Abstract
In-demand fresh-cut fruits are highly perishable and require shelf stability. Starch, such as sago, is a naturally available polysaccharide with good gas barrier properties. The study aimed to develop an edible coating and examine the effect of its application on the quality parameters of fresh-cut apples. The coating solution was prepared with sago and soy oil in concentrations of 3, 4, and 5% and 0, 0.25, and 0.50%, respectively. Lecithin (0.50%) was used as an emulsifier and glycerol (1.5%) as a plasticizer. Coated fresh-cut apples were evaluated for physicochemical properties (TSS, pH, non-enzymatic browning index, moisture content, weight loss, headspace gas, and color attributes) during a 12-day shelf-life study at 4 °C. Sensory analysis was also performed to assess consumer acceptability, and microbial analysis to investigate its inhibiting effect against yeast and mold. Compared to the control, developed coatings reduced browning, respiration rate, moisture, weight loss, and microbial load in fresh-cut apples. The study indicates that the blend of 5% sago and 0.5% soy oil produced the best coatings which were most effective for retaining the original quality attributes and in extending the shelf life of apple slices for 12 days in storage at 4 °C.
1. Introduction
In recent years, there has been a significant increase in the consumption of fresh-cut fruits and vegetables. This trend is attributed to the low caloric content of these foods, which makes them a key element of a healthy, balanced diet [1]. Major factors driving this demand include heightened health awareness, busy lifestyles, and increased consumer purchasing power, all of which contribute to the growing preference for minimally processed foods [2]. As consumers become more aware of the advantages of healthy eating and have less time for food preparation, the production and economic value of fresh-cut fruits have become crucial for food processors [3,4].
Fresh-cut fruit, despite its nutritional benefits, is inherently delicate. Peeling or cutting the fruit can cause moisture and nutrient loss, accelerate enzyme activity, lead to changes in color and texture, promote microbial growth, and result in weight loss, all of which contribute to a decrease in product quality [4]. Various methods have been developed to mitigate these issues, including low-temperature storage, high relative humidity, and modified atmosphere packaging. Key quality factors for marketable fresh produce include appearance, texture, color, flavor, nutritional value, and microbial safety. Although minimal processing can shorten the shelf life of fresh produce, further research is needed to evaluate the effectiveness of postharvest techniques, such as modified atmosphere packaging and dipping treatments, in delaying softening and browning [5].
Apple, a pomaceous fruit from the apple tree Malus domestica of the rose family (Rosaceae), is widely cultivated and valued for its taste, flavor, and juiciness. However, its shelf life can be diminished by factors such as color and textural changes, juice leakage, off-flavors, enzymatic browning, moisture loss, appearance issues, and microbial growth [6,7,8]. Secondary processing can significantly reduce firmness in fruit tissues due to the enzymatic breakdown of cell walls, which are primarily made of pectin, cellulose, and hemicellulose [9]. To address these issues, texture enhancers, like calcium salts, can be incorporated into edible coatings to manage softening and reduce moisture loss in fresh-cut fruit [10]. Edible coatings create a barrier on the fruit surface, lowering the water vapor transmission rate, and thus limiting moisture loss and texture deterioration [11].
Edible coatings offer a practical method for extending the shelf life of fresh-cut fruit by acting as a semi-permeable barrier to water vapor and gases, which helps maintain the fruit’s structural integrity [12]. Additionally, these coatings can deliver active compounds, such as antimicrobial agents to prevent spoilage and control pathogenic microorganisms [13]. While edible coatings provide several benefits over other preservation methods, their effectiveness depends on appropriate storage conditions. Enhancing the quality of coatings by adjusting their composition and incorporating food additives can improve their performance. However, minimal processing techniques, like peeling, cutting, and slicing, can disrupt tissue integrity and cellular metabolism, making fresh produce more susceptible to spoilage [14,15]. Consequently, maintaining the quality of fresh produce remains a significant challenge for the food industry. The growing recognition of the sago palm’s significance has led to considerable research on sago starch [16,17]. Sago starch has emerged as a promising alternative for producing edible films due to its low production cost and high yield compared to other starch types. There is a notable link between amylose content and the film-forming capacity of starch solutions. Starches with higher amylose content (about 30% amylose) exhibit better film-forming properties than those with lower amylose content [18,19]. It is reported that sago starch contains 27%–35% amylose, making it suitable for creating effective biodegradable films. Additionally, sago starch offers excellent oxygen barrier properties and high mechanical strength. Thus, the relatively high amylose concentration in sago starch makes it a viable raw material for edible film production [19].
This research aimed to develop an edible coating and evaluate its impact on the quality of fresh-cut apples during storage, focusing on aspects such as color, sensory attributes, weight loss, total soluble solids, pH, microbial growth, and respiration rate. Post-cutting, fruits undergo enzymatic and non-enzymatic browning, which can diminish market acceptability [20]. To address these issues, composite coatings incorporating sago, soy oil, glycerol, and lecithin were created. Sago-based coatings are advantageous due to their low cost and safety for consumption. Additionally, such coatings help preserve nutritional value, maintain quality, and extend the shelf life of fresh-cut apples. This study aims to assess how the quality of fresh-cut apples is maintained over 12 days of storage at 4 °C, compare the effectiveness of chemical treatments and edible coatings, and identify the optimal formulation for use in dipping treatments.
2. Materials and Methods
2.1. Materials
In this study, the Red Delicious variety of apples was used along with food grade sago and soy oil. Apples of uniform size and shape with an average weight of 104 g were selected for the study. The sago was processed into a powder for use in coating preparation. Glycerol (Moly Chemicals, Mumbai, India) and lecithin (Acro Organics, New Jersey, USA) were utilized in making the edible coatings. For the pretreatment process, calcium chloride (Qualiken Fine Chemicals Pvt Ltd., Delhi, India) and ascorbic acid (Central Drug House, Delhi, India) were employed. PET (polyethylene terephthalate) trays, used for storing the cut apples, and 20 µm polyethylene foil, used for covering the trays, were sourced from a packaging store.
2.2. Methods
2.2.1. Preparation of Coating Solutions
The coating was based on sago and soy oil as the major constituents, whereas glycerol and lecithin act as the plasticizer and emulsifier. The formulation was developed based on preliminary trials. The composition of the sago (3, 4, 5%) and soy oil (0, 0.25, 0.50%) was varied while maintaining a fixed composition of lecithin (0.50%) and glycerol (1.50%) in the water solution. A total of nine coatings were developed, as shown in Table 1. The solutions were heated to 70 °C with continuous stirring followed by homogenization at 10,000 rpm. The solutions were brought to room temperature before applying the apple slices as a coating

Table 1.
Combinations of sago powder and oil are used to prepare coating solutions.
