ULPING-Based Titanium Oxide as a New Cathode Material for Zn-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
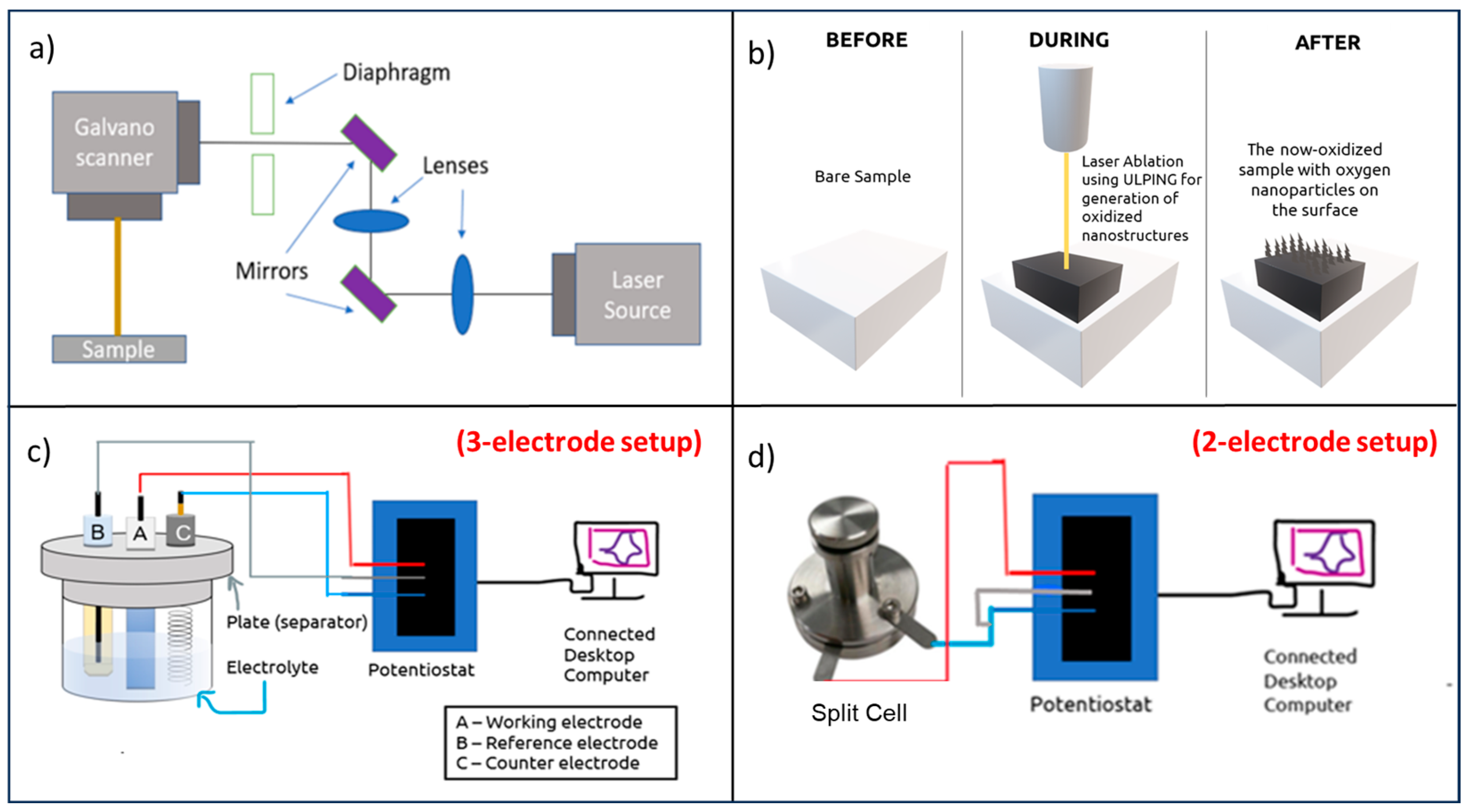
2.2. Sample Preparation Using ULPING
2.3. Electrochemical Characterizations
2.4. Material Characterization Tests
3. Results and Discussion
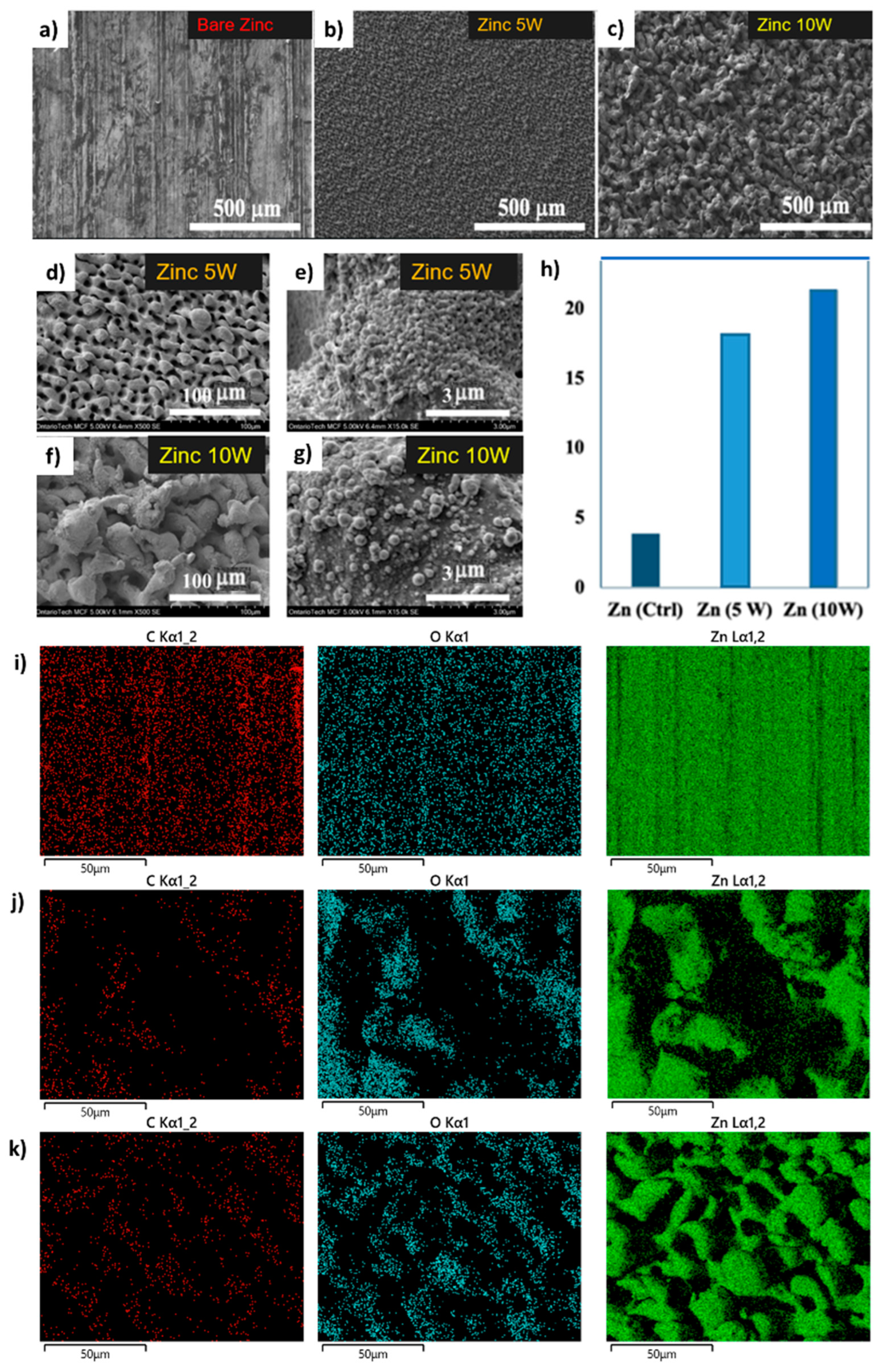
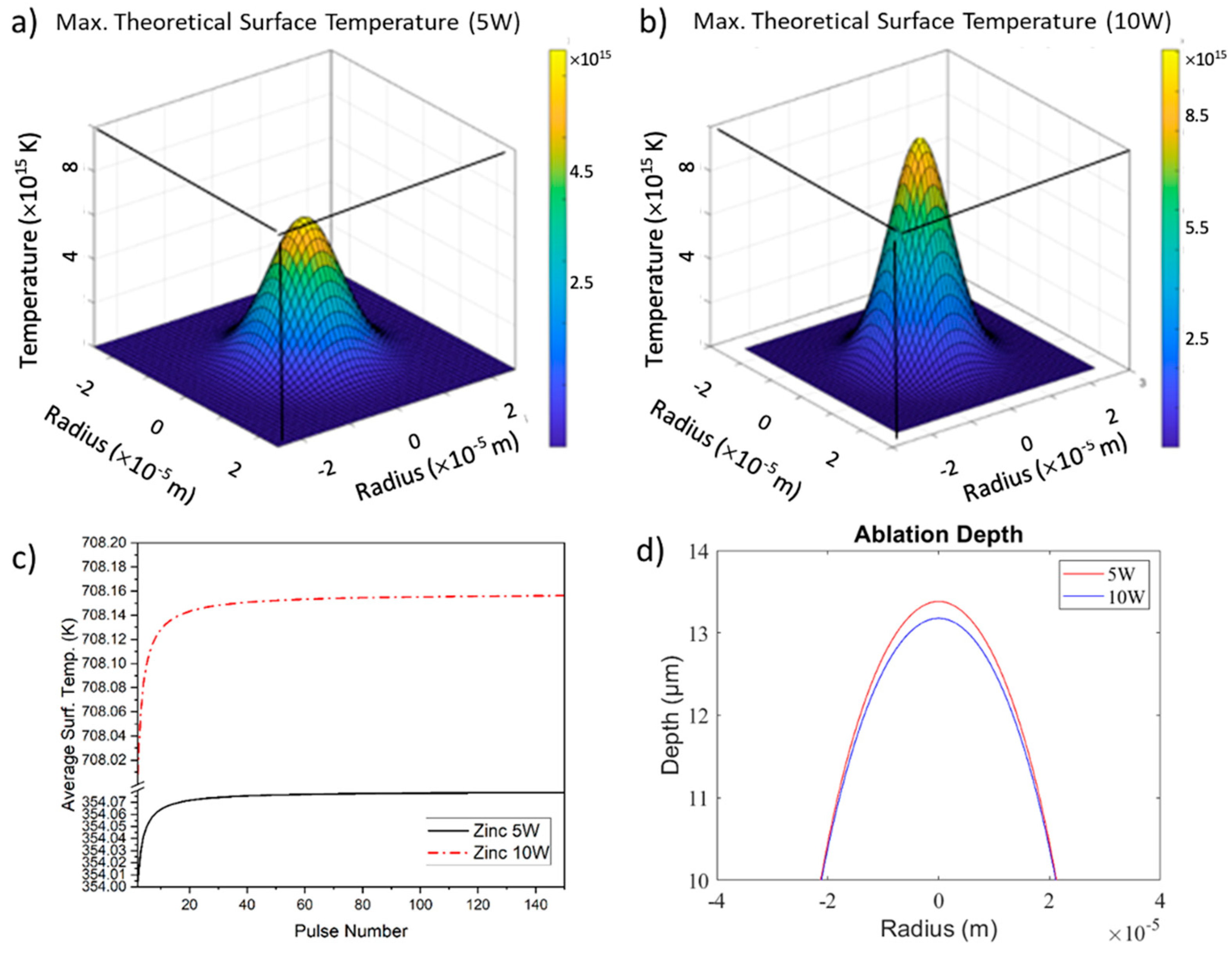
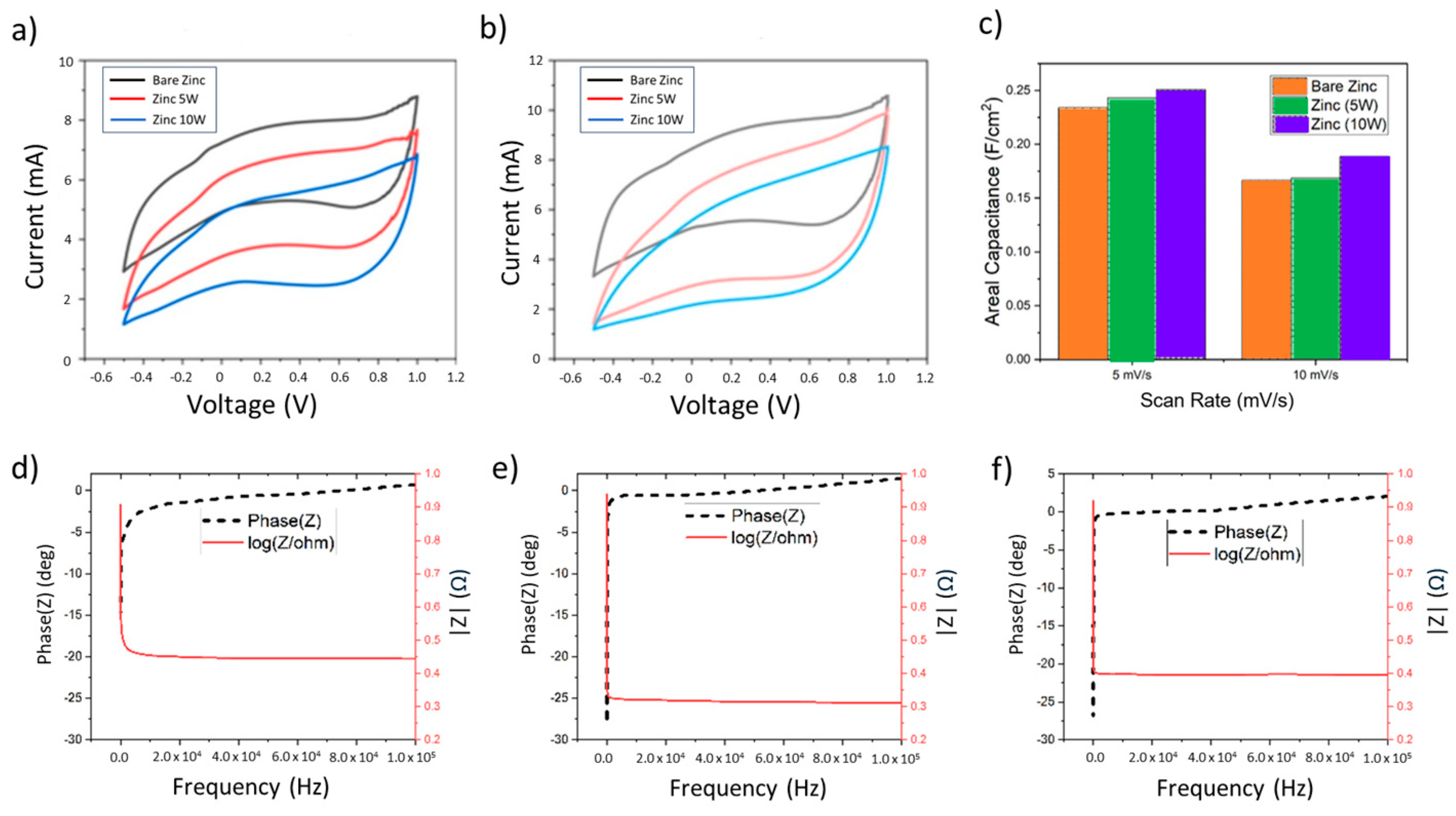
3.1. Zinc
3.1.1. Results from Material Characterization
3.1.2. Thermal Modeling and Theoretical Results
3.1.3. Electrochemical Tests
3.2. Titanium
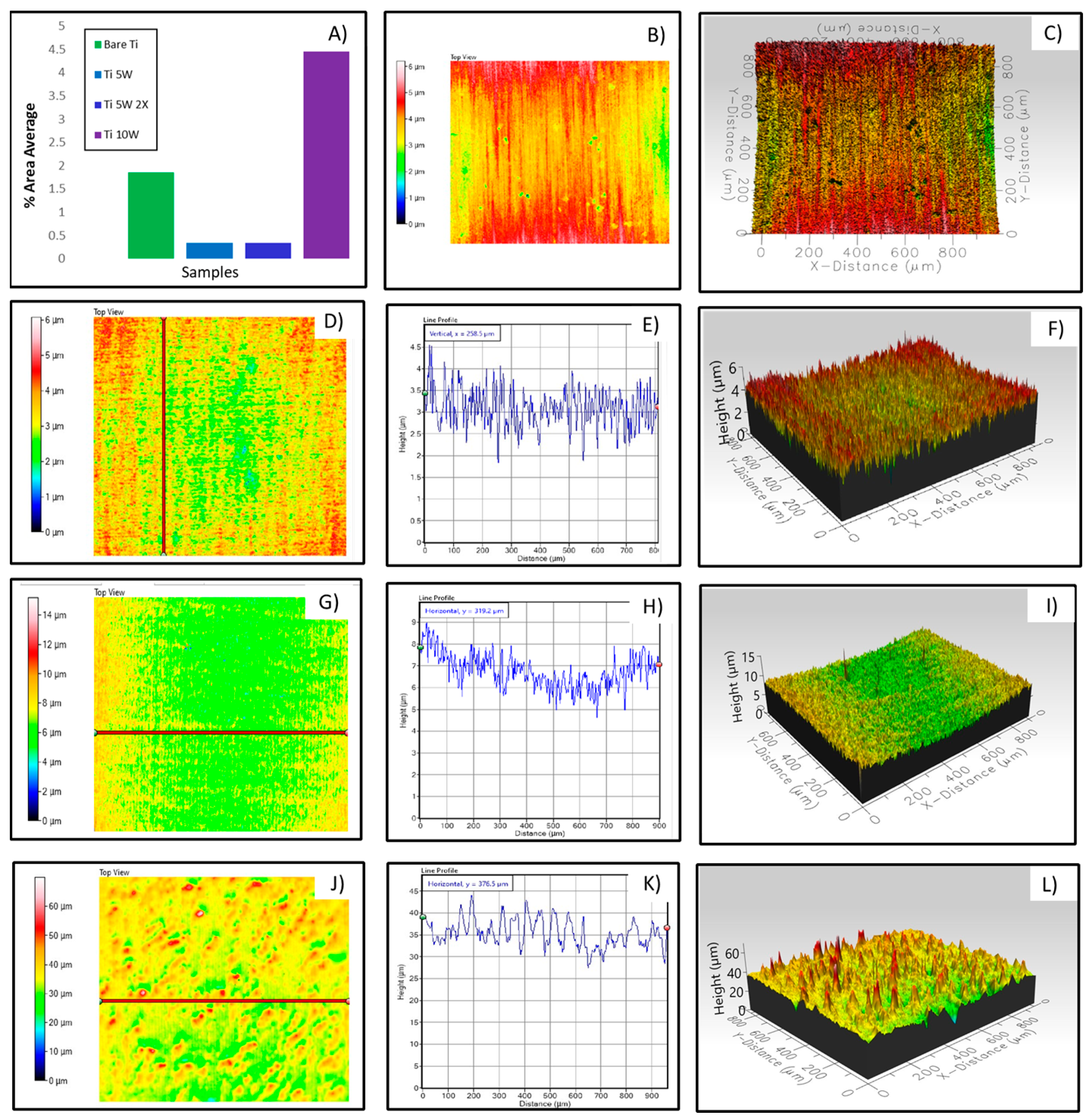
3.2.1. Results from Material Characterization
3.2.2. MATLAB Results
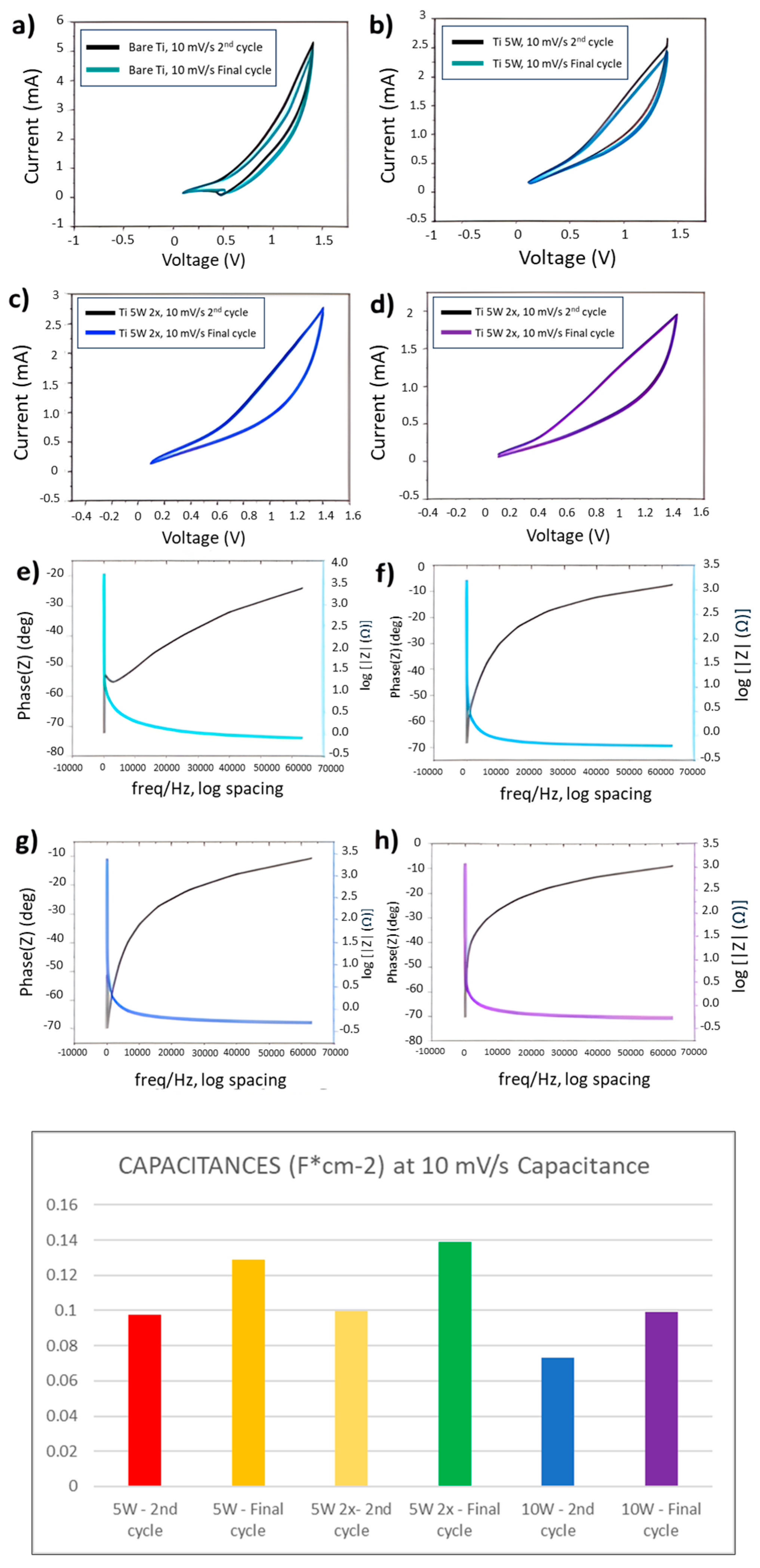
3.2.3. Electrochemical Tests (Three-Electrode)
3.2.4. Electrochemical Tests (Two-Electrode)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominković, D.F.; Bačeković, I.; Pedersen, A.S.; Krajačić, G. The Future of Transportation in Sustainable