Corrosion and Protection of Chinese Bronze Relics: A Review
Abstract
1. Introduction
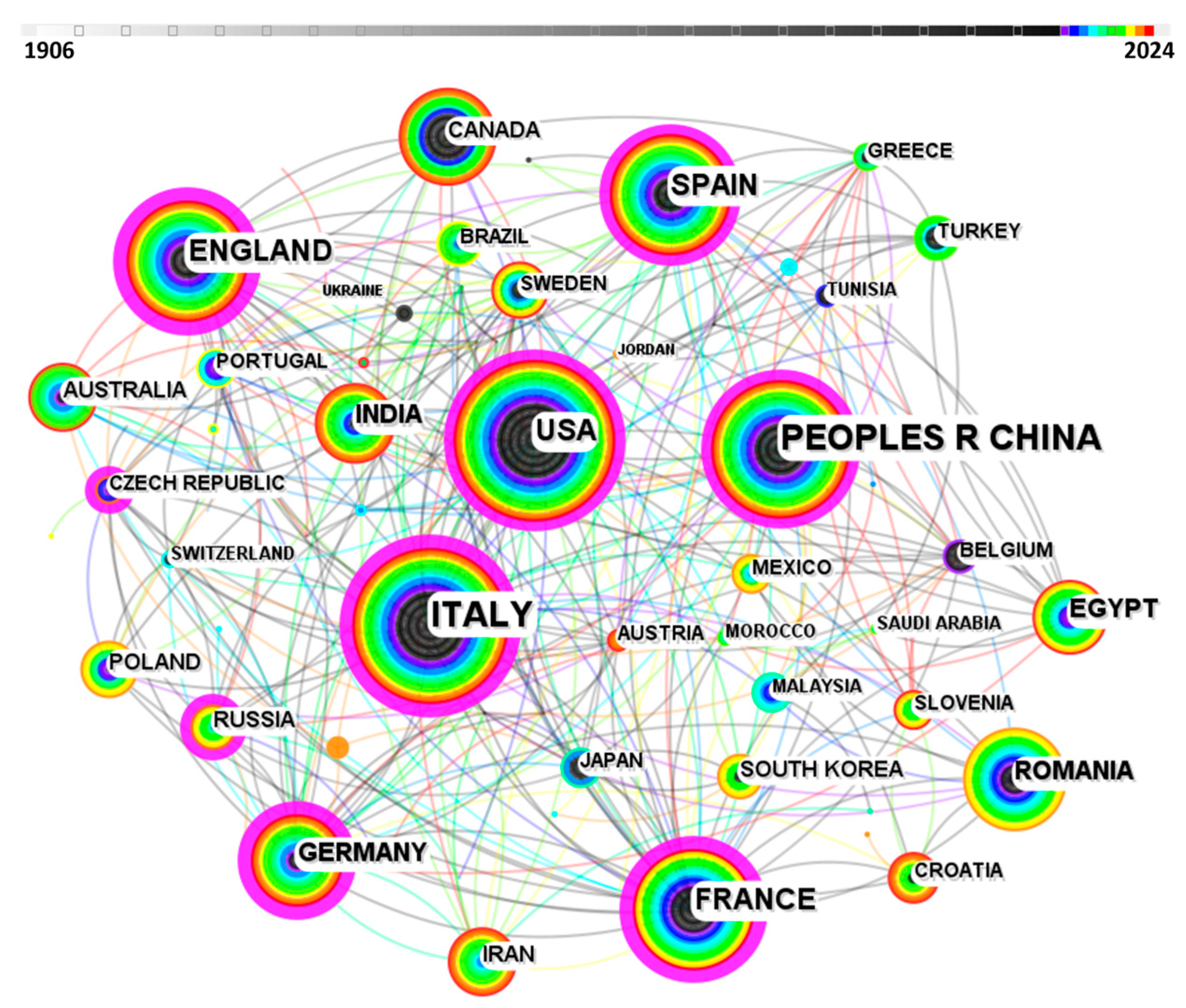
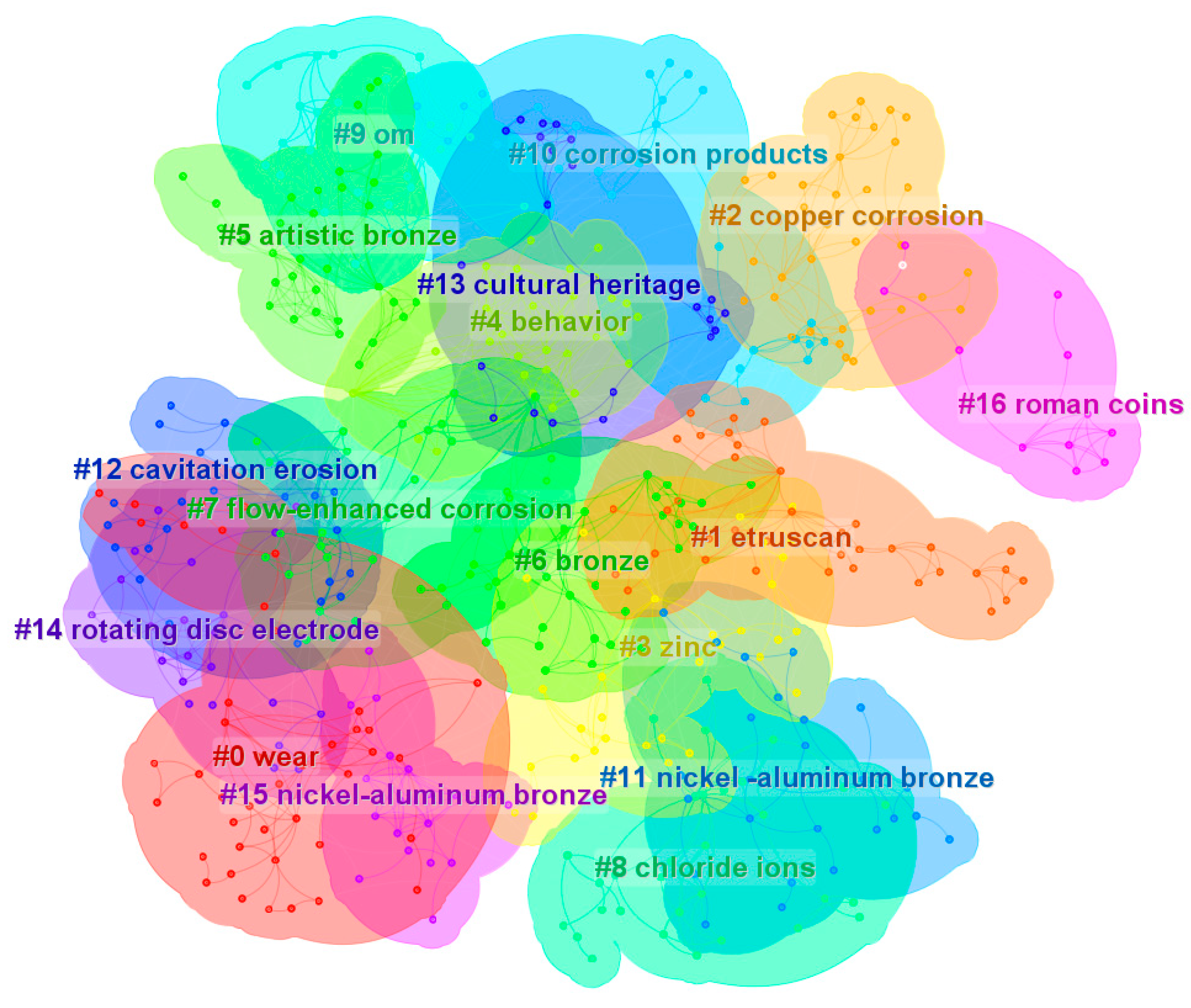
2. Research Statistics on the Corrosion and Protection of Bronze Relics
3. Corrosion and Detection of Bronze Relics
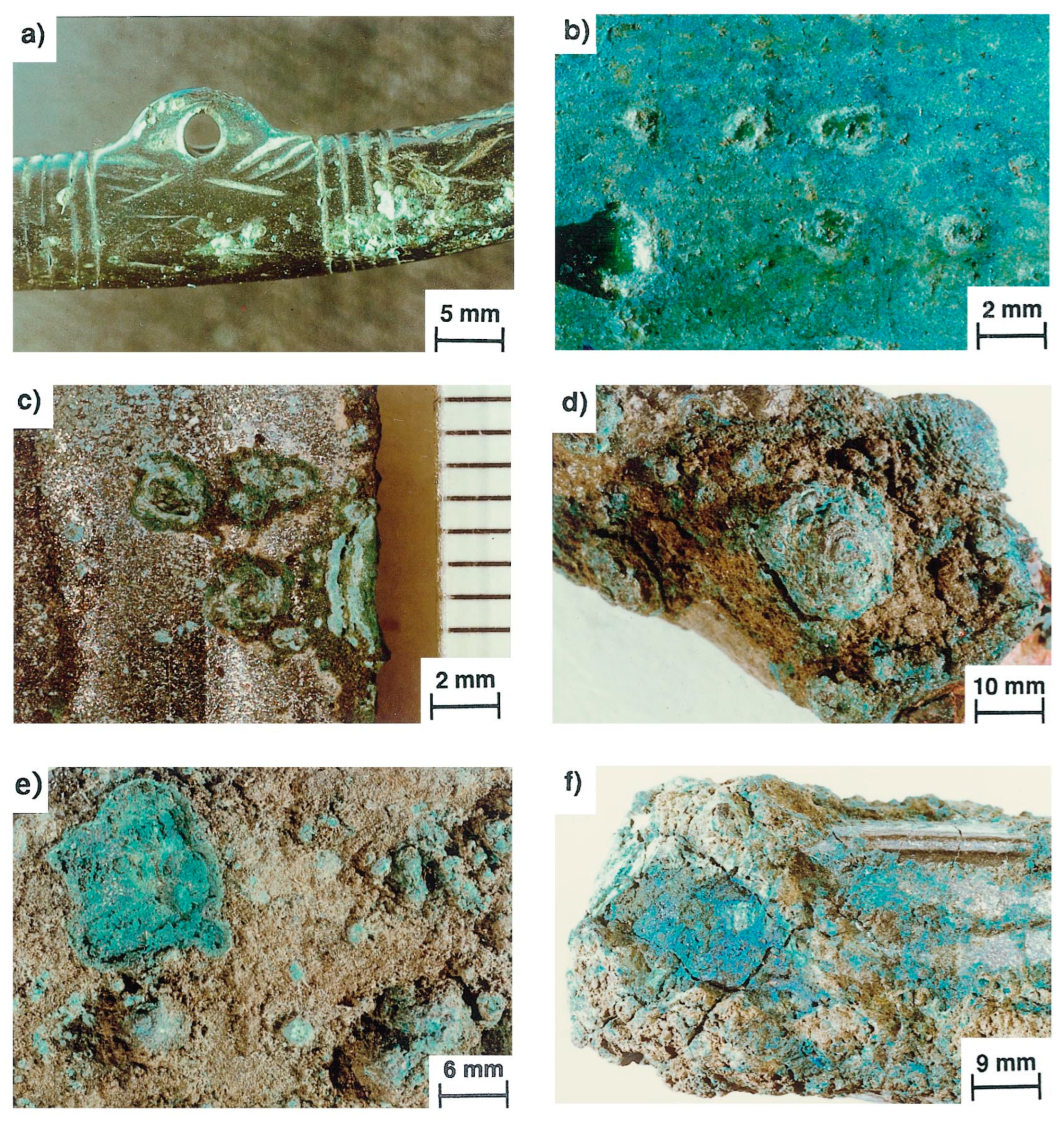
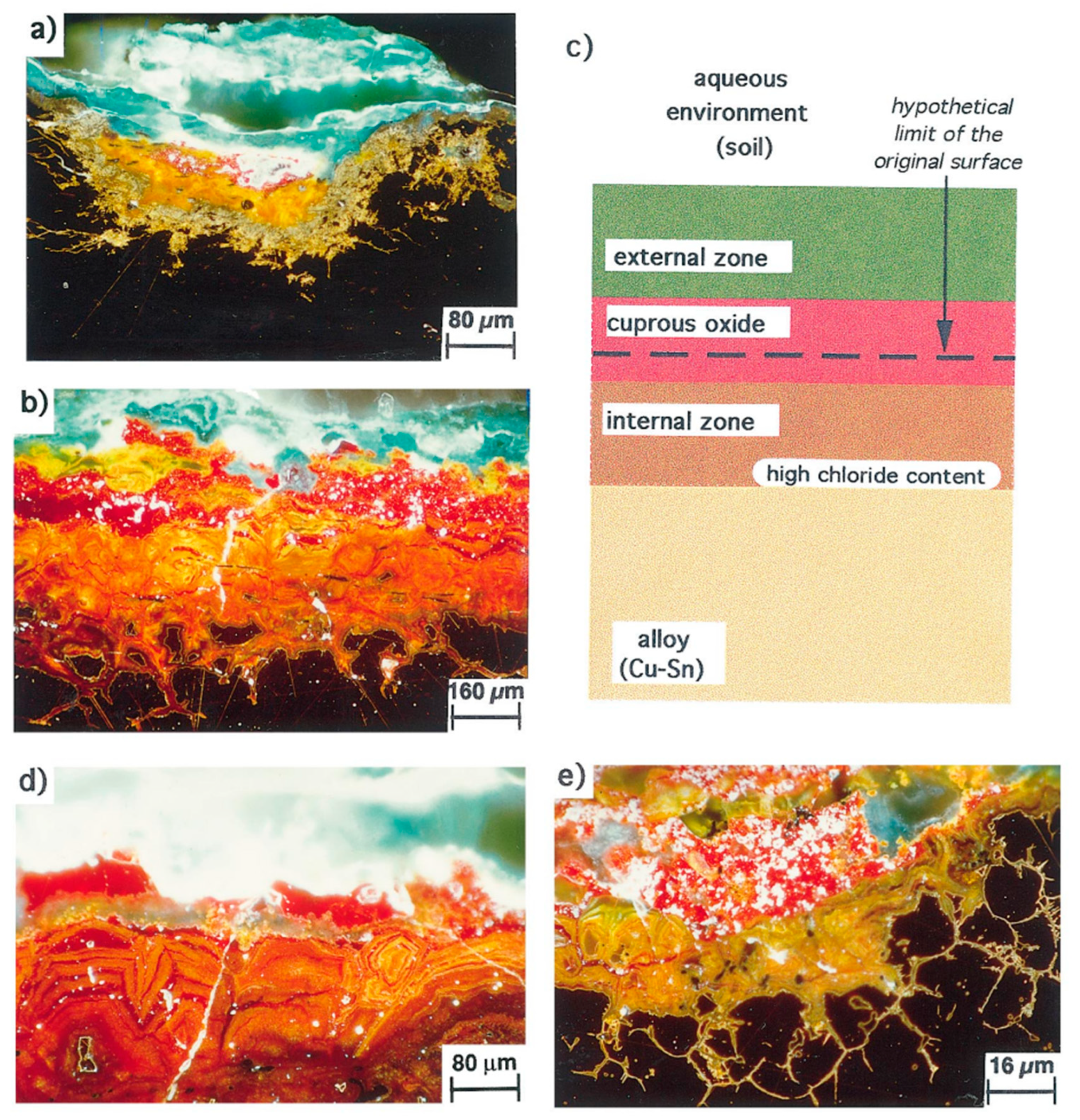
3.1. Corrosion Mechanism
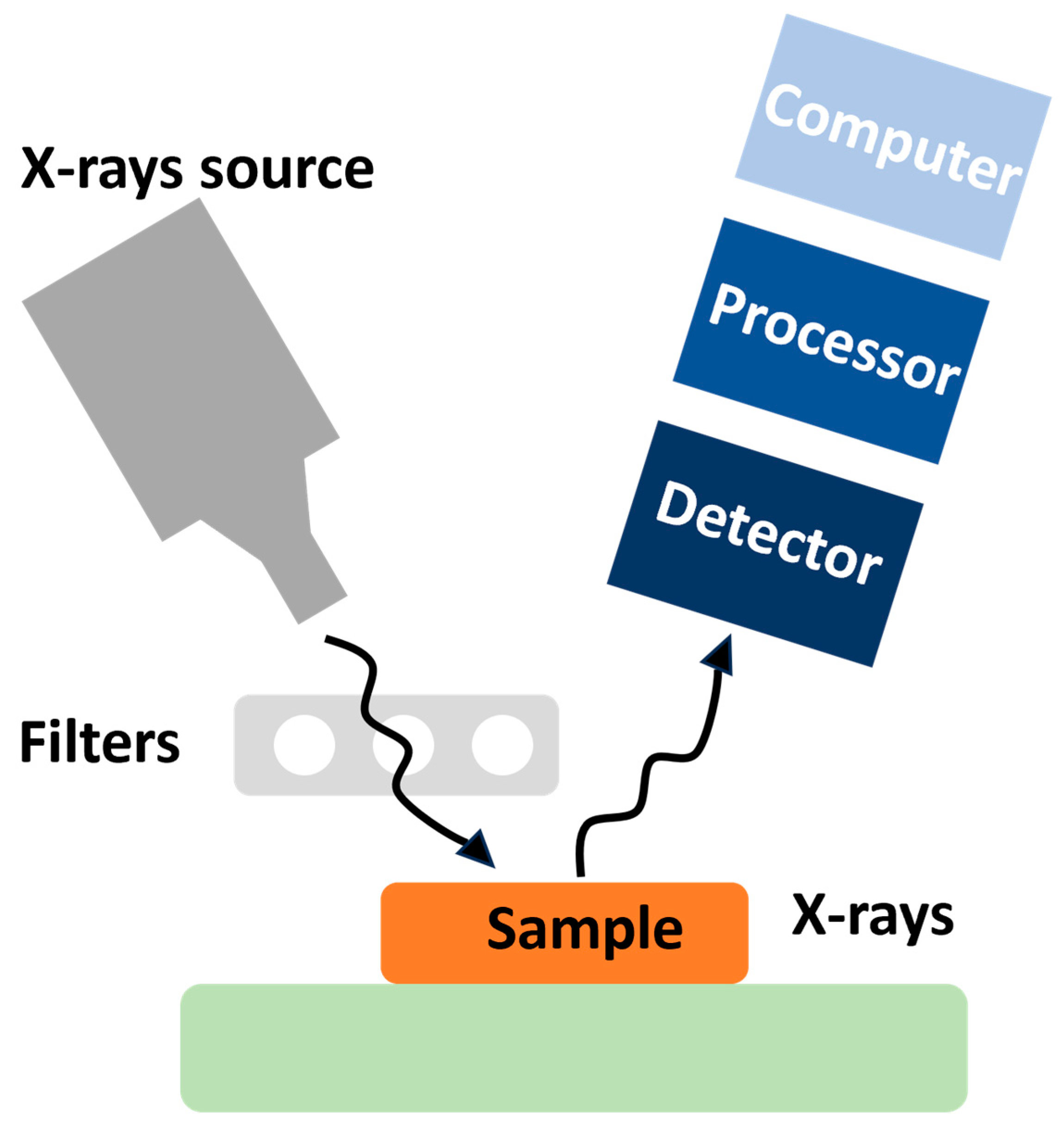
3.2. Corrosion Detection Methods
3.3. Corrosion-Influencing Factors
4. Corrosion Protection Strategy
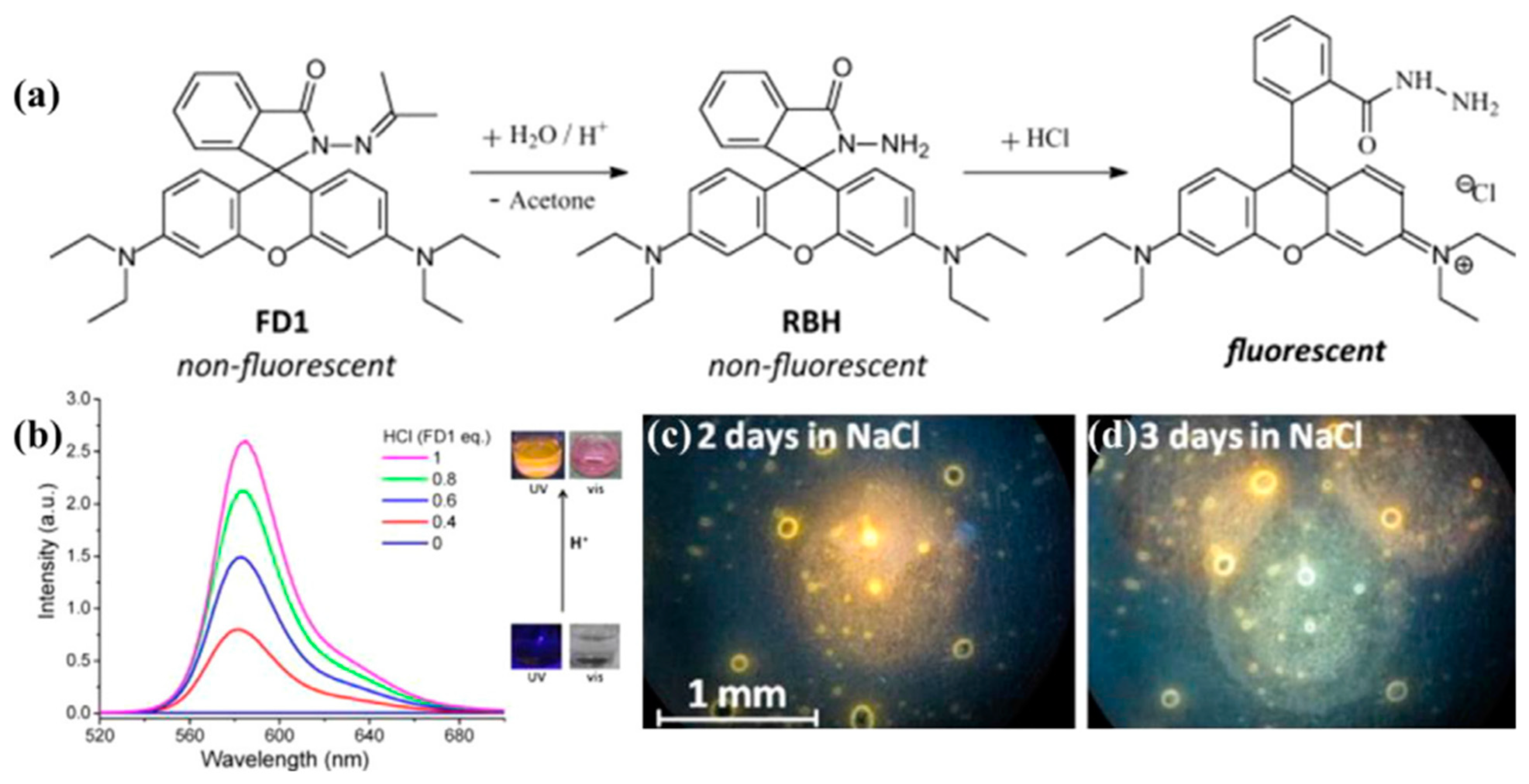
4.1. Surface Corrosion Inhibitor
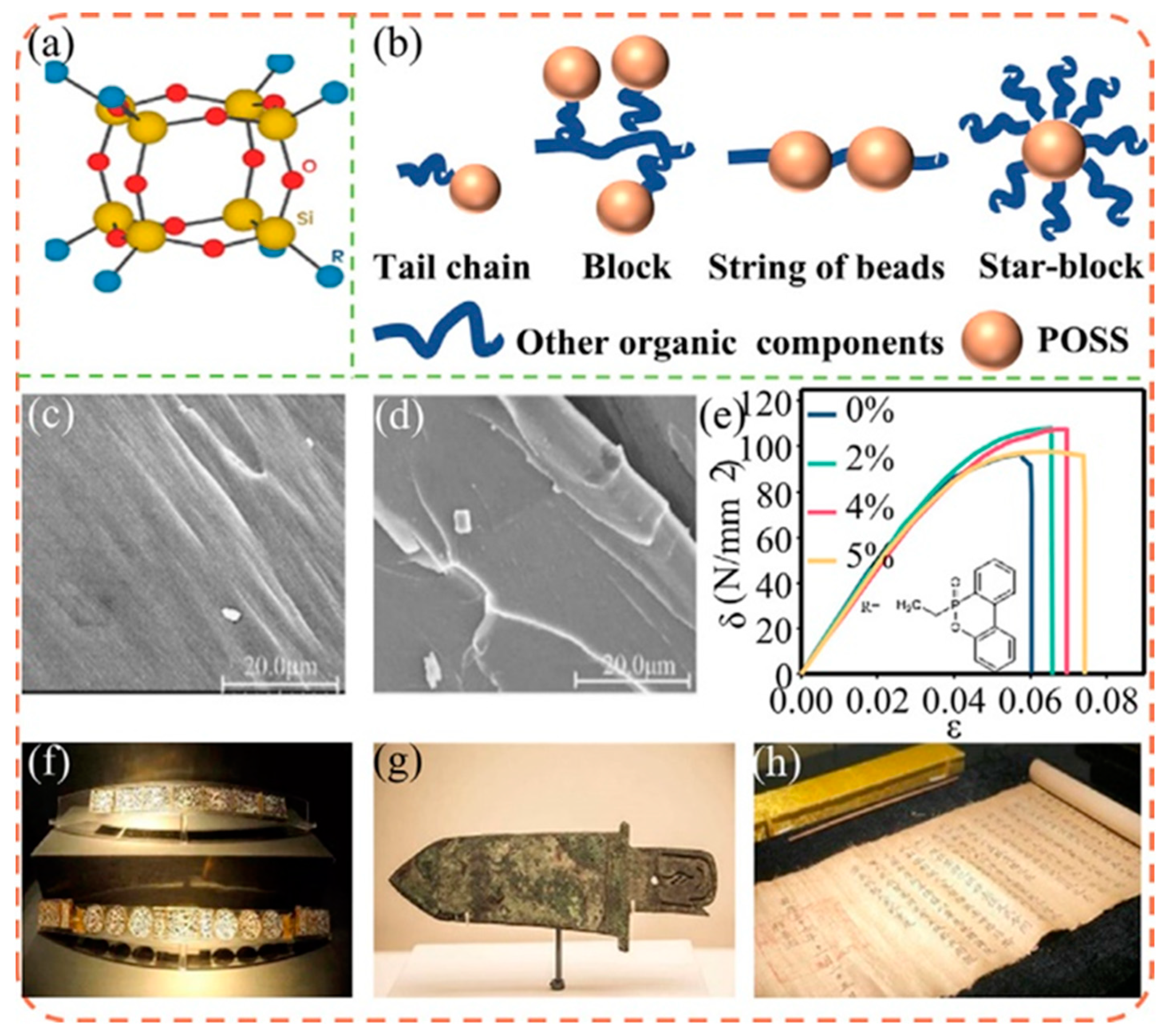
4.2. Organic Resin Coating
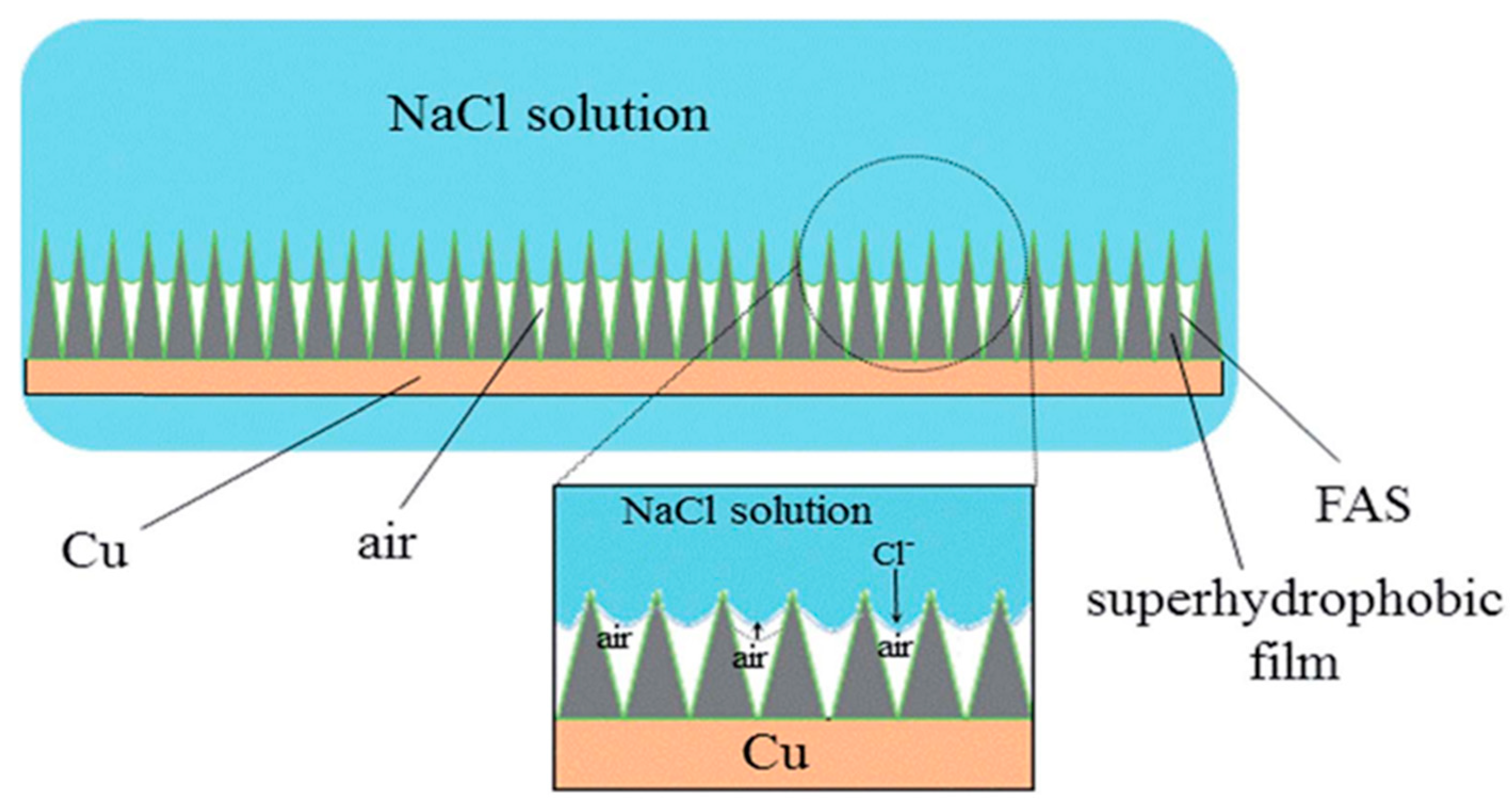
4.3. Bionic Superhydrophobic Surface
5. Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Wei, Y.; Li, L.; Zhang, J.; Chen, J. Same site, different corrosion phenomena caused by chloride: The effect of the archaeological context on bronzes from Sujialong Cemetery. China J. Cult. Herit. 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Quaranta, M.; Catelli, E.; Prati, S.; Sciutto, G.; Mazzeo, R. Chinese archaeological artefacts: Microstructure and corrosion behaviour of high-leaded bronzes. J. Cult. Herit. 2014, 15, 283–291. [Google Scholar] [CrossRef]
- Chiavari, C.; Martini, C.; Balbo, A.; Monticelli, C.; Velino, C.; Masi, G.; Bernardi, E. Atmospheric corrosion of Cu-Si-Mn bronze for contemporary art under simulated runoff and continuous immersion conditions. Corros. Sci. 2022, 205, 110442. [Google Scholar] [CrossRef]
