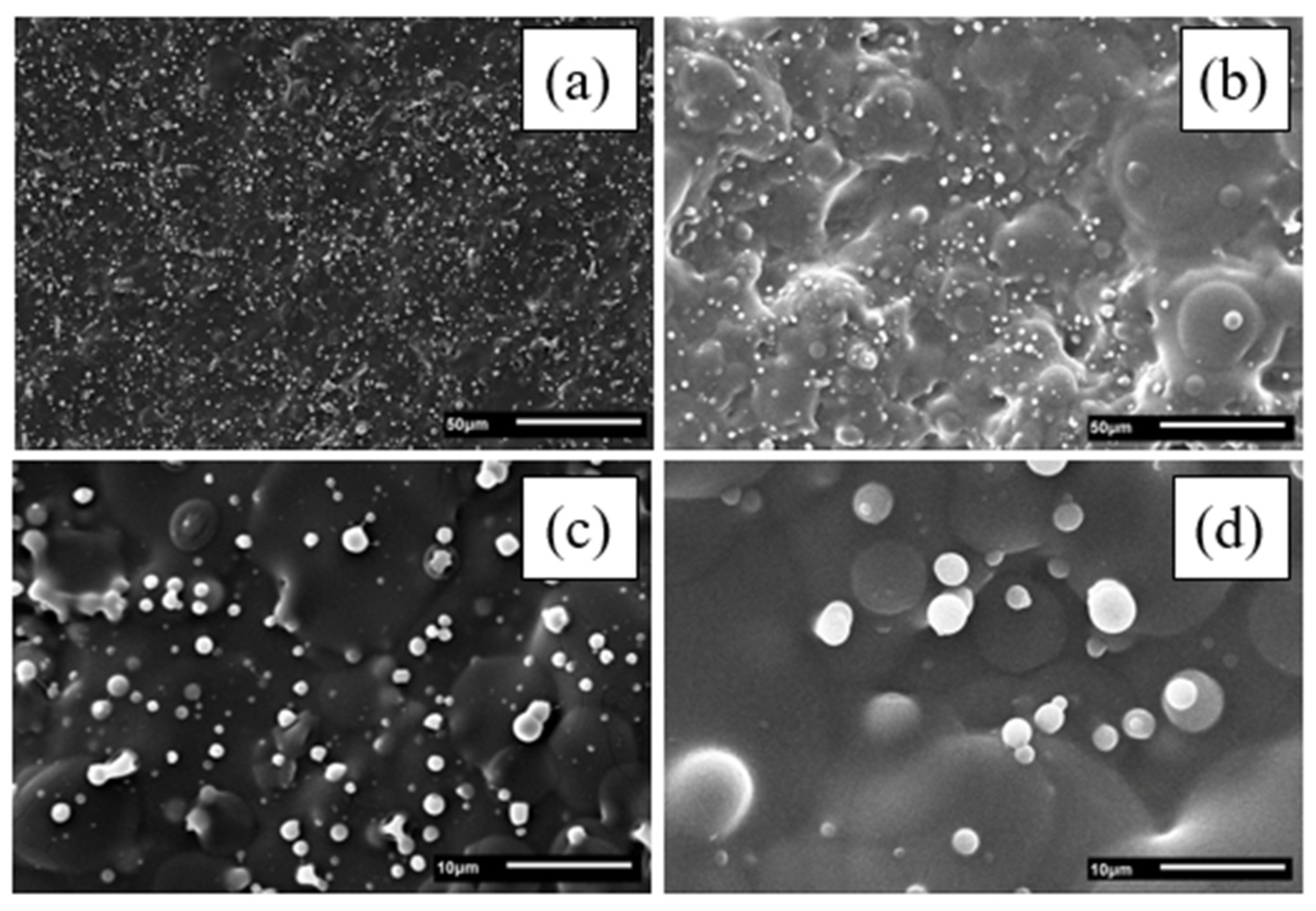
2.3. Morphology of Electrosprayed Chitosan-Based Films
2.4. Structural Modifications of Electrosprayed Chitosan-Based Films
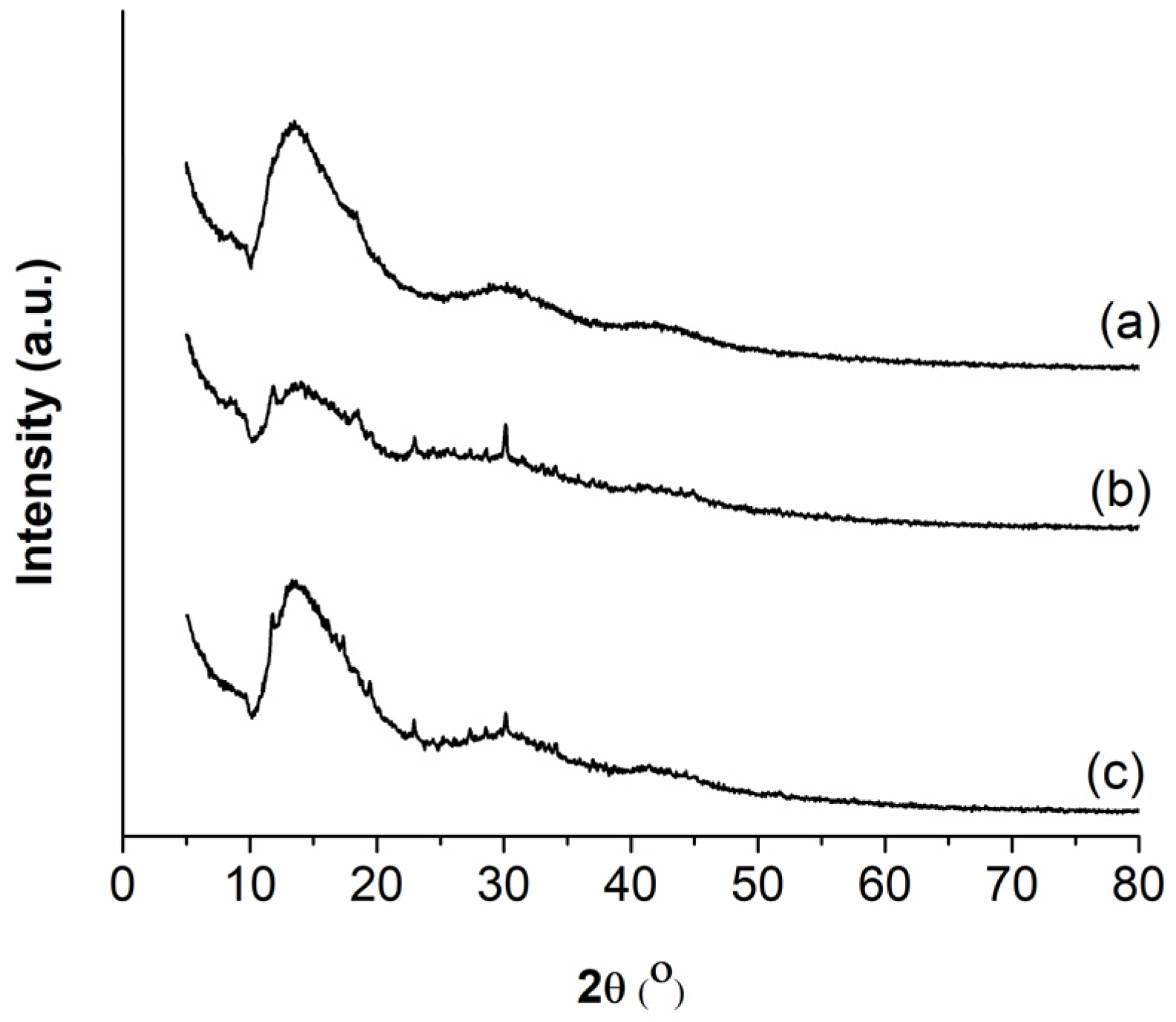
2.5. Crystallinity of Electrosprayed Chitosan-Based Films
2.6. Thermal Properties of Electrosprayed Chitosan-Based Films
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Chitosan
3.2. Morphology of Electrosprayed Chitosan-Based Films
3.3. Structural Evaluation of Electrosprayed Chitosan-Based Films
3.4. Crystallinity Evaluation of Electrosprayed Chitosan-Based Films
3.5. Thermal Properties of Electrosprayed Chitosan-Based Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almajidi, Y.Q.; Ponnusankar, S.; Chaitanya, M.V.N.L.; Marisetti, A.L.; Hsu, C.-Y.; Dhiaa, A.M.; Saadh, M.J.; Pal, Y.; Thabit, R.; Adhab, A.H.; et al. Chitosan-Based Nanofibrous Scaffolds for Biomedical and Pharmaceutical Applications: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 264, 130683. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S.; Machado, B.R.; Farias, B.S.; Santos, L.O.; Duarte, S.H.; Cadaval Junior, T.R.S.; Pinto, L.A.A.; Diaz, P.S. Development of Microstructured Chitosan Nanocapsules with Immobilized Lipase. J. Polym. Environ. 2024, 32, 3627–3639. [Google Scholar] [CrossRef]
- Elnaggar, E.M.; Abusaif, M.S.; Abdel-Baky, Y.M.; Ragab, A.; Omer, A.M.; Ibrahim, I.; Ammar, Y.A. Insight into Divergent Chemical Modifications of Chitosan Biopolymer: Review. Int. J. Biol. Macromol. 2024, 277, 134347. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; Dey, S.C.; Molla, M.A.I.; Hossain, M.R.; Rahman, M.M.; Quddus, M.S.; Moniruzzaman, M.; Shamsuddin, S.M.; Sarker, M. Facile Synthesis of TiO2/Chitosan Nanohybrid for Adsorption-Assisted Rapid Photodegradation of an Azo Dye in Water. React. Kinet. Mech. Catal. 2021, 133, 1121–1139. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Antos, D.; Górak, A. Review on the Application of Chitin and Chitosan in Chromatography. React. Funct. Polym. 2020, 152, 104606. [Google Scholar] [CrossRef]
- Yang, Y.; Aghbashlo, M.; Gupta, V.K.; Amiri, H.; Pan, J.; Tabatabaei, M.; Rajaei, A. Chitosan Nanocarriers Containing Essential Oils as a Green Strategy to Improve the Functional Properties of Chitosan: A Review. Int. J. Biol. Macromol. 2023, 236, 123954. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Araújo, G.M.S.; Rodrigues, J.L.; Yurgel, V.C.; Silva, C.; Paulo, A.M.C.; Loureiro, A.I.S.; Dora, C.L. Designing and Characterization of Curcumin-Loaded Nanotechnological Dressings: A Promising Platform for Skin Burn Treatment. Int. J. Pharm. 2023, 635, 122712. [Google Scholar] [CrossRef]