2.2.2. Apple Processing and Application of Coating
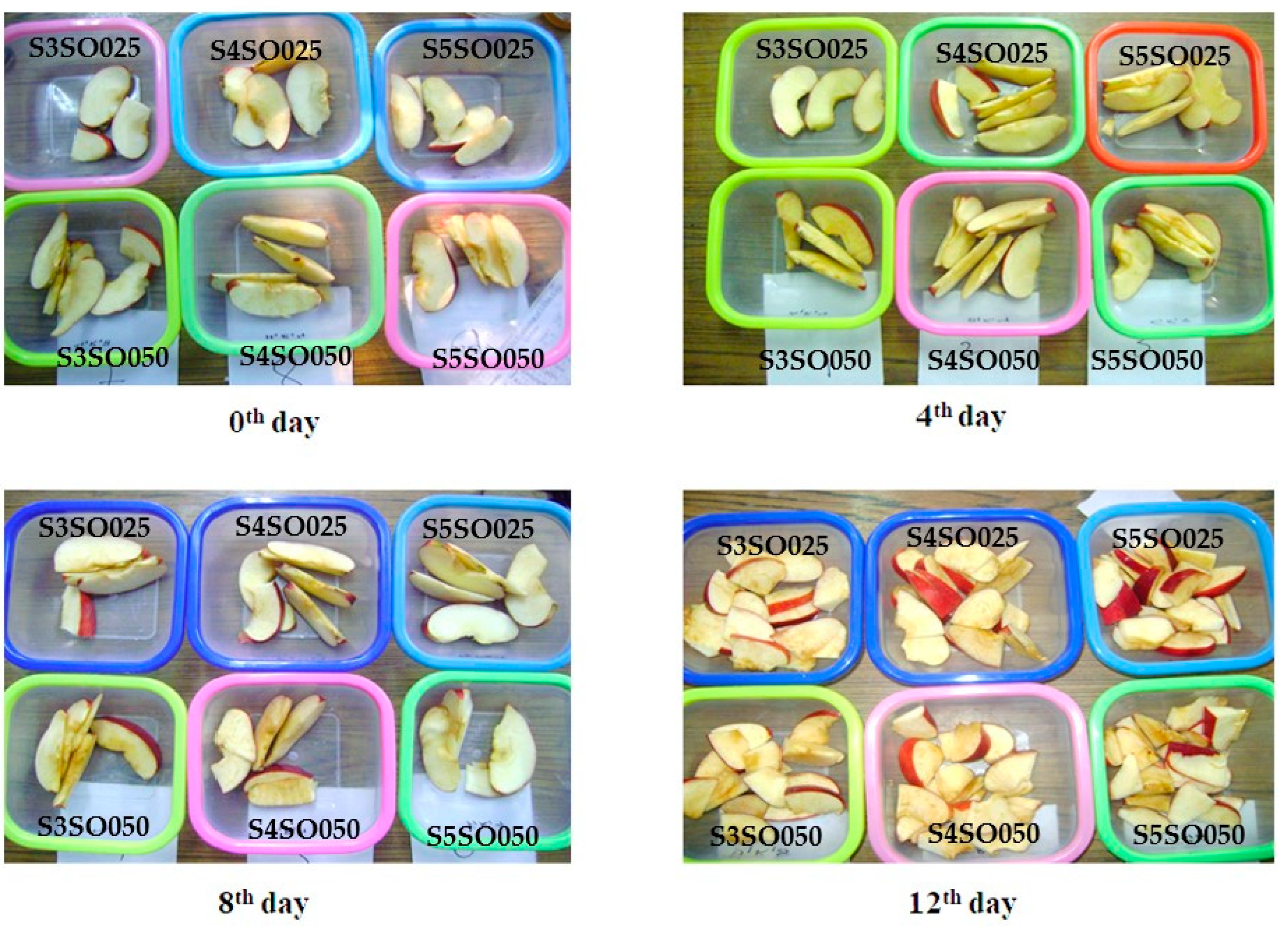
Freshly harvested and firm apples were screened for bruises and cuts, and the best among them were considered for the study. The apples were rinsed and cleaned with distilled water and then cut into equal-sized slices of approximately 2 cm using a sterile slicer. These pieces were then pretreated with the 2% calcium chloride and 2% ascorbic acid solutions sequentially for 2 min each. Afterward, these pretreated samples were dipped in their respective coating solutions for 3–5 min and strained and left on the tray for straining excessive coating solution. The coated apple pieces were then weighed and packed in the PET containers, covered with polyethylene film, and marked as per their coating and storage time (0, 4, 8, and 12 days). The control sample was also prepared for comparison. For each coating solution, 4 containers were made with 3 replicates (Figure 1). All the packs were kept at 4 °C for the whole study. The samples were drawn every fourth day for analysis.

Figure 1.
Packaged coated and control samples.
2.3. Physicochemical Analysis
2.3.1. pH
pH was determined by following the AOAC method 981.12 [21]. A piece of apple was sliced and homogenized in a grinder. A total of 10 g of ground apple was mixed and filtered in 100 mL of distilled water. The pH of the filtered solution was measured using a pH meter (Model pH-1500, Eutech Instruments, Singapore).
2.3.2. Total Soluble Solids (TSSs)
Total soluble solids (TSSs) content was determined using a method described by [22]. Apple slices from each treatment were ground in an electric juice extractor to obtain juice filtered through Whatman No. 1 filter paper. The soluble solids content was measured using a hand Refractometer (Metzer Optical Instruments, Mathura, India) in Brix at 20 °C.
2.3.3. Non-Enzymatic Browning Index (NEBI)
The browning index of the samples was assessed using the method outlined by [23]. In this procedure, 5 mL of 95% ethyl alcohol was mixed with 5 mL of apple juice and then centrifuged at 5000 rpm for 10 min using a Remi centrifuge. The resulting supernatant was filtered through Whatman No.1 filter paper and its absorbance was measured at 420 nm with a Spectronic 20D+ digital spectrophotometer (USA). This absorbance value was used to determine the non-enzymatic browning index (NEBI) [24].
2.3.4. Moisture Content
The moisture content of apple slices was determined using the hot air oven method [21]. In this method, the initial weight of the samples was measured, and the sample was crushed into small pieces and placed in low-bottomed Petri dishes. The Petri dishes containing the samples were placed in a hot air oven (Yorco, UP, India) and maintained at 70 °C for 16–18 h for drying until the weight became constant. Petri dishes were then taken out from the oven, covered with their lids placed in the desiccators, and allowed to cool before weighing. The moisture content was calculated using the formula:
where = Initial sample weight before drying; = Final sample weight after drying.
2.3.5. Weight Loss
Weight loss of apple slices was calculated by a method followed by [25]. In this method, apple pieces from each coating treatment and container were taken and weighed on a weighing balance (PGB 300, Wensar, Bengaluru, India) having a capacity of 300 g and a least count of 200 mg. Each sample was weighed on the 0th day and after an interval of four days. The percentage weight loss for each treatment was calculated using the corresponding weight of the sample on day 0 and determined by the following formula:
where = Initial weight of the sample at the 0th day; = weight of the sample after storage on a particular day of study.
2.3.6. Headspace Analysis
The package headspace gas composition was examined using a gas analyzer (Gaspace Advance, Systech Instruments, Thame, UK) integrated with a thermal conductivity detector as per the method followed by [26]. The gas concentration was determined by inserting the equipment needle into the fruit case through a septum attached to the packaging surface for about 60 s or until the appropriate amount of gas was sucked in. After a few seconds, the percentage of gas was displayed on the screen, indicating the oxygen and carbon dioxide percentage in the case’s headspace. Three trays per treatment were analyzed as fresh and after 4, 8, and 12 days of storage. The gas concentration in the packages was measured to see whether the packaging, which was used to protect the samples, altered the surrounding atmosphere of the product [27].
2.3.7. Color Analysis
The color values (L*, a*, b*) of the fruit were measured using a Hunter Lab Mini Scan XE Plus (45/0-L model) from Hunter Associates Laboratory Inc., Reston, VA, USA. The instrument was calibrated with standard black and white plates and configured for illuminant D65 and a 10° observer angle. Color measurements were taken using the CIE L*, a*, b* scale, where L* represents lightness, a* indicates chromaticity on a green (−) to red (+) axis, and b* denotes chromaticity on a blue (−) to yellow (+) axis.
The browning index (BI) was calculated to evaluate the intensity of the brown color, as described by [28].
where
Browning of the apple slices was also investigated by conversion of L*, a*, and b* values into whiteness index (WI) [7] using the following formula:
2.4. Sensory Analysis
Sensory evaluations were conducted every fourth day during storage to assess color, texture, taste, aroma, juiciness, and overall acceptability of both coated and uncoated apples. The sensory panel consisted of 12 semi-trained individuals, including research scholars and staff members. A semi-trained panelist in sensory analysis is familiar with a product and can distinguish differences but may not have formal training. Sensory analysis of food involves evaluating food attributes using human senses and requires controlled conditions for accurate results. The room considered for this had neutral lighting, a stable temperature (25 °C), good ventilation, minimal noise, and cleanliness. Samples were uniformly prepared and served at the correct temperature in neutral containers. To neutralize sensory effects between samples, panelists used palate cleansers, like water or plain crackers, and had short breaks to reset their senses. Panelists were provided with coded samples and asked to rate the sensory parameters. Various attributes were assessed using a 9-point Hedonic scale: 9 for “like extremely”, 8 for “like very much”, 7 for “like moderately”, 6 for “like slightly”, 5 for “neither like nor dislike”, 4 for “dislike slightly”, 3 for “dislike moderately” 2 for “dislike very much”, and 1 for “dislike extremely” [29].