- Liu, Y.W.; Yu, G.C.; Cao, G.W.; Wang, C.; Wang, Z.Y. Characterization of corrosion products formed on tin-bronze after 29 years of exposure to Shenyang, China. J. Mater. Res. Technol. 2023, 23, 5270–5279. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu-Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Lin, J.L.; Wang, Y.K.; Liu, S.R.; Qian, W. Evaluating accuracy and inter-laboratory reproducibility of the compositional analysis of ancient bronzes. AIA 2023, 4, 100027. [Google Scholar] [CrossRef]
- Fan, M.; Cui, Y.; Zhang, Z.; Liu, L.; Li, Q.; Luo, Q. Enhanced oxidation resistance in Mg-Y-Zn-Ho alloys via introducing dense low-oxygen-diffusion Ho2O3 oxide film. Corros. Sci. 2023, 213, 110970. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wu, N.; Liu, S.; Chen, J. A new application of fiber optics reflection spectroscopy (FORS): Identification of “bronze disease” induced corrosion products on ancient bronzes. J. Cult. Herit. 2021, 49, 19–27. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Rayner, A.J.; Bishop, D.P.; Nasiri, A. Perspectives on the unusual electrochemical corrosion of Nickel Aluminum Bronze (NAB) alloy fabricated through laser-powder bed fusion additive manufacturing. Corros. Sci. 2024, 228, 111846. [Google Scholar] [CrossRef]
- Bannister, C.O.; Newcomer, J.A. Examination of Bronze Implements. Nature 1925, 116, 786–789. [Google Scholar] [CrossRef]
- Silveira, P.; Falcade, T. Applications of energy dispersive X-ray fluorescence technique in metallic cultural heritage studies. J. Cult. Herit. 2022, 57, 243–255. [Google Scholar] [CrossRef]
- Augustyniak, A.; Ming, W.H. Early detection of aluminum corrosion via “turn-on” fluorescence in smart coatings. Prog. Org. Coat. 2011, 71, 406–412. [Google Scholar] [CrossRef]