- Jamali, A.A.; Mahar, F.K.; Hussain, N.; Khatri, M.; Ullah, A.; Ahmed, F.; Khatri, Z.; Kim, I.S. Fabrication of Chitosan-β-Cyclodextrin-Fe Nanofibers for the Adsorption of As-III from Aqueous Solution Using a Lab-Scale Filtration System. J. Appl. Polym. Sci. 2023, 140, e54367. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Farias, B.S.; Cadaval Junior, T.R.S.; Pinto, L.A.A.; Diaz, P.S. Chitosan–Based Nanofibers for Enzyme Immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of Chain Entanglements on Fiber Formation during Electrospinning of Polymer Solutions: Good Solvent, Non-Specific Polymer-Polymer Interaction Limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun Nanofibers: Solving Global Issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Electrospraying of Polymers with Therapeutic Molecules: State of the Art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Farias, B.S.; Cadaval Junior, T.R.S.; Pinto, L.A.A. Chitosan-Functionalized Nanofibers: A Comprehensive Review on Challenges and Prospects for Food Applications. Int. J. Biol. Macromol. 2019, 123, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Bowlin, G.L. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011, 5, 13403. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Sanchez, G.; López-Rubio, A. Impact of Molecular Weight on the Formation of Electrosprayed Chitosan Microcapsules as Delivery Vehicles for Bioactive Compounds. Carbohydr. Polym. 2016, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, J.; Fu, X.; Lin, H.; Zhang, W.; Wang, X.; Liu, F. Electrosprayed Thin Film Nanocomposite Polyamide Nanofiltration with Homogeneous Distribution of Nanoparticles for Enhanced Separation Performance. Desalination 2023, 546, 116206. [Google Scholar] [CrossRef]
- Nunnenkamp, M.; Nieuwenhuijzen, K.J.H.; Elshof, J.E. Multilayer Films of Exfoliated 2D Oxide Nanosheets by Electrospray Deposition. Sci. Rep. 2022, 12, 8673. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; Salgado-Cruz, M.P.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable Electrosprayed Pectin Films: An Alternative to Valorize Coffee Mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Bodnár, E.; Grifoll, J.; Rosell-Llompart, J. Polymer Solution Electrospraying: A Tool for Engineering Particles and Films with Controlled Morphology. J. Aerosol Sci. 2018, 125, 93–118. [Google Scholar] [CrossRef]
