Enhancement of the Corrosion and Wear Resistance of an Epoxy Coating Using a Combination of Mullite Powder and PVB
Abstract
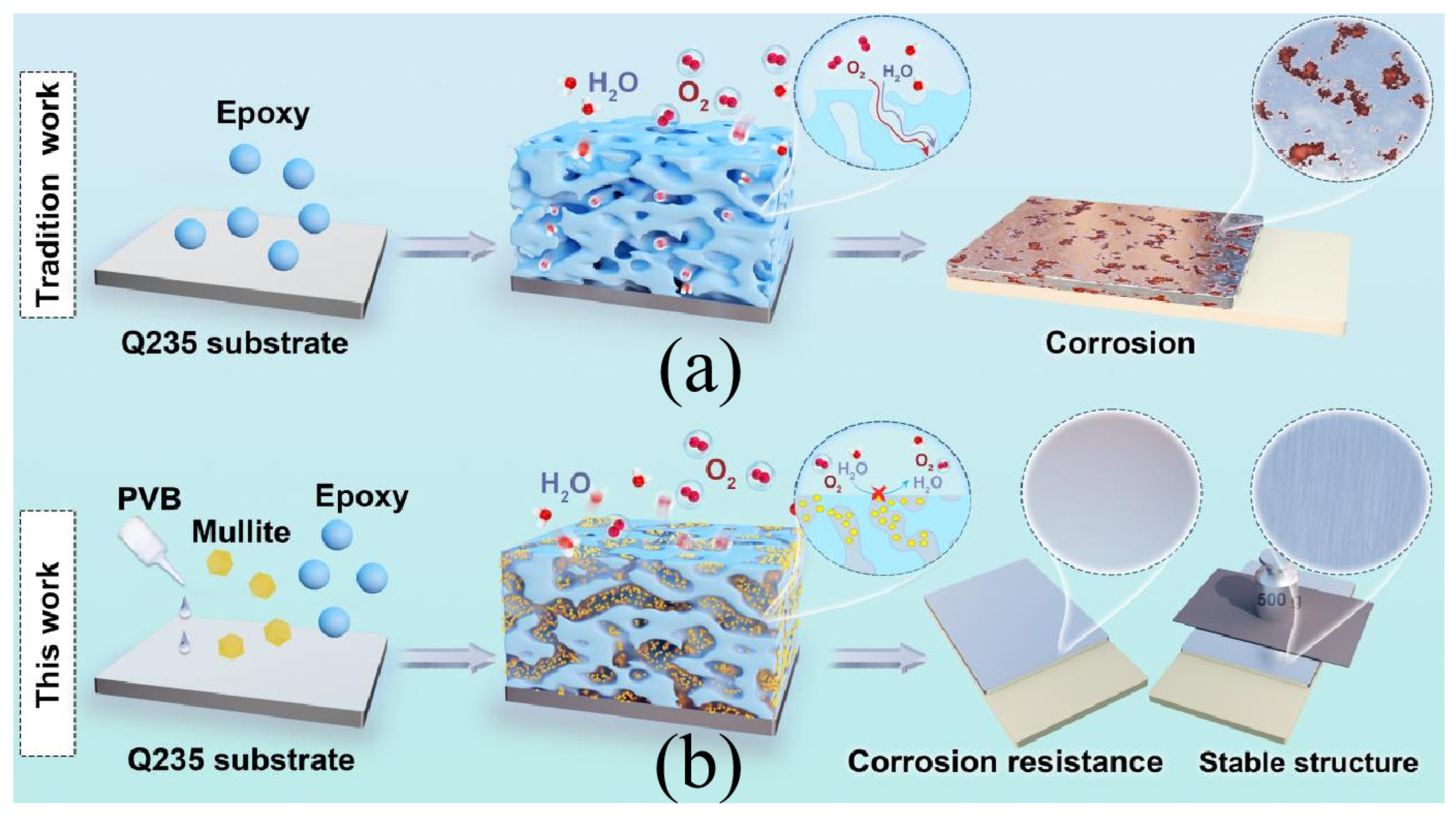
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Composite Coatings
2.3. Surface Characterization
2.4. Electrochemical Measurement and Salt Spray Test
2.5. Evaluation of Mechanical Durability
3. Results and Discussion
3.1. Characterization of Samples and Coatings
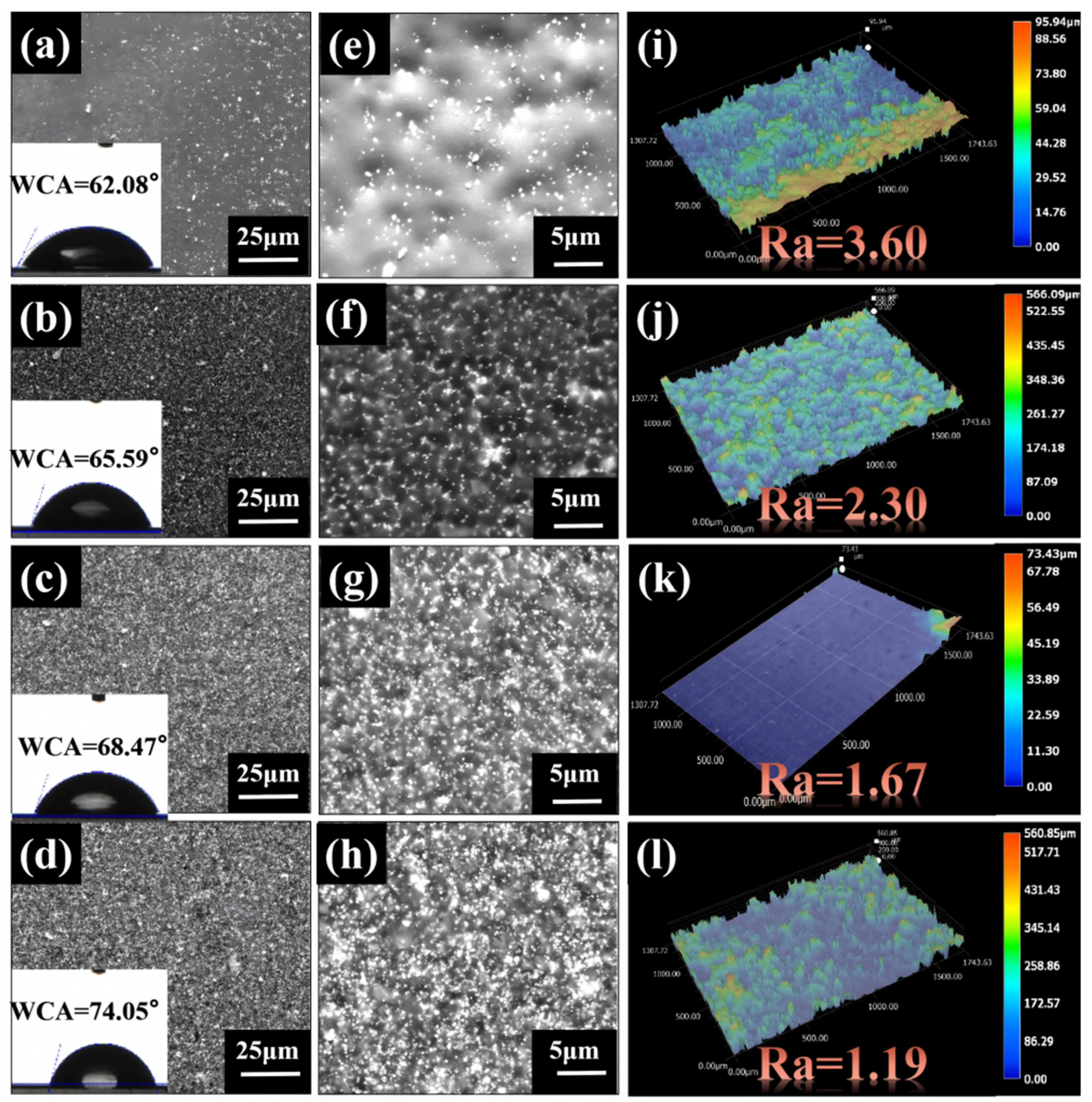
3.1.1. Morphology Characterization of Samples and Coatings
3.1.2. Structural Characterization of Samples and Coatings
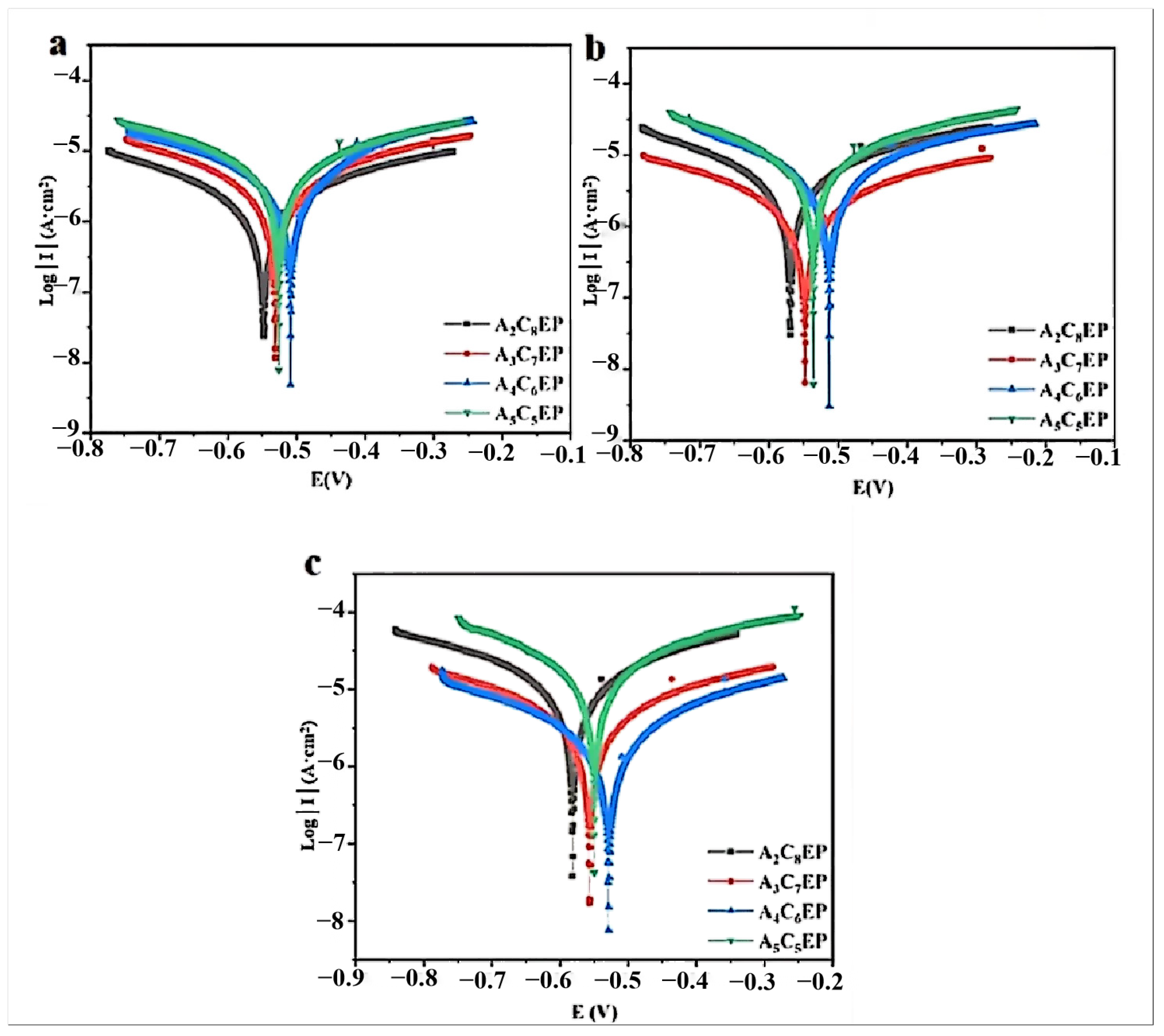
3.1.3. Electrochemical Test
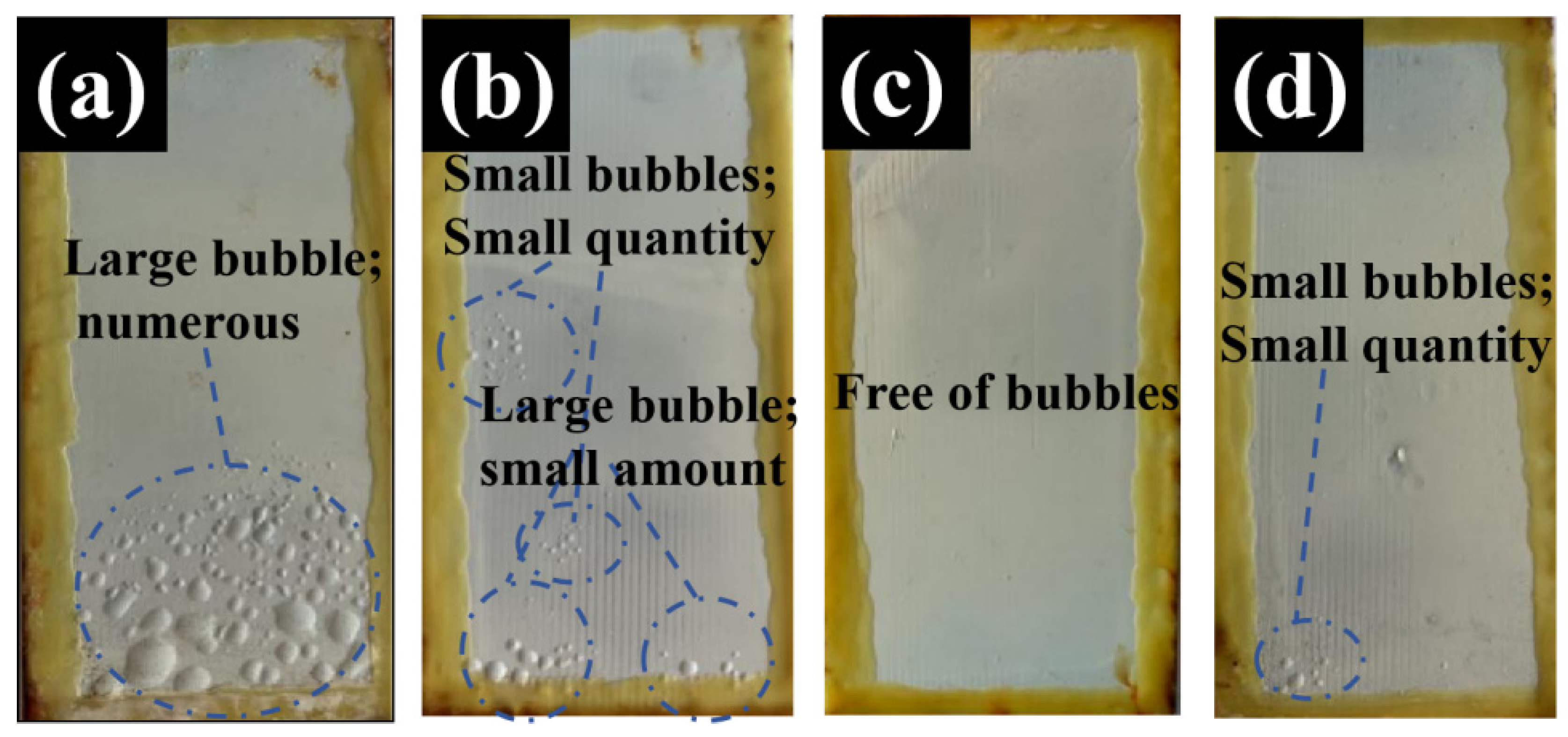
3.1.4. Salt Spray Test
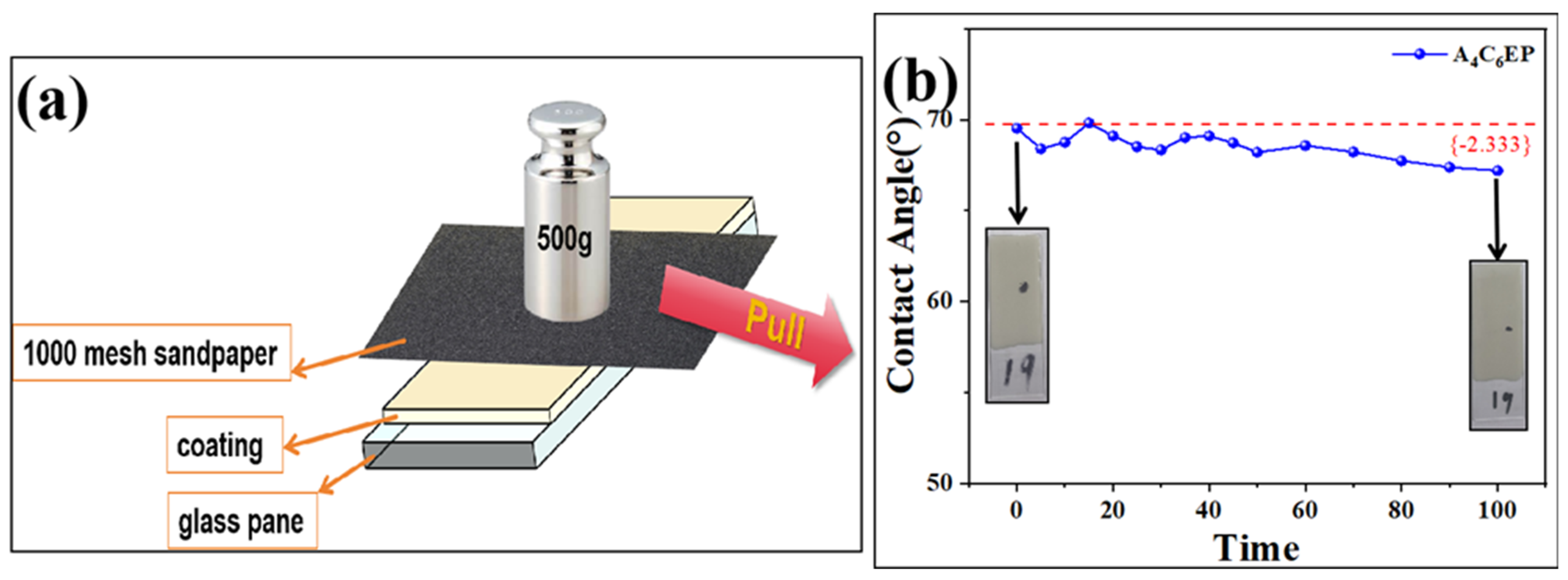
3.2. Mechanical Durability Test
3.3. Corrosion Resistance Mechanism of Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zehra, S.; Mobin, M.; Aslam, J. An overview of the corrosion chemistry. In Environmentally Sustainable Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–23. [Google Scholar]
- Akpan, E.D.; Singh, A.K.; Lgaz, H.; Quadri, T.W.; Shukla, S.K.; Mangla, B.; Dwivedi, A.; Dagdag, O.; Sheetal; Inyang, E.E.; et al. Coordination compounds as corrosion inhibitors of metals: A review. Coord. Chem. Rev. 2024, 499, 215503. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Zhou, G. Corrosion behaviors and mechanical properties of Q235 steel pipes collapsing due to corrosion after 25-year service in urban industrial atmospheric environment. Constr. Build. Mater. 2023, 395, 132342. [Google Scholar] [CrossRef]