2.5. Microbial Analysis
Yeast and mold count, among other microbiological characteristics, were assessed while the product was being stored. Using serial dilution, apple slices from each container were analyzed to determine the microbial burden [27]. Potato dextrose agar and agar were used to produce the medium (Hi-Media Ltd., Mumbai, India). To maintain a sterile environment, hot media were put into the Petri plates near the flame. For serial dilution, 1 mL of the sample was taken and put into test tubes along with 9 mL of regular saline solution. The sample was labeled 10−1. A cyclomixer was used to homogenize the sample-containing test tubes. Using a micropipette, 1 mL of the material was homogenized and transferred from a 10−1 test tube to a 10−2 test tube for each successive dilution. Repeat this process with each test tube that is left. To initiate the inoculation process for every dilution, a standard saline sample was aseptically transferred, using a micropipette (0.1) from the test tube labeled 10−1, to a Petri dish with nutrient agar media labeled 10−1. As a control plate, a Petri dish containing nutrient agar media was maintained without inoculation. In a B.O.D. incubator, Petri dishes were inoculated and then kept at 25 °C. The colonies were counted after 5 days of inoculation. The product’s logarithm of colony-forming units per gram (CFU/g) was used to calculate the results.
Yeast and Mold count (log cfu/g) = No. of colonies × Dilution factor × 10
2.6. Statistical Analysis
A factorial design was employed to evaluate the impact of independent variables—sago concentration, oil concentration in the coating solutions, and storage duration—on the quality parameters of fresh-cut apple slices. Analysis was conducted using a three-way ANOVA (Analysis of Variance) implemented in Microsoft Office Excel. Data were reported as means ± standard deviation (SD), with statistical significance determined at 5% and 1% confidence levels.
3. Results and Discussion
The edible coating made from sago and soy oil effectively preserves the quality of fresh-cut apples over 12 days of refrigerated storage. Figure 2 illustrates the condition of the coated apples on the 0th, 4th, 8th, and 12th days of storage. This section discusses the evaluation of various quality parameters of the fresh-cut apples throughout the storage period.
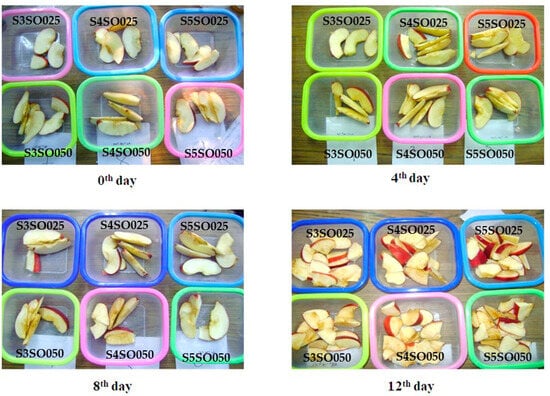
Figure 2.
Coated fresh-cut apple slices during 12 days of storage.
3.1. pH
During storage for 12 days, all samples, including the control, showed an increasing pH trend (Table 2), between 4.226 and 4.941. A drop in titrable acidity may correlate with a rise in pH. The starch coating on the apple slices may have changed the endogenous CO2 and O2 concentrations, which led to the utilization of organic acid as a respiration substrate and an elevation in pH [30]. Similar findings were observed by [31] for fresh-cut pineapples coated with honey and soy protein isolate-based coatings. Increased pH has been associated with a decrease in malic acid due to increased respiration rate after peeling and cutting. The increase in pH was found to be more pronounced in coated samples than in control, probably due to less dependence on organic acids for respiration in control samples, which further strengthens the above-cited factor. This result showed that the changes in pH during storage and the effects of different coating treatments were different. The storage effect was significant at both the 5% and 1% confidence levels, according to the ANOVA results but, for sample S3SO025 storage days 4 and 8, for sample S4SO025 storage days 4 and 8, for sample S4SO050 storage days 8 and 12, and control sample S0SO000 storage days 4 and 8, the results seemed to show no significant differences between them. Additionally, it was demonstrated that the combined effect of all independent parameters, oil–sago, oil-storage duration, sago-storage period, and combinations of these sources, was significant at both confidence levels. Because starches have better gas barrier properties than oil [32,33], they have a relatively more pronounced effect on the modified internal atmosphere at the cellular level. This is likely to be why the effect of sago concentration has a higher F value than that of oil.

Table 2.
Effect of coating treatments and storage days on pH, TSS, and non-enzymatic browning index (NEBI) of fresh-cut apples.
3.2. Total Soluble Solids (TSSs)
Up to 4 days of storage, the TSSs remained stable in most of the samples; however, they increased during further storage except in those treated with only 3% sago solution and control. During 12 days of storage, an increase in mean values of TSSs from 11 to 14.5° Brix was observed in apple slices coated with sago and oil-based edible coating solutions (Table 2). Refs. [26,34] have also reported an increase in TSSs of chitosan-coated mangoes and psyllium gum-coated fresh-cut papaya during storage. The effect of the parameter of storage on TSSs was most significant, followed by the parameter of oil. TSSs significantly increased as sago and oil levels rose. This sample that was coated with 0.5% oil and 4% sago solution showed the greatest increase in TSSs. Even after 12 days of storage, there was no difference in TSSs between the sample coated with 3% sago and the control. Total soluble solids (TSSs) in living tissues can be influenced by several variables, including moisture content loss, which can result in a rise in TSSs. Other factors impacting the TSSs include variations in respiration rate and metabolic changes, such as the conversion of protopectin into soluble pectin [35]. TSSs concentration in fruit increases during storage due to the breakdown of cell wall polysaccharides and the hydrolysis of starch into sugars [36]. A faster respiration rate that would have counteracted the effects of moisture loss and soluble pectin formation could be the reason for the lack of apparent change in the TSSs of the control sample. At 5% and 1% levels of confidence, the impact of oil was found to have a greater significance on TSSs than that of storage. It was discovered that there was considerable interaction between the two and all three components, as well as the individual effects of sago.
3.3. Non-Enzymatic Browning Index
All the samples, including the control, showed an increase in the Non-Enzymatic Browning Index during the storage period of 12 days. (Table 2). Browning was considerably decreased by dipping apple slices in ascorbic acid (2%) before coating as ascorbic acid is an antioxidant. Browning started to showcase, mostly in the control slices, and it became more pronounced towards the conclusion of the storage time. Apple slices preserved their fresh appearance after being coated with a coating solution and ascorbic acid. The samples treated with the 0.5% oil solution had the least amount of browning overall. The ANOVA clearly showed that, at both the 5% and 1% confidence levels, the effects of sago and the interaction between sago and storage period on the non-enzymatic browning index were not significant. The remaining variables had a major impact on the NEBI. The storage period had the most pronounced effect on the non-enzymatic browning index, followed by oil concentration. It was also observed that the replication had a significant effect, indicating that variation in the non-enzymatic browning index occurred within replicates, which could be attributed to fruit individuality.
The Non-Enzymatic Browning Index (NEBI) of apple samples is significantly impacted by the application of coatings based on sago and soy oil. By limiting moisture loss and decreasing water activity, which slows down browning reactions, sago-based coatings can lower NEBI values. However, if the oil stays stable, soy oil coatings offer a lipid barrier that can lessen exposure to oxygen and moisture and, thus, lessen browning. On the other hand, if soy oil oxidation is not controlled, it can result in more browning. Coatings made of sago may function as barriers to the movement of moisture. Reduced NEBI values are typically the consequence of slower browning rates caused by reduced moisture levels [37]. Sago-based coatings have the potential to lessen the amount of non-enzymatic browning on apples by binding water and reducing the water activity of the apple surface. Sago starch’s gel-like consistency has the potential to create a barrier that affects heat dispersion and thermal conductivity while being stored. This may have an impact on how quickly browning reactions take place. Further influencing NEBI levels, the protective layer may lessen the effects of temperature changes and oxygen exposure [38]. Rich in lipids, soy oil coatings can also have a major impact on apple sample NEBI levels over storage. Apples coated with soy oil can be shielded from moisture and oxygen by a lipid barrier. This barrier has the potential to lessen the rate of non-enzymatic browning by slowing down oxidation reactions. However, the thickness of the coating and the makeup of the lipids determine how effective this barrier is [39]. Oxidation is a common problem with soy oil, particularly when it is stored near heat sources and lights. Free radicals produced by oxidized oil have the potential to worsen browning reactions. Consequently, oxidized soy oil may hasten browning while fresh soy oil can assist in preventing it, resulting in greater NEBI values [40].