- Gaona-Sánchez, V.A.; Calderón-Domínguez, G.; Morales-Sánchez, E.; Chanona-Pérez, J.J.; Velázquez-de la Cruz, G.; Méndez-Méndez, J.V.; Terrés-Rojas, E.; Farrera-Rebollo, R.R. Preparation and Characterisation of Zein Films Obtained by Electrospraying. Food Hydrocoll. 2015, 49, 1–10. [Google Scholar] [CrossRef]
- Moura, J.M.; Farias, B.S.; Rodrigues, D.A.S.; Moura, C.M.; Dotto, G.L.; Pinto, L.A.A. Preparation of Chitosan with Different Characteristics and Its Application for Biofilms Production. J. Polym. Environ. 2015, 23, 470–477. [Google Scholar] [CrossRef]
- Dotto, G.L.; Souza, V.C.; Moura, J.M.; Moura, C.M.; Pinto, L.A.A. Influence of Drying Techniques on the Characteristics of Chitosan and the Quality of Biopolymer Films. Dry. Technol. 2011, 29, 1784–1791. [Google Scholar] [CrossRef]
- Weska, R.F.; Moura, J.M.; Batista, L.M.; Rizzi, J.; Pinto, L.A.A. Optimization of Deacetylation in the Production of Chitosan from Shrimp Wastes: Use of Response Surface Methodology. J. Food Eng. 2007, 80, 749–753. [Google Scholar] [CrossRef]
- Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Cadaval Junior, T.R.S.; Pinto, L.A.A. Production of Low Molecular Weight Chitosan by Acid and Oxidative Pathways: Effect on Physicochemical Properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhong, W. A New Linear Potentiometric Titration Method for the Determination of Deacetylation Degree of Chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Masuelli, M.A. Mark-Houwink Parameters for Aqueous-Soluble Polymers and Biopolymers at Various Temperatures. J. Polym. Biopolym. Phys. Chem. 2014, 2, 37–43. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Silverstein, R.W.; Bassler, G.C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- ASTM D7426; Standard Test Method for Assignment of the DSC Procedure for Determining Tg of a Polymer or an Elastomeric Compound. American Society for Testing & Material: West Conshohocken, PA, USA, 2013.
- ASTM D3850-12; Standard Test Method for Rapid Thermal Degradation of Solid Electrical Insulating Materials By Thermogravimetric Method (TGA). American Society for Testing & Material International: West Conshohocken, PA, USA, 2012.