Energy Systems: Opportunities and barriers in a clean energy transition. Renew. Sustain. Energy Rev. 2018, 82, 1823–1838. [Google Scholar] [CrossRef]
- Lund, P.D. Clean Energy Systems as mainstream energy options. Int. J. Energy Res. 2015, 40, 4–12. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for Clean Energy Systems. J. Power Sources 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. A review on Clean Energy Solutions for better sustainability. Int. J. Energy Res. 2015, 39, 585–606. [Google Scholar] [CrossRef]
- Desideri, U.; Yan, J. Clean Energy Technologies and Systems for a Sustainable World. Appl. Energy 2012, 97, 1–4. [Google Scholar] [CrossRef]
- Garcia-Diaz, B.L.; Olson, L.; Martinez-Rodriguez, M.; Fuentes, R.; Colon-Mercado, H.; Gray, J. High temperature electrochemical engineering and clean energy systems. J. South Carol. Acad. Sci. 2016, 14, 4. [Google Scholar]
- Mangla, S.K.; Luthra, S.; Jakhar, S.; Gandhi, S.; Muduli, K.; Kumar, A. A step to clean energy-sustainability in energy system management in an emerging economy context. J. Clean. Prod. 2020, 242, 118462. [Google Scholar] [CrossRef]
- Gui, E.M.; MacGill, I. Typology of future Clean Energy Communities: An exploratory structure, opportunities, and challenges. Energy Res. Amp. Soc. Sci. 2018, 35, 94–107. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical Energy Storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Amp. Environ. Sci. 2012, 5, 7854. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial design of dendrite-Free Zinc Anodes for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 13280–13291. [Google Scholar] [CrossRef]
- Gür, T.M. Review of Electrical Energy Storage Technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Amp. Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The rise of organic electrode materials for Energy Storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Ao, H.; Zhao, Y.; Zhou, J.; Cai, W.; Zhang, X.; Zhu, Y.; Qian, Y. Rechargeable