- Li, B.; Fang, H.; Yang, K.; Zhang, X.; Du, X.; Wang, N.; Guo, X. Impact of erosion voids and internal corrosion on concrete pipes under traffic loads. Tunn. Undergr. Space Technol. 2022, 130, 104761. [Google Scholar] [CrossRef]
- Bender, R.; Féron, D.; Mills, D.; Ritter, S.; Bäßler, R.; Bettge, D.; De Graeve, I.; Dugstad, A.; Grassini, S.; Hack, T.; et al. Corrosion challenges towards a sustainable society. Mater. Corros. 2022, 73, 1730–1751. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. Corrosion in seawater desalination industry: A critical analysis of impacts and mitigation strategies. Chemosphere 2022, 307, 135640. [Google Scholar] [CrossRef]
- Singh, P.; Rana, A.; Karak, N.; Kumar, I.; Rana, S.; Kumar, P. Sustainable smart anti-corrosion coating materials derived from vegetable oil derivatives: A review. RSC Adv. 2023, 13, 3910–3941. [Google Scholar] [CrossRef]
- Kumar, S.; Bheel, N.; Zardari, S.; Alraeeini, A.S.; Almaliki, A.H.; Benjeddou, O. Effect of graphene oxide on mechanical, deformation and drying shrinkage properties of concrete reinforced with fly ash as cementitious material by using RSM modelling. Sci. Rep. 2024, 14, 18675. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Y.; Sun, J.; Guo, Z.; Fang, Z.; Chen, P.; Li, J. Multi-element hybrid flame retardants balance flame retardancy and mechanical performance of epoxy coatings. Prog. Org. Coat. 2024, 188, 108219. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Liu, X.; Wan, D.; Lv, K. Controllable size of graphite nanosheets and functionalized SiO2 microspheres with excellent anti-corrosive/wear function for superhydrophobic coating. Mater. Today Commun. 2024, 40, 110026. [Google Scholar] [CrossRef]
- Lv, K.; Ma, H.; Liu, X.; Zhu, Y.; Wan, D. Exfoliated MoS2 with tunable size effect towards anticorrosion application. J. Alloys Compd. 2024, 1005, 176225. [Google Scholar] [CrossRef]
- Fabrichnaya, O.; Seifert, H.; Weiland, R.; Ludwig, T.; Aldinger, F.; Navrotsky, A. Phase equilibria and thermodynamics in the Y2O3–Al2O3–SiO2 system. Int. J. Mater. Res. 2022, 92, 1083–1097. [Google Scholar]
- Zhi, W.; Wang, F.; Chen, X.; Tian, Y.; Yang, B. Phase equilibria and thermodynamic assessment of the Al2O3-SiO2-Sc2O3 system. Ceram. Int. 2023, 49, 9735–9747. [Google Scholar] [CrossRef]
- Lima, L.K.S.; Silva, K.R.; Menezes, R.R.; Santana, L.N.L.; Lira, H.L. Microstructural characteristics, properties, synthesis and applications of mullite: A review. Cerâmica 2022, 68, 126–142. [Google Scholar] [CrossRef]
- Philips, N.R.; Carl, M.; Cunningham, N.J. New opportunities in refractory alloys. Metall. Mater. Trans. A 2020, 51, 3299–3310. [Google Scholar] [CrossRef]