3.4. Moisture Content
The initial moisture content in samples was 86.1%. The water retention was much better in coated samples, while moisture loss can be seen in control samples. During the experimental period, the moisture content of both coated and uncoated samples decreased, but the rate of decrease was decelerated by the coating (Table 3). In the case of samples coated with 0.5% oil and 5% sago solution, the weight loss was negligible. It is also significant that almost all coating showed the same result for moisture content. At the end of storage (12 days), the moisture content ranged between 86% and 81.25%, with the coated samples having the higher values. In the control case, a significant amount of moisture was lost during storage, which was evident on the last day. As the moisture content decreased, the texture of the apple slices was also lost as the samples turned soft or wilted. As per the results, it was observed that the coated samples were firm, as the moisture content was still very good until the last day of the study, but shrinkage in apple slices was seen in the case of control. The sago-based coating was successful in reducing moisture loss in apple slices. The addition of oil to the sago coating mixture resulted in a significant improvement in the moisture content of the sample. The decrease in moisture during storage could be due to evaporative losses and physiological factors, especially due to respiration. The ANOVA showed that only the storage period significantly affected moisture content. The combined effect of all three parameters was significant only at a 5% confidence level, while the effect of other sources on moisture content was insignificant. It is also to be mentioned here that, in the sago-coated samples which are free of oil, the moisture content starts to decrease first till the 8th day of storing but, by the end of the 12th day, the moisture has increased, which may be attributed to the change in texture and the gain in moisture from the atmosphere, as there was no oil layer coating to stop the absorption of moisture.

Table 3.
Effect of coating treatments and storage days on moisture content, weight loss, and headspace concentration (O2 and CO2) of fresh-cut apples.
The moisture content of coated apple slices after 12 days has been seen to be significantly affected by the usage of coatings based on sago and soy oil. Sago-based coatings work largely as a moisture barrier when applied to apple slices. Sago-coated apple slices tend to hold moisture better than uncoated ones throughout 12 days. This is explained by the development of a layer that resembles gel and reduces water loss. Sago starch coatings, according to the research of [41], assist retain texture and minimize moisture loss by delaying the evaporation process and forming a semi-permeable surface [41]. Though sago-based coatings do offer a protective barrier, their efficacy may wane with time as a result of possible coating layer breakdown or interactions with the fruit’s natural enzymes. While sago coatings initially prevent moisture loss, [12] pointed out that their effectiveness can vary depending on ambient factors, including humidity and temperature [12]. Another coating substance used to extend the shelf life of apple slices is soy oil, which is well known for its calming effects. The mechanism by which coatings based on soy oil decrease moisture migration and slow down dehydration is known as a hydrophobic barrier. When compared to uncoated apple slices, apple slices coated with soy oil typically show less moisture loss for 12 days. According to research by [42] soy oil coatings work well to reduce moisture loss because of their lipid content, which creates a barrier that protects the apple slices [42]. Because soy oil coatings form a flexible, protective layer rather than a solid film, like sago-based coatings, they also have the advantage of being less vulnerable to mechanical harm. This can be very helpful in preserving the quality of apple slices while they are being handled and stored. However, [7] point out that, although soy oil coatings are good at retaining moisture, their effectiveness might vary depending on the thickness of the coating and the particular formulation applied. Overall, sago and soy-oil-based coatings offer notable advantages, each having its own, in terms of maintaining the moisture content of apple slices. Numerous variables, such as the coatings’ particular formulation and the surrounding environment, might affect how effective these coatings are.
3.5. Weight Loss
During the storage period, weight loss occurred in all samples but was comparatively less in coated samples compared to control samples. (Table 3). The effect of sago and oil on weight loss was significant. Among the coated samples, weight loss was found to have decreased with an increase in sago concentration, but no definite effect of oil concentration on weight loss was observed; however, in many cases, weight loss increased with an increase in oil concentration, which may be attributed to physiological weight loss accompanied by water loss. Physiological weight loss usually occurs due to loss in respiration substrates utilized by living tissues to accomplish respiration. Weight loss occurs during fruit storage because of the respiratory process, humidity transfer and, in a few cases, oxidation [31]. Sago, being a starch, offers better oxygen resistance, lowering respiration, eventually leading to less weight loss. In contrast, oil does not offer as much resistance to oxygen as starch. Researchers found a similar weight loss in fuji apples stored at 4 °C for 14 days. Furthermore, despite the increased transpiration rate through the peeled surface, the closed plastic boxes used for sample storage created a saturated or nearly saturated atmosphere in water vapor, minimizing water loss [43].
The control samples experienced significantly higher weight loss (3.18 ± 0.08) than the coated samples. Edible coatings can effectively minimize water loss in fresh-cut fruit [44]. For instance, research indicated that gellan and sodium alginate-based coatings, when combined with sunflower oil, effectively reduced water loss in fresh-cut apples [45]. Researchers observed a 19% reduction in weight loss in Palmer mangoes coated with hydroxypropyl methylcellulose and beeswax compared to uncoated fruit after 15 days of storage [46]. Another study reported a 42% reduction in weight loss in sweet cherries coated with an aloe vera-based solution after 16 days at 1 °C [47]. Similarly, researchers found a significant difference in weight loss between coated and uncoated papayas stored in PP bags at 5 °C for 15 days [27]. The results of this study confirm that the sago and oil-coated samples experienced less weight loss than the control. ANOVA indicated that only the storage period had a significant effect on weight loss, with no substantial impact from other factors within the treated samples, while the control samples showed notable weight loss during storage.
3.6. Headspace Analysis
The coatings had a notable effect on the gas composition within the package headspace of coated fresh-cut apple slices during storage. The oxygen (O2) concentration in the headspace of coated apple slices decreased slightly, while a more substantial decrease of 13.85% was observed in uncoated samples. Additionally, carbon dioxide (CO2) levels remained stable in samples with high oil concentrations but increased significantly in other samples. These findings suggest that the rate of CO2 production and O2 consumption is significantly influenced by the high oil content in the sago coating (Table 3). After harvest, fruits continue to respire through their skin, and cutting accelerates this process, altering the fruit’s flavor and aroma compounds [48]. Edible coatings can modify the atmosphere around the fruit by acting as a barrier to oxygen, carbon dioxide, and water vapor, thus reducing the respiration rate [49]. Research on fresh-cut pear packages showed a 5%–8% reduction in O2 levels and a 15%–20% increase in CO2 concentrations due to coating effects. However, according to a study, coatings with selective permeability to gases can reduce the exchange of O2 and CO2 between coated fruit and the environment, reducing metabolism by decreasing internal O2 concentration and increasing CO2 [3]. It is important to mention here that the coatings produced a substantial rate reduction in the gas, compared to uncoated cut apples. This suggests that the diffusion of the headspace O2 to the tissue was inhibited by the high O2 resistance of the coating. Peeling and cutting cause physical damage or wounding, which increases respiration rate within minutes, so lowering respiration rate is critical for extending the shelf life of minimally processed fruit.
The ANOVA for the effect of coating on O2 concentration indicates that all independent parameters, and their two-way and three-way interaction, significantly affected O2 concentration in the headspace. Of all sources, the storage period was affected most significantly, as evidenced by its highest F value, followed by oil concentration in the treatment solution. The ANOVA for the effect of coating on CO2 concentration indicates that the individual effect of all independent parameters was significant at 5% and 1% confidence levels, and the interactive effect of oil and storage and all the independent parameters (i.e., three-way interaction) on CO2 concentration was significant.
3.7. Browning Index (BI) and Whiteness Index (WI)
The color changes in fresh-cut apples were assessed by measuring L* (lightness), a* (redness), b* (yellowness), browning index (BI), and whiteness index (WI) over 12 days of storage at 4 °C. An increase in enzymatic browning of apple slices was associated with higher a* and b* values and lower lightness (L*). The Browning index, calculated as part of this study, was considered a key indicator of color change throughout storage. Compared to control samples, all coatings significantly reduced the BI (Table 4). Among the coated and uncoated samples, those with 0.5% oil and various sago combinations exhibited the smallest rise in the browning index and the smallest reduction in the whiteness index. Additionally, apple slices coated with oil and sago showed an improved L* value. The sago and oil coatings protected the fruit as the barrier for the fruit, thereby reducing its respiration (in terms of headspace analysis) and transpiration rates (in terms of moisture loss), as discussed above in the sections on headspace analysis and moisture loss. The L* value, which indicates color on the light–dark axis, declined with storage time for all samples, signaling that the fresh-cut fruit was becoming duller. Overall, the coatings were more effective at preserving the lightness of the fruit.