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Materials and Their Applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Lee, S.J.; Gwak, M.A.; Chathuranga, K.; Lee, J.S.; Koo, J.; Park, W.H. Multifunctional Chitosan/Tannic Acid Composite Films with Improved Anti-UV, Antioxidant, and Antimicrobial Properties for Active Food Packaging. Food Hydrocoll. 2023, 136, 108249. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des. Devel Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Shajahan, A.; Shankar, S.; Sathiyaseelan, A.; Narayan, K.S.; Narayanan, V.; Kaviyarasan, V.; Ignacimuthu, S. Comparative Studies of Chitosan and Its Nanoparticles for the Adsorption Efficiency of Various Dyes. Int. J. Biol. Macromol. 2017, 104, 1449–1458. [Google Scholar] [CrossRef]
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef]
- Gomez, A.; Bingham, D.; De Juan, L.; Tang, K. Production of Protein Nanoparticles by Electrospray Drying. J. Aerosol Sci. 1998, 29, 561–574. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR Studies of Chitosan Blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and Mechanism of the Thermal Degradation of Biopolymers Chitin and Chitosan Using Thermogravimetric Analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the Degree of Deacetylation of Chitin and Chitosan by X-Ray Powder Diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Jayakumar, R.; Tamura, H. Synthesis, Characterization and Thermal Properties of Chitin-g-Poly(ɛ-Caprolactone) Copolymers by Using Chitin Gel. Int. J. Biol. Macromol. 2008, 43, 32–36. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, Z.; Liu, Q.; Chen, Q.; Chen, M. Amorphous Electrode: From Synthesis to Electrochemical Energy Storage. Energy Environ. Mater. 2023, 6, e12573. [Google Scholar] [CrossRef]
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 2022, 14, 2202. [Google Scholar] [CrossRef]
- Stachurski, Z.H. On Structure and Properties of Amorphous Materials. Materials 2011, 4, 1564–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Valenzuela, L.D.; Sammalkorpi, M.; Lutkenhaus, J.L. Effect of Water on the Thermal Transition Observed in Poly(Allylamine Hydrochloride)-Poly(Acrylic Acid) Complexes. Macromolecules 2016, 49, 7563–7570. [Google Scholar] [CrossRef]
- Hariri, H.H.; Lehaf, A.M.; Schlenoff, J.B. Mechanical Properties of Osmotically Stressed Polyelectrolyte Complexes and Multilayers: Water as a Plasticizer. Macromolecules 2012, 45, 9364–9372. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. Structural Relaxations of Amorphous Lactose and Lactose-Whey Protein Mixtures. J. Food Eng. 2016, 173, 106–115. [Google Scholar] [CrossRef]