aqueous hybrid ion batteries: Developments and prospects. J. Mater. Chem. A 2019, 7, 18708–18734. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhou, J.; Cai, W.; Zhang, X.; Zhu, Y.; Qian, Y. Advanced Energy Storage Devices: Basic principles, analytical methods, and Rational Materials Design. Adv. Sci. 2017, 5, 1700322. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Riaz, A.; Sarker, M.R.; Saad, M.H.M.; Mohamed, R. Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations. Sensors 2021, 21, 5041. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef]
- Al-Amin, M.; Islam, S.; Shibly, S.U.A.; Iffat, S. Comparative review on the aqueous zinc-ion batteries (AZIBs) and flexible zinc-ion batteries (FZIBs). Nanomaterials 2022, 12, 3997. [Google Scholar] [CrossRef] [PubMed]
- De Cachinho Cordeiro, I.M.; Li, A.; Lin, B.; Ma, D.X.; Xu, L.; Eh, A.L.S.; Wang, W. Solid Polymer Electrolytes for Zinc-Ion Batteries. Batteries 2023, 9, 343. [Google Scholar] [CrossRef]
- Jin, T.; Singer, G.; Liang, K.; Yang, Y. Structural batteries: Advances, challenges and perspectives. Mater. Today 2023, 62, 151–167. [Google Scholar] [CrossRef]
- Khot, M.; Kiani, A. Effects of surface morphology with pulse duration variation for supercapacitor electrode fabrication using ULPING technique. Lasers Manuf. Mater. Process. 2023, 10, 548–562. [Google Scholar] [CrossRef]
- Kiani, A.; Venkatakrishnan, K.; Tan, B. Direct laser writing of amorphous silicon on si-substrate induced by high repetition femtosecond pulses. J. Appl. Phys. 2010, 108, 074907. [Google Scholar] [CrossRef]
- Colpitts, C.; Ektesabi, A.M.; Wyatt, R.A.; Crawford, B.D.; Kiani, A. Mammalian fibroblast cells avoid residual stress zone caused by nanosecond laser pulses. J. Mech. Behav. Biomed. Mater. 2017, 74, 214–220. [Google Scholar] [CrossRef]