Table 4.
Effect of coating treatments and storage days on browning index, whiteness index, and yeast and mold count (log cfu/g) of fresh-cut apples.
Browning reactions are linked to the oxidation of phenolic compounds, which produce o-quinones that eventually form brown pigments [50]. Ascorbic acid and some of its derivatives can inhibit browning by reducing the quinone complex; however, they are unstable and prone to oxidation [51]. Applying a coating to apple slices significantly slows down browning. This preservative effect is likely due to the presence of calcium chloride, which inhibits polyphenol oxidase (PPO) through interaction with copper [52], as well as to the coatings’ role as oxygen barrier, necessary for browning reactions. Studies have shown that apple slices coated with carrageenan and whey protein-based films containing calcium chloride and anti-browning agents effectively preserve both texture and color [53,54]. ANOVA showed that the effect of the storage period on the browning index was most pronounced, followed by the combined effect of all the three independent parameters, significant at both 5% and 1% level of confidence, but that of oil was only slight, with significance at only 5% level of confidence. The effect of other sources on the browning index was found to be insignificant.
The increment in surface browning of the apples was accompanied by a decrease in WI. Table 4 shows the trend of the whiteness index on coated apples. Other experimental results obtained with various apple cultivars corroborated the findings [8,54,55]. The effect