- Ye, J.W.; Zhao, L.M.; Bogale, R.F.; Gao, Y.; Wang, X.X.; Qian, X.M.; Guo, S.; Zhao, J.Z.; Ning, G.L. Highly selective detection of 2,4,6-trinitrophenol and Cu2+ ions based on a fluorescent cadmium-pamoate metal-organic framework. Chem.-Eur. J. 2015, 21, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Y.; Tian, L.M.; Zhao, J.; Ren, L.Q. Colorimetric/fluorescent dual channel sensitive coating for early detection of copper alloy corrosion. Mater. Lett. 2020, 265, 127419. [Google Scholar] [CrossRef]
- Figueiredo, E.; Silva, R.J.C.; Fernandes, F.M.B.; Araújo, M.F. Some long term corrosion patterns in archaeological metal artefacts. MSF 2010, 636–637, 1030–1035. [Google Scholar] [CrossRef]
- Mabille, I.; Bertrand, A.; Sutter, E.M.M.; Fiaud, C. Mechanism of dissolution of a Cu–13Sn alloy in low aggressive conditions. Corros. Sci. 2003, 45, 855–866. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, H.; Mehta, V.; Welter, G.J.; Giammar, D.E. Impact of galvanic corrosion on lead release from aged lead service lines. Water. Res. 2012, 46, 5049–5060. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhou, H.R.; Song, J.L.; Fan, Z.L.; Zhang, L.M.; Shi, J.R.; Chen, J.C.; Xiao, K. Mechanism of corrosion behavior between Pb-rich phase and Cu-rich structure of high Sn–Pb bronze alloy in neutral salt spray environment. J. Mater. Res. Technol. 2024, 29, 881–896. [Google Scholar] [CrossRef]
- Ren, X.Y.; Shi, Z.H.; Zhang, G.W.; Chen, N.N.; Zhang, Y.G.; Guo, Z.M.; Hu, J.Z.; Xu, H. Strengthening mechanism of sub-nano Ni3P and Cu3P phases particles on the Pb–Sn bronze alloy. Heliyon 2024, 10, e34624. [Google Scholar] [CrossRef]
