Facile Synthesis of Iron Phosphide Nanoparticles in 3D Porous Carbon Framework as Superior Anodes for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
- 1.
- Synthesis of Fe3C@PCF
- 2.
- Synthesis of FeP@PCF
- 3.
- Synthesis of pure FeP NPs
3. Materials Characterization
4. Electrochemical Measurements
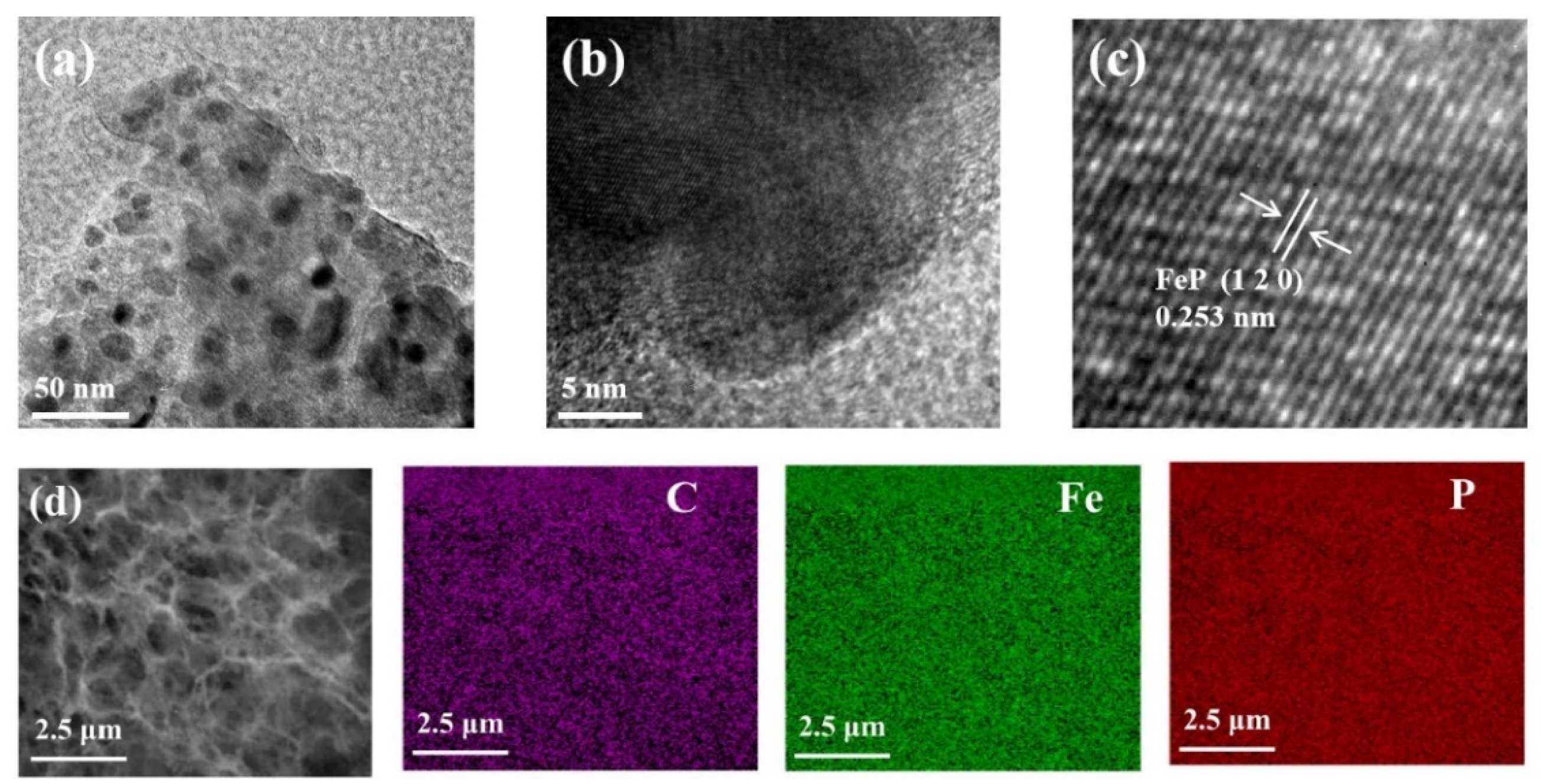