of the storage period on the whiteness index was most pronounced, followed by that of oil. The interactive effect of two and three parameters was significant at both 5% and 1% confidence levels. In contrast, the individual effect of sago was only significant at a 5% confidence level.
It is pertinent to mention here that the overall browning index was reduced by coating the apples with sago and oil-based coating but some results have shown a higher browning index than the control sample at different storage days, which may have been caused if the coating is too thick or unevenly applied, possibly creating a microenvironment that traps moisture against the apple’s surface, potentially leading to increased enzymatic activity and browning, which may also further effect the whiteness index.
3.8. Sensory Analysis
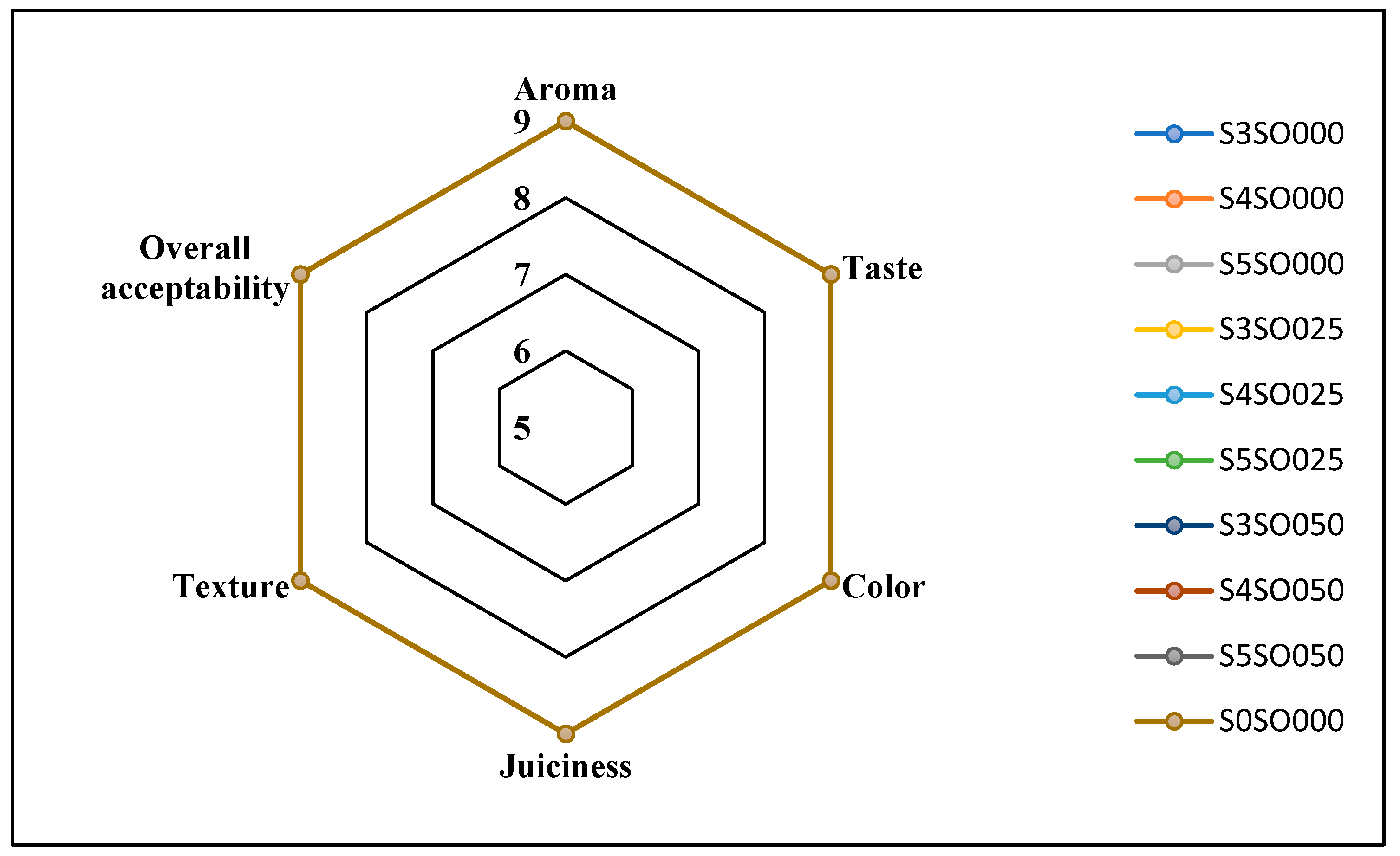
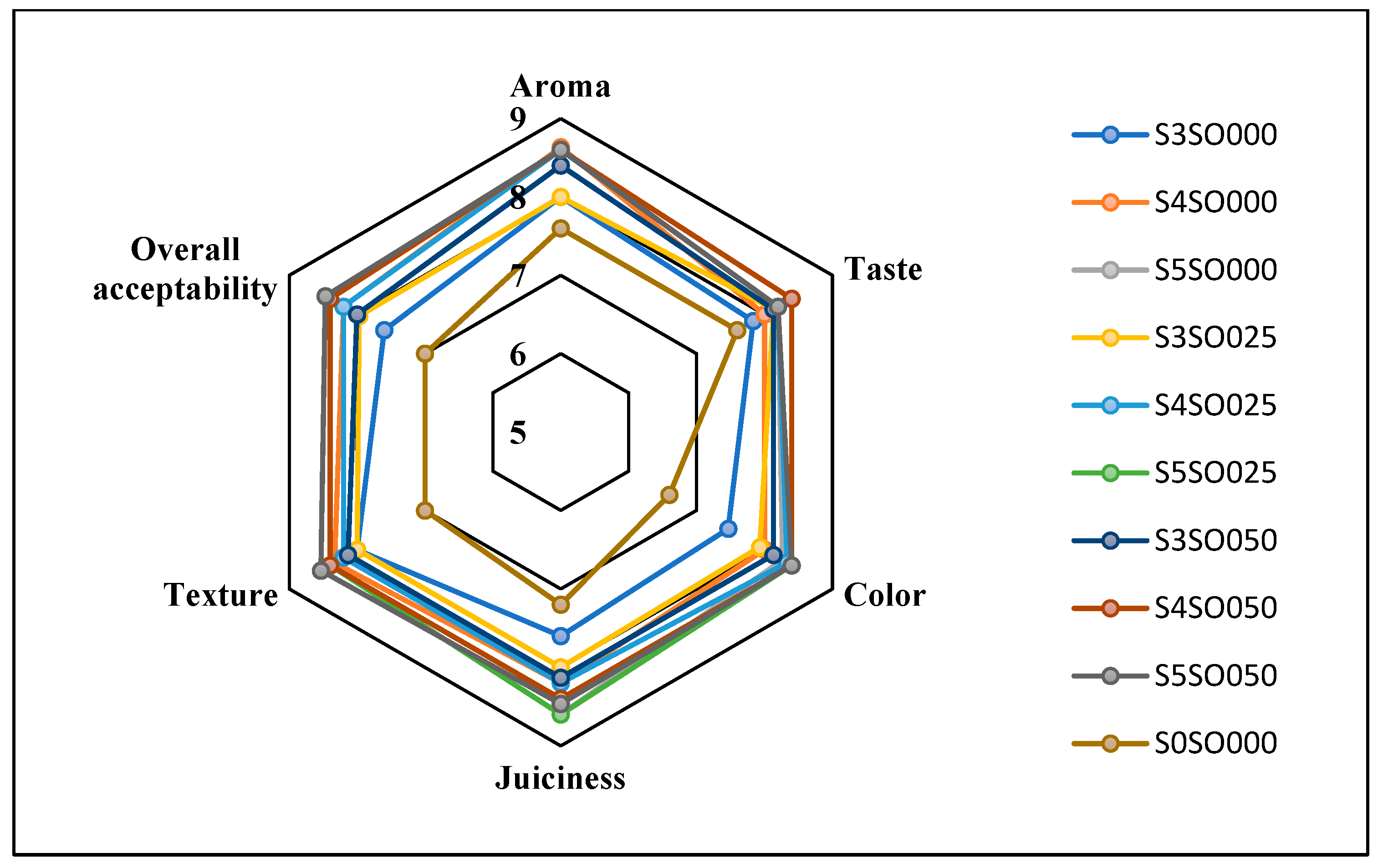
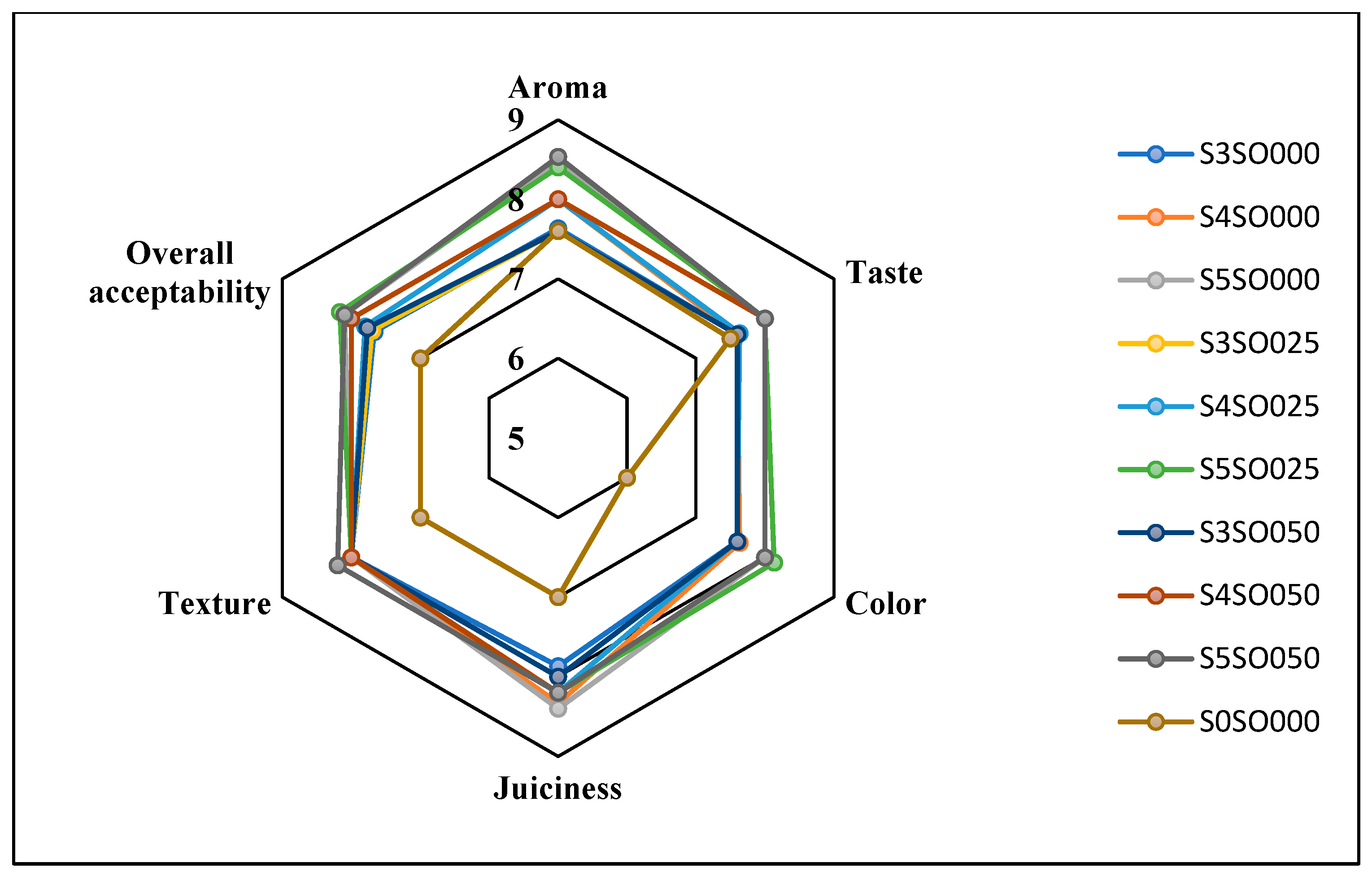
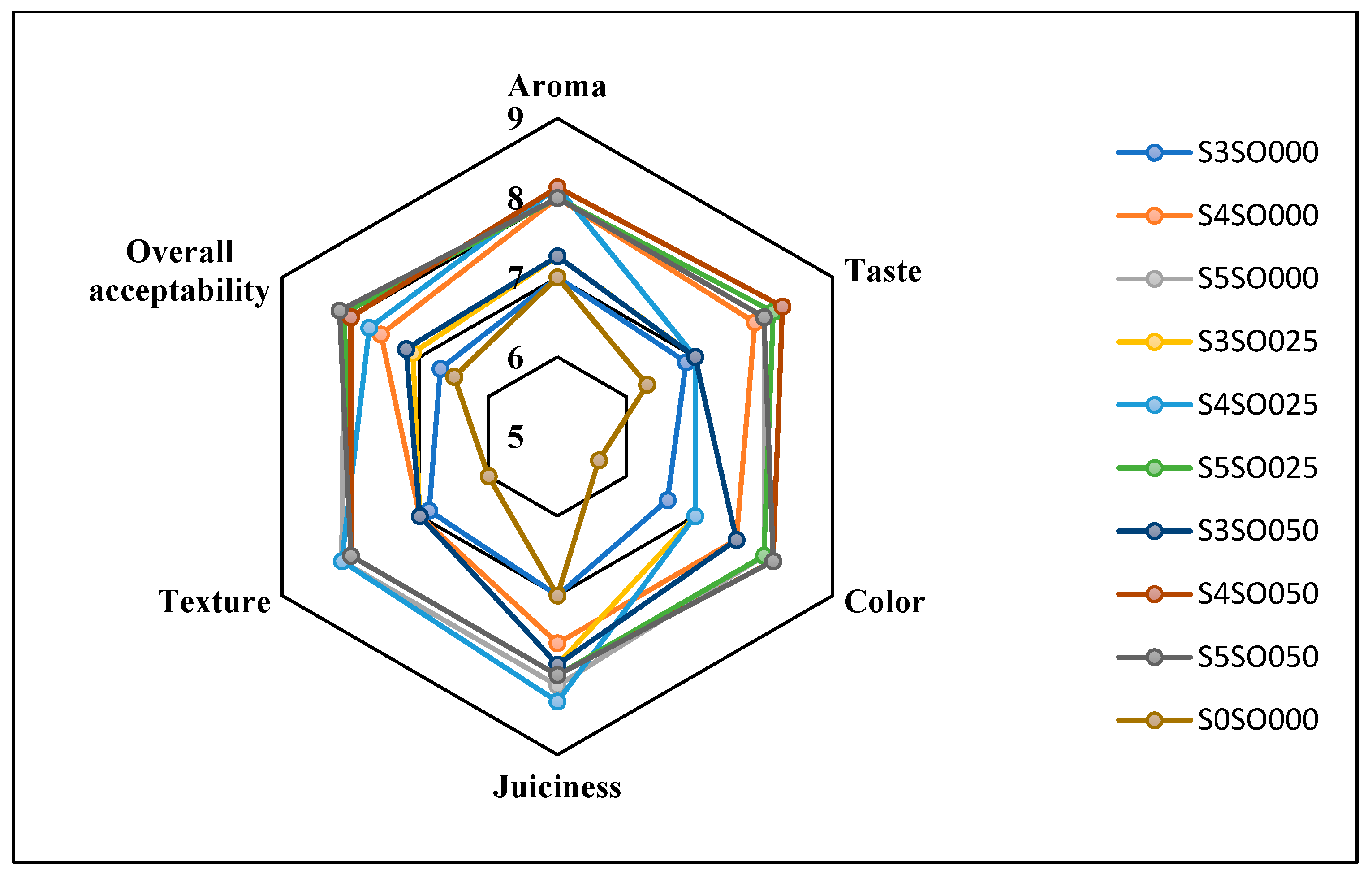
Figure 3, Figure 4, Figure 5 and Figure 6 display the changes in sensory attributes—color, aroma, texture, taste, juiciness, and overall acceptability—of coated and uncoated apple slices over 0, 4, 8, and 12 days of storage. All edible coatings resulted in higher sensory scores across all quality parameters compared to uncoated apple slices. Edible coatings that are nearly tasteless are preferred to avoid detection during consumption [56]. Coated apple slices had superior sensory qualities compared to uncoated ones, with a notable difference in overall sensory quality favoring the coated slices.