- Boccaccini, F.; Riccucci, C.; Messina, E.; Pascucci, M.; Bosi, F.; Chelazzi, D.; Guaragnone, T.; Baglioni, P.; Ingo, G.M.; Carlo, G.D. Reproducing bronze archaeological patinas through intentional burial: A comparison between short- and long-term interactions with soil. Heliyon 2023, 9, e19626. [Google Scholar] [CrossRef]
- Liang, Z.P.; Jiang, K.X.; Zhang, T.A. Corrosion behaviour of lead bronze from the Western Zhou Dynasty in an archaeological-soil medium. Corros. Sci. 2021, 191, 109721. [Google Scholar] [CrossRef]
- Oudbashi, O.; Emami, S.; Ahmadi, H.; Davami, P. Micro-stratigraphical investigation on corrosion layers in ancient Bronze artefacts by scanning electron microscopy energy dispersive spectrometry and optical microscopy. Herit. Sci. 2013, 1, 21. [Google Scholar] [CrossRef]
- Turo, F.D.; Coletti, F.; Vito, C.D. Investigations on alloy-burial environment interaction of archaeological bronze coins. Microchem. J. 2020, 157, 104882. [Google Scholar] [CrossRef]
- Said, M.; Nazeri, M.F.M.; Sharif, N.M.; Kheawhom, S.; Mohamad, A.A. Corrosion properties of Cu/Sn–3.0Ag–0.5Cu/Cu solder butt joints fabricated by conventional reflow and microwave hybrid heating. Corros. Sci. 2022, 208, 110641. [Google Scholar] [CrossRef]
- Amegroud, H.; Boudalia, M.; Elhawary, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M.A. Electropolymerized conducting polyaniline coating on nickel-aluminum bronze alloy for improved corrosion resistance in marine environment. Colloid. Surface A 2024, 691, 133909. [Google Scholar] [CrossRef]
- Novakovic, J.; Papadopoulou, O.; Vassiliou, P.; Filippaki, E.; Bassiakos, Y. Plasma reduction of bronze corrosion developed under long-term artificial ageing. Anal. Bioanal. Chem. 2009, 395, 2235–2244. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Niu, L.; Zhao, S.; Li, S.; Li, D. Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. J. Appl. Electrochem. 2002, 32, 65–72. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Wang, Y.; Cao, C.; Li, L. Research progress on copper corrosion protection and corrosion inhibitors. Mater. Protect. 2021, 54, 160–166. [Google Scholar]
- Han, Z.F.; Huang, X.; Chen, J.C.; Chen, J.Y. 2-Mercaptob enzimidazole compounded with the conventional sealer B72 for the protection of rusted bronze. J. Cult. Herit. 2024, 67, 42–52. [Google Scholar] [CrossRef]
- Jin, J.Y.; Yin, H.; Shi, N.Q.; Luo, H.X.; Li, X.; Li, H.Y. Efficient self-healing coatings embedded with polydopamine modified BTA@DMSNs for corrosion protection. Prog. Org. Coat. 2024, 191, 108426. [Google Scholar] [CrossRef]
- Messali, M.; Larouj, M.; Lgaz, H.; Rezki, N.; Al-Blewi, F.F.; Aouad, M.R.; Chaouiki, A.; Salghi, R.; Chung, I.M. A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: Experimental and computer simulations studies. J. Mol. Struct. 2018, 1168, 39–48. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, D.S.; Chauhan, S.S.; Singh, G.; Quraishi, M.A. Chemically modified expired Dapsone drug as environmentally benign corrosion inhibitor for mild steel in sulphuric acid useful for industrial pickling process. J. Mol. Liq. 2019, 286, 110903. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Wu, Y. The inhibition effect and mechanism of L-cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Wang, Y.; Tang, J.L.; Wang, Y.Y.; Wang, H. Molecular dynamic simulation and experimental investigation on the synergistic mechanism and synergistic effect of oleic acid imidazoline and l-cysteine corrosion inhibitors. Corros. Sci. 2021, 185, 109414. [Google Scholar] [CrossRef]