- Randis, R.; Darmadi, D.B.; Gapsari, F.; Sonief, A.A.; Akpan, E.D.; Ebenso, E.E. The potential of nanocomposite-based coatings for corrosion protection of metals: A review. J. Mol. Liq. 2023, 390, 123067. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.; Li, X. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Xie, H.; Ma, D.; Zhang, L. A PPy modified Al2O3-mullite coating on Al alloys for anti-static electricity and corrosion resistance. Ceram. Int. 2023, 49, 18885–18895. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Wan, D.; Liu, X.; Lv, K.; Wang, X. Fabrication of a Hydrophilic Al2O3/AlN EP Coating with Self-Cleaning and High-Efficiency Anti-Corrosion Properties. ChemistrySelect 2024, 9, e202402728. [Google Scholar] [CrossRef]
- Wu, B.; Cui, X.; Jiang, H.; Wu, N.; Peng, C.; Hu, Z.; Liang, X.; Yan, Y.; Huang, J.; Li, D. A superhydrophobic coating harvesting mechanical robustness, passive anti-icing and active de-icing performances. J. Colloid Interface Sci. 2021, 590, 301–310. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, X.; Liu, Z.; Zheng, Q.; Zheng, B. The role of hot extrusion in improving electrochemical properties of low-cost commercial Al alloy as anode for Al-air battery. J. Electroanal. Chem. 2022, 909, 116127. [Google Scholar] [CrossRef]