Figure 3.
Effect of coating on sensory attributes of fresh-cut apples on 0th day of storage.
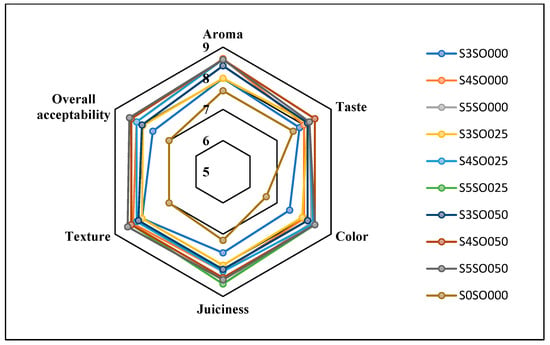
Figure 4.
Effect of coating on sensory attributes of fresh-cut apples on 4th day of storage.
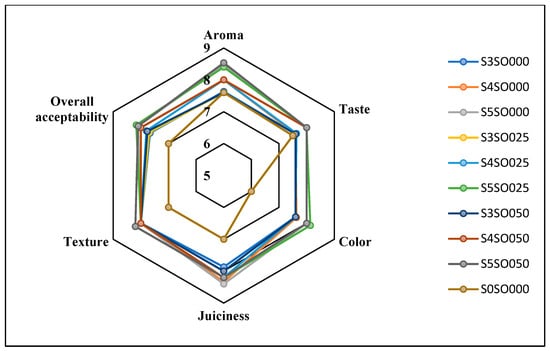
Figure 5.
Effect of coating on sensory attributes of fresh-cut apples on the 8th day of storage.
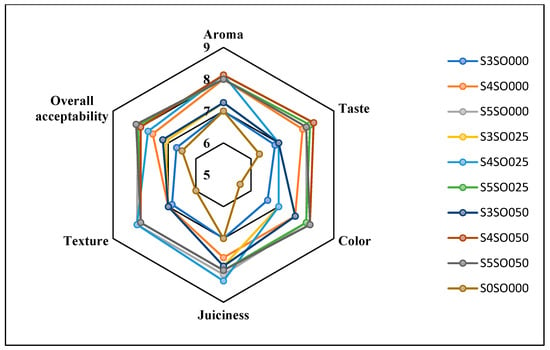
Figure 6.
Effect of coating on sensory attributes of fresh-cut apples on the 12th day of storage.
The color of the apple slices plays a significant role in sensory attributes, with coated slices exhibiting better color compared to uncoated slices, likely due to the ascorbic acid treatment and coating. The aroma and taste of the coated samples were also rated highly. Coatings with sago and oil did not affect the taste or aroma significantly and maintained the apple slices’ natural characteristics. The coatings used imparted little to no taste or odor. Juiciness and texture were well-preserved, due to the Calcium Chloride solution and sago-oil coating. Even by the 12th day, the texture of the coated apple slices remained intact. In terms of overall acceptability, coatings without oil were least preferred, whereas coatings with 0.5 percent soy oil were rated the highest. Coatings with higher oil percentages were preferred overall. Consequently, the coated apple slices maintained their sensory attributes better than the uncoated slices throughout the study.
3.9. Microbial Analysis
Fresh-cut fruit in storage is prone to spoilage by yeasts and molds due to the exposure of the cut surface to the environment, as well as the high moisture and nutrient content making them a suitable substrate for the growth of microorganisms. The sago and oil coating strongly suppressed apple slices’ yeast and mold growth. Coating without oil resulted in a significant reduction in yeast and mold count. In contrast, growth was completely inhibited and did not exceed 3 log CFU/g at the end of 12 days of storage in samples coated with the highest concentration of oil (0.5%). as shown in Table 4. In a minimally processed fruit, a 6-log CFU/g yeast count is deemed appropriate [57]. A noticeable difference in yeast and mold counts between coated and uncoated fresh-cut apples was observed. The study found that an edible coating applied to fresh-cut apples reduced yeast and mold counts significantly compared to uncoated apple pieces. A high number of yeast and mold counts in uncoated apples can also be justified by headspace gas concentration, which shows a significant decrease in O2 and a concurrent increase in CO2 concentration because of microbial respiration. The coated samples considerably reduced the yeast and molds by 3 log CFU/g, while in uncoated apple slices it reached as high as 4.48 log CFU/g at the end of the 12-day storage. The storage effect on yeast and mold count was most significant at 5% and 1% confidence levels. The anti-browning agent used in the coatings may have facilitated the observed antimicrobial effect. Comparable results were found for minimally processed fresh-cut papaya coated with an alginate-based coating infused with thyme essential oil [58].
4. Conclusions
The study showed that the effect of sago-oil coating on the physicochemical properties of fresh-cut apple samples, like TSS and pH, were concentration and time-dependent. During initial storage periods, these properties were recorded similarly for coating treated and control samples; however, at the later stages of the storage period, a difference in the properties was observed depending upon the concentration of sago and oil. TSS and pH were higher, while non-enzymatic browning was lower in samples coated with solutions with a higher oil concentration. Moisture and weight loss were considerably reduced due to the coating’s effectiveness as a barrier against moisture loss. Sago-oil coatings reduced respiration rate, evidenced by a lesser depletion of O2 concentration and low amounts of CO2 formation in the headspace of boxes containing coated samples compared to those containing control. The browning index was less in samples treated with a higher oil-containing solution and vice versa for the whiteness index. The sensory attributes, such as odor, taste, color, texture, and juiciness, were better maintained in coated samples than in controls throughout storage. Overall acceptability showed that the apple slices coated with the highest oil concentration were the most acceptable. The findings also showed that the coating inhibited yeast and mold growth in fresh-cut fruit. In conclusion, sago 5% and 0.5% oil produced the best coatings. This treatment was ideal for retaining the fresh-like quality attributes of apples for longer, gained the highest acceptability among panelists, and met the shelf-life extension criterion of 12 days in storage at 4 °C.