- Han, Z.F.; Huang, X.; Chen, J.C.; Chen, J.Y.; Zhou, H.R. Study on the compounding of cysteine modified Schiff base and decanoic acid as corrosion inhibitors for bronze with patina. Surf. Interfaces 2024, 46, 103996. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Dagdag, O.; Bouchti, M.E.; Nouneh, K.; Assouag, M.; Briche, S.; Zarrouk, A.; Elharfi, A. Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: Outlooks from experimental and computational investigations. J. Colloid. Interf. Sci. 2020, 574, 43–60. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.L.; Gu, H.B.; Colorado, H.A.; Wei, S.Y.; Guo, Z.H. Flame-Retardant Electrical Conductive Nanopolymers Based on Bisphenol F Epoxy Resin Reinforced with Nano Polyanilines. ACS Appl. Mater. Inter. 2013, 5, 898–910. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Diraki, A.; Omanovic, S. Smart PANI/epoxy anti-corrosive coating for protection of carbon steel in sea water. Prog. Org. Coat. 2022, 168, 106835. [Google Scholar] [CrossRef]
- Zhou, C.G.; Chen, Q.; Zhao, J.J.; Wang, S.H.; Li, J.Y.; Ai, L.Q.; Li, T.Z.; Hu, C.B. Superhydrophobic epoxy resin coating with composite nanostructures for metal protection. Mater. Today. Commun. 2024, 38, 107803. [Google Scholar] [CrossRef]
- Chen, Z.R.; Liu, X.H.; Chen, H.N.; Li, J.L.; Wang, X.F.; Zhu, J.F. Application of epoxy resin in cultural relics protection. Chin. Chem. Lett. 2024, 35, 109194. [Google Scholar] [CrossRef]