- Jamali, S.S.; Mills, D. An assessment of intrinsic noise of pseudo-reference electrodes and instrumental noise to enable reliable electrochemical noise measurements in situ on organically coated metal. Electrochim. Acta 2021, 398, 139279. [Google Scholar] [CrossRef]
- Jha, S.; Singh, V.; Kumar, V.; Bansal, A.; Singh, J.; Singla, A.K.; Singla, J.; Goyal, D.K. Microstructure, wettability, cavitation and corrosion performance of aluminum (Al6061) coated with RF-sputtered AlN thin film. Surf. Coat. Technol. 2024, 489, 131168. [Google Scholar] [CrossRef]
- Toro, L.; A Zuleta, A.; Correa, E.; Echeverría, F. Hydrothermal Sealing of Plasma Electrolytic Oxidation Coatings Developed on AZ31 Alloy. J. Mater. Eng. Perform. 2022, 31, 9768–9776. [Google Scholar] [CrossRef]
- Chen, A.; Zhao, Y.; Chen, H.; Ma, H.; Lv, K. Facile design of PTFE-kaolin-based ternary nanocomposite as a hydrophobic and high corrosion-barrier coating. Rev. Adv. Mater. Sci. 2024, 63, 20240019. [Google Scholar] [CrossRef]
- Lv, K.; Bao, Y.; Ma, H.; Liu, X.; Zhu, Y.; Wan, D. Size Effect of Graphite Nanosheet-Induced Anti-Corrosion of Hydrophobic Epoxy Coatings. Coatings 2024, 14, 769. [Google Scholar] [CrossRef]
- Sohail, M.G.; Kahraman, R.; Alnuaimi, N.A.; Gencturk, B.; Alnahhal, W.; Dawood, M.; Belarbi, A. Electrochemical behavior of mild and corrosion resistant concrete reinforcing steels. Constr. Build. Mater. 2020, 232, 117205. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Qu, D.; Chen, W.; Qiu, Z.; Qiu, X.; Zhu, L.; Chen, M. Multifunctional Epoxy Coatings Reinforced with LDHs-Modified Silica Microspheres: Synergistic Effects on Corrosion Resistance, Thermal Insulation, and Flame Retardancy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134578. [Google Scholar] [CrossRef]
- Vignesh, S.; Bensam Raj, J.; Viswanath, K.; Natarajan, N. Experimental investigation on the behavior of epoxy/peepal composites fortified with silica and silicon carbide nanoparticles for industrial applications. Wood Mater. Sci. Eng. 2024, 19, 718–724. [Google Scholar] [CrossRef]
- Singh, J.K.; Rout, A.K. Experimental investigation on tribo-performance of rice husk nanoparticles blend epoxy composites reinforced by Borassus flabellifer L. fibre using neural network approach. Mater. Today Commun. 2024, 38, 107971. [Google Scholar] [CrossRef]
- MR, H.; Pal, B.; SP, S. Influence of MoS2 nano-filler additions on wear behavior of carbon fiber reinforced epoxy composites. Adv. Compos. Mater. 2024, 1–15. [Google Scholar] [CrossRef]
- Kousar, H.; Umer, M.A.; Shehzad, K.; Ferdous, R.; Mehmood, K.; Basit, A.; Shahbaz, T.; Yasir, M.; Shakoor, A. Exceptional mechanical properties and wear behavior of Al2O3 nanoparticle reinforced Ni-WP coatings. Tribol. Int. 2024, 194, 109533. [Google Scholar] [CrossRef]