- Khosravinia, K.; Kiani, A. Protocol for fabricating pseudocapacitor electrodes using ultra short laser pulses for in situ nanostructure generation. STAR Protoc. 2023, 4, 102469. [Google Scholar] [CrossRef]




| Conditions | Conventional Methods | ULPING |
|---|---|---|
| Complexity of experimental setup | Complicated/many steps | Single, one step in ambient condition |
| Technological route | Mostly separate products from substrate | Add modification directly on substrate material |
| Electrode pattern? | No | Yes |
| Target material | All materials | All solid materials and polymers, particularly transition metals |
| Collection after procedure | Post-processing, binding | N/A |
| Environmentally friendly? | No | Yes |
| Experimental cost | High | Low |
| Time to conduct experiments | Slow and time-consuming | Quick |
| Sample | Scan Speed (mm/s) | Frequency (kHz) | Power (W) | Pulse Width (ps) | Number of Times Laser Test Was Repeated |
|---|---|---|---|---|---|
| Zn-1 (Bare Zn) | N/A | N/A | N/A | N/A | N/A |
| Zn-2 (Zn-10W) | 100 | 1200 | 10 | 150 | 1 |
| Zn-3 (Zn-5W) | 100 | 1200 | 5 | 150 | 1 |
| Ti-4 (Ti-5W) | 50 | 1200 | 5 | 150 | 1 |
| Ti-5 (Ti-5W 2×) | 50 | 1200 | 5 | 150 | 2 |
| Ti-6 (Ti-10W) | 50 | 1200 | 10 | 150 | 1 |
| Ti-7 (Bare Ti) | 50 | 1200 | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiam, S.S.; Rath, J.; Gutiérrez Vera, E.; Kiani, A. ULPING-Based Titanium Oxide as a New Cathode Material for Zn-Ion Batteries. Coatings 2024, 14, 1163. https://doi.org/10.3390/coatings14091163
Shiam SS, Rath J, Gutiérrez Vera E, Kiani A. ULPING-Based Titanium Oxide as a New Cathode Material for Zn-Ion Batteries. Coatings. 2024; 14(9):1163. https://doi.org/10.3390/coatings14091163
Chicago/Turabian StyleShiam, Suben Sri, Jyotisman Rath, Eduardo Gutiérrez Vera, and Amirkianoosh Kiani. 2024. "ULPING-Based Titanium Oxide as a New Cathode Material for Zn-Ion Batteries" Coatings 14, no. 9: 1163. https://doi.org/10.3390/coatings14091163