- Jerman, I.; Vuk, A.Š.; Koželj, M.; Orel, B.; Kovac, J. A Structural and Corrosion Study of Triethoxysilyl Functionalized POSS Coatings on AA 2024 Alloy. Langmuir 2008, 24, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sue, H.J.; Thompson, Z.J.; Bates, F.S.; Dettloff, M.; Jacob, G.; Verghese, N.; Pham, H. Nanocavitation in Self-Assembled Amphiphilic Block Copolymer-Modified Epoxy. Macromolecules 2008, 41, 7616–7624. [Google Scholar] [CrossRef]
- Pang, V.; Thompson, Z.J.; Joly, G.D.; Francis, L.F.; Bates, F.S. Block Copolymer and Nanosilica-Modified Epoxy Nanocomposites. ACS Appl. Polym. Mater. 2021, 3, 4156–4167. [Google Scholar] [CrossRef]
- Briffa, S.M.; Vella, D.A. The behaviour of as-applied and artificially weathered silica–epoxy consolidants on a typical Mediterranean porous limestone: A comparison with TEOS. Herit. Sci. 2019, 7, 30. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Xiao, F.; Yuan, S.J.; Liang, B.; Li, G.Q.; Pehkonenc, S.O.; Zhang, T.J. Superhydrophobic CuO nanoneedle-covered copper surfaces for anticorrosion. J. Mater. Chem. A 2015, 3, 4374. [Google Scholar] [CrossRef]
- Ueda, E.; Levkin, P.A. Emerging Applications of Superhydrophilic-Superhydrophobic Micropatterns. Adv. Mater. 2013, 25, 1234–1247. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.Y.; Zhao, X.Z.; Shen, Z.; Wen, M.; Zhao, C.C.; Sheng, L.Y.; Wang, Y.G.; Xu, D.K.; Zheng, Y.F.; et al. Superhydrophobic surface on MAO-processed AZ31B alloy with zinc phosphate nanoflower arrays for excellent corrosion resistance in salt and acidic environments. Mater. Design. 2024, 239, 112769. [Google Scholar] [CrossRef]
- Fu, X.; He, X. Fabrication of super-hydrophobic surfaces on aluminum alloy substrates. Appl. Surf. Sci. 2008, 255, 1776–1781. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Yu, X.; Liu, H.; Fu, Y.; Wang, Z.; Jiang, L.; Li, X. Polyelectrolyte multilayer as matrix for electrochemical deposition of gold clusters: Toward superhydrophobic surface. J. Am. Chem. Soc. 2004, 126, 3064. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.T.; Shen, Z.Q. Fabrication of Superhydrophobic Surfaces by Dislocation-Selective Chemical Etching on Aluminum, Copper, and Zinc Substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.Z.; Guo, Y.; Zhang, Z.; Ren, L. Fabrication of super-hydrophobic nano-sized copper films by electroless plating. Thin Solid Films 2010, 518, 3731–3734. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.G.; Fu, H.Y.; Yang, S.N.; Zhu, Y.M.; Yue, H.R.; Jiang, W.; Liang, B. A stable eco-friendly superhydrophobic/superoleophilic copper mesh fabricated by one-step immersion for efficient oil/water separation. Surf. Coat. Tech. 2019, 359, 108–116. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically stable and corrosion resistant superhydrophobic sol gel coatings on copper substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, C.; Guo, Y. Corrosion and Protection of Chinese Bronze Relics: A Review. Coatings 2024, 14, 1196. https://doi.org/10.3390/coatings14091196
Zhang L, Yang C, Guo Y. Corrosion and Protection of Chinese Bronze Relics: A Review. Coatings. 2024; 14(9):1196. https://doi.org/10.3390/coatings14091196
Chicago/Turabian StyleZhang, Lingling, Chao Yang, and Yingzhi Guo. 2024. "Corrosion and Protection of Chinese Bronze Relics: A Review" Coatings 14, no. 9: 1196. https://doi.org/10.3390/coatings14091196
APA StyleZhang, L., Yang, C., & Guo, Y. (2024). Corrosion and Protection of Chinese Bronze Relics: A Review. Coatings, 14(9), 1196. https://doi.org/10.3390/coatings14091196


.jpg)