| Samples | Immersion Duration in 3.5% NaCl (Days) | Coating Thickness (μm) | Rc (Ω cm2) | CPEc (F) | Reference |
|---|---|---|---|---|---|
| A4C6EP | 7 | 25 ± 2 | 6.74 × 107 | 7.78 × 10−11 | This Work |
| EG1500SO0.1SN0.9EP | 30 (min) | 25 ± 0.5 | 4.65 × 106 | 7.54 × 10−8 | 10 |
| AO0.3AN0.7EP | 7 | 25 ± 1 | 2.42 × 107 | 7.22 × 10−11 | 20 |
| EG5000/C1P0.2EP | 7 | 25 ± 2 | 2.58 × 108 | 3.94 × 10−11 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Ma, H.; Gao, Z.; Huang, Z.; Wu, Y.; Lv, K. Enhancement of the Corrosion and Wear Resistance of an Epoxy Coating Using a Combination of Mullite Powder and PVB. Coatings 2025, 15, 41. https://doi.org/10.3390/coatings15010041
Zhao Y, Ma H, Gao Z, Huang Z, Wu Y, Lv K. Enhancement of the Corrosion and Wear Resistance of an Epoxy Coating Using a Combination of Mullite Powder and PVB. Coatings. 2025; 15(1):41. https://doi.org/10.3390/coatings15010041
Chicago/Turabian StyleZhao, Yan, Huachao Ma, Zhenglu Gao, Ziyan Huang, Yuanyuan Wu, and Kuilin Lv. 2025. "Enhancement of the Corrosion and Wear Resistance of an Epoxy Coating Using a Combination of Mullite Powder and PVB" Coatings 15, no. 1: 41. https://doi.org/10.3390/coatings15010041
APA StyleZhao, Y., Ma, H., Gao, Z., Huang, Z., Wu, Y., & Lv, K. (2025). Enhancement of the Corrosion and Wear Resistance of an Epoxy Coating Using a Combination of Mullite Powder and PVB. Coatings, 15(1), 41. https://doi.org/10.3390/coatings15010041