- Farias, B.S.; Vidal, É.M.; Ribeiro, N.T.; Silveira, N.; Vaz, B.S.; Kuntzler, S.G.; Morais, M.G.; Cadaval, T.R.S.A.; Pinto, L.A.A. Electrospun Chitosan/Poly(Ethylene Oxide) Nanofibers Applied for the Removal of Glycerol Impurities from Biodiesel Production by Biosorption. J. Mol. Liq. 2018, 268, 365–370. [Google Scholar] [CrossRef]
- Ero-Phillips, O.; Jenkins, M.; Stamboulis, A. Tailoring Crystallinity of Electrospun Plla Fibres by Control of Electrospinning Parameters. Polymers 2012, 4, 1331–1348. [Google Scholar] [CrossRef]
- Thanisha, F.; Koliyoor, J.; Ismayil; Monteiro, J. Amorphous Magnesium-Doped Chitosan Films as Solid Polymer Electrolytes for Energy Storage Device Applications. Mater. Sci. Eng. B 2024, 301, 117149. [Google Scholar] [CrossRef]
- Yang, C.C.; Lo, C.T.; Luo, Y.L.; Venault, A.; Chang, Y. Thermally Stable Bioinert Zwitterionic Sulfobetaine Interfaces Tolerated in the Medical Sterilization Process. ACS Biomater. Sci. Eng. 2021, 7, 1031–1045. [Google Scholar] [CrossRef]
- Wang, F.; Wei, D.; Li, Y.; Chen, T.; Mu, P.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Chitosan/Reduced Graphene Oxide-Modified Spacer Fabric as a Salt-Resistant Solar Absorber for Efficient Solar Steam Generation. J. Mater. Chem. A Mater. 2019, 7, 18311–18317. [Google Scholar] [CrossRef]


| Type | Molecular Weight (kDa) | Deacetylation Degree (%) |
|---|---|---|
| CH–D | 220.1 ± 3.7 a | 72.5 ± 3.8 a |
| CH–O8 | 124.5 ± 2.3 b | 84.2 ± 0.8 b |
| CH–O100 | 52.7 ± 1.5 c | 86.5 ± 0.4 b |
| Sample | Tg (°C) | ∆HR (J g−1) | TID (°C) |
|---|---|---|---|
| CH–D | 117 ± 2 | 247 ± 9 | 275 ± 4 |
| CH–O8 | 117 ± 1 | 197 ± 4 | 245 ± 6 |
| CH–O100 | 80 ± 2 | 397 ± 6 | 260 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, B.; Rizzi, F.; Gerhardt, R.; Ribeiro, E.; Dias, D.; Cadaval, T.R.; Pinto, L.A. Electrosprayed Chitosan Nanospheres-Based Films: Evaluating the Effect of Molecular Weight on Physicochemical Properties. Coatings 2024, 14, 1159. https://doi.org/10.3390/coatings14091159
Farias B, Rizzi F, Gerhardt R, Ribeiro E, Dias D, Cadaval TR, Pinto LA. Electrosprayed Chitosan Nanospheres-Based Films: Evaluating the Effect of Molecular Weight on Physicochemical Properties. Coatings. 2024; 14(9):1159. https://doi.org/10.3390/coatings14091159
Chicago/Turabian StyleFarias, Bruna, Francisca Rizzi, Rafael Gerhardt, Eduardo Ribeiro, Daiane Dias, Tito Roberto Cadaval, and Luiz Antonio Pinto. 2024. "Electrosprayed Chitosan Nanospheres-Based Films: Evaluating the Effect of Molecular Weight on Physicochemical Properties" Coatings 14, no. 9: 1159. https://doi.org/10.3390/coatings14091159