Author Contributions
Software, K.Y.; Supervision, O.Y.; Writing—original draft, M.Z., D.X., C.Z. and O.Y.; Writing—review and editing, K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Guangdong Provincial Higher Education Enhancement Plan of “Charging to the First Class, Making up for Shortcomings and Strengthening Characteristics” (2021–2025) Revitalization Plan for Colleges and Universities in Northwest Guangdong Province (Jiaying College); 2024 University-level Teaching Quality and Teaching Reform Project (ZLGC2024301).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Giacalone, G. Effect of Antibrowning Agents on Color and Related Enzymes in Fresh-Cut Apples During Cold Storage. J. Food Process. Preserv. 2012, 36, 133–140. [Google Scholar] [CrossRef][Green Version]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible Coatings for Fresh-Cut Fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, O.; Singh, A. An innovative approach of development of edible coating for fresh cut apple fruit for value addition of horticultural produce. J. Pharmacogn. Phytochem. 2018, 7, 2353–2355. [Google Scholar]
- Cortellino, G.; Gobbi, S.; Bianchi, G.; Rizzolo, A. Modified atmosphere packaging for shelf life extension of fresh-cut apples. Trends Food Sci. Technol. 2015, 46 Pt B, 320–330. [Google Scholar] [CrossRef]
- Graça, A.; Santo, D.; Esteves, E.; Nunes, C.; Abadias, M.; Quintas, C. Evaluation of microbial quality and yeast diversity in fresh-cut apple. Food Microbiol. 2015, 51, 179–185. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the shelf life of fresh-cut “Royal Delicious” apple with edible coatings and anti-browning agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef]
- Rux, G.; Bohne, K.; Huyskens-Keil, S.; Ulrichs Ch Hassenberg, K.; Herppich, W.B. Effects of modified atmosphere and sugar immersion on physiology and quality of fresh-cut “Braeburn” apples. Food Packag. Shelf Life 2021, 29, 100726. [Google Scholar] [CrossRef]
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Chapter 8—Biopolymer Packaging Materials for Food Shelf-Life Prolongation. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 223–277. [Google Scholar] [CrossRef]
- Oduro, K.O.-A. Edible Coating. In Postharvest Technology; Ahiduzzaman, M., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of Le Conte pear. RSC Adv. 2021, 11, 9572–9585. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-based coatings for preservation of fruits and vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 248935. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, D.; Xie, J.; Feng, S.; Wang, Y. Effects of Heat Treatment on Quality and Browning of Fresh-Cut Sugarcane. J. Food Process. Preserv. 2015, 39, 688–696. [Google Scholar] [CrossRef]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Ahmad, A.; Dubey, P.; Younis, K.; Yousuf, O. Mosambi (Citrus limetta) peel and Sago based biodegradable film: Development and characterization of physical, water barrier and biodegradation properties. Bioresour. Technol. Rep. 2022, 18, 101016. [Google Scholar] [CrossRef]
- Kasim, R.; Bintoro, N.; Rahayoe, S.; Pranoto, Y. Physiological activity of banana coated with sago starch and cellulose nanofiber edible coating. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 24–25 August 2021; Volume 653, p. 012019. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Mechanical and thermal properties of yam starch films. Food Hydrocoll. 2005, 19, 157–164. [Google Scholar] [CrossRef]
- Polnaya, F.; Talahatu, J.; Haryadi, H.; Marseno, D. Properties of biodegradable films from hydroxypropyl sago starches. Asian J. Food Agro-Ind. 2012, 5, 183–192. [Google Scholar]
- Jiang, Y.; Duan, X.; Qu, H.; Zheng, S. Browning: Enzymatic Browning. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 508–514. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis: Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality los of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Zhu, D.; Ji, B.; Eum, H.L.; Zude, M. Evaluation of the non-enzymatic browning in thermally processed apple juice by front-face fluorescence spectroscopy. Food Chem. 2009, 113, 272–279. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Zahedi, S.M.; Abadía, J.; Karimi, M. Effects of postharvest treatments with chitosan and putrescine to maintain quality and extend shelf-life of two banana cultivars. Food Sci. Nutr. 2018, 6, 1328–1337. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A. Psyllium (Plantago) Gum as an Effective Edible Coating to Improve Quality and Shelf Life of Fresh-cut Papaya (Carica papaya). Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015, 9, 702–707. [Google Scholar]
- Waghmare, R.; Annapure, U. Combined effect of chemical treatment and/or modified atmosphere packaging (MAP) on quality of fresh-cut papaya. Postharvest Biol. Technol. 2013, 85, 147–153. [Google Scholar] [CrossRef]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G.V. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Charles Oluwaseun, A.; Kayode, A.; Oluyemisi Bolajoko, F.; Bunmi, A.J.; Ar, O. Effect of edible coatings of carboxy methyl cellulose and corn starch on cucumber stored at ambient temperature. Asian J. Agric. Biol. 2013, 1, 133–140. [Google Scholar]
- Yousuf, B.; Srivastava, A.K. Impact of honey treatments and soy protein isolate-based coating on fresh-cut pineapple during storage at 4 °C. Food Packag. Shelf Life 2019, 21, 100361. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Talens, P.; Chiralt, A. Thermoplastic starch: Improving their barrier properties. Agron. Colomb. 2016, 34, S73. [Google Scholar]
- Noiwan, D.; Sutenan, K.; Yodweingchai, C.; Rachtanapun, P. Postharvest Life Extension of Fresh-Cut Mango (Mangifera indica cv. Fa-Lun) Using Chitosan and Carboxymethyl Chitosan Coating. J. Agric. Sci. 2018, 10, 438. [Google Scholar] [CrossRef]
- Sharma, M.; Saini, C.S. Postharvest shelf-life extension of fresh-cut guavas (Psidium guajava) using flaxseed protein-based composite coatings. Food Hydrocoll. Health 2021, 1, 100015. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. In Starch/Staerke; Wiley-VCH: Hoboken, NJ, USA, 2016; Volume 68, pp. 1026–1037. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; De Luca Sarantópoulos CI, G.; Hubinger, M.D. Effect of antimicrobial starch edible coating on shelf-life of fresh strawberries. Packag. Technol. Sci. 2012, 25, 413–425. [Google Scholar] [CrossRef]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible Coating of Fruits and Vegetables: A Review. Int. J. Sci. Res. Mod. Educ. 2016, 1, 188–204. Available online: https://www.researchgate.net/publication/331298687 (accessed on 13 July 2024).
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Rao Karri, R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Solís-Contreras, G.A.; Rodríguez-Guillermo, M.C.; de la Luz Reyes-Vega, M.; Aguilar, C.N.; Rebolloso-Padilla, O.N.; Corona-Flores, J.; de Abril Alexandra Soriano-Melgar, L.; Ruelas-Chacon, X. Extending shelf-life and quality of minimally processed golden delicious apples with three bioactive coatings combined with cinnamon essential oil. Foods 2021, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Mohamed AM, A.; Ramaswamy, H.S. Effect of Soybean Oil on the Improvement of the Functionality of Edible Membrane-Type Food Packaging Films Based on Caseinate–Carboxymethyl Chitosan Compositions. Membranes 2024, 14, 104. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef]
- Viacava, G.E.; Cenci, M.P.; Ansorena, M.R. Effect of Chitosan Edible Coatings Incorporated with Free or Microencapsulated Thyme Essential Oil on Quality Characteristics of Fresh-Cut Carrot Slices. Food Bioprocess Technol. 2022, 15, 768–784. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. Lwt—Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Sousa, F.F.; Pinsetta Junior, J.S.; Oliveira, K.T.E.F.; Rodrigues, E.C.N.; Andrade, J.P.; Mattiuz, B.-H. Conservation of ‘Palmer’ mango with an edible coating of hydroxypropyl methylcellulose and beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Ansah, F.A.; Amodio, M.L.; Colelli, G. Quality of fresh-cut products as affected by harvest and postharvest operations. J. Sci. Food Agric. 2018, 98, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Al-Amrani, M.; Al-Alawi, A.; Al-Marhobi, I. Assessment of Enzymatic Browning and Evaluation of Antibrowning Methods on Dates. Int. J. Food Sci. 2020, 8380461. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Fan, X. Chemical inhibition of polyphenol oxidase and cut surface browning of fresh-cut apples. Crit. Rev. Food Sci. Nutr. 2022, 63, 8737–8751. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT—Food Sci. Technol. 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Serra, M.; del Río, M.A. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol. Technol. 2006, 39, 84–92. [Google Scholar] [CrossRef]
- Rocha, A.; de Morais, A.M.M.B. Shelf life of minimally processed apple (cv. Jonagored) determined by colour changes. Food Control 2003, 14, 13–20. [Google Scholar] [CrossRef]
- Zhao, Y.; McDaniel, M. Sensory quality of foods associated with edible film and coating systems and shelf-life extension. Innov. Food Packag. 2005, 434–453. [Google Scholar] [CrossRef]
- Bell, C. Institute of Food Science and Technology of the United Kingdom. Development and Use of Microbiological Criteria for Foods; IFST: London, UK, 1999. [Google Scholar]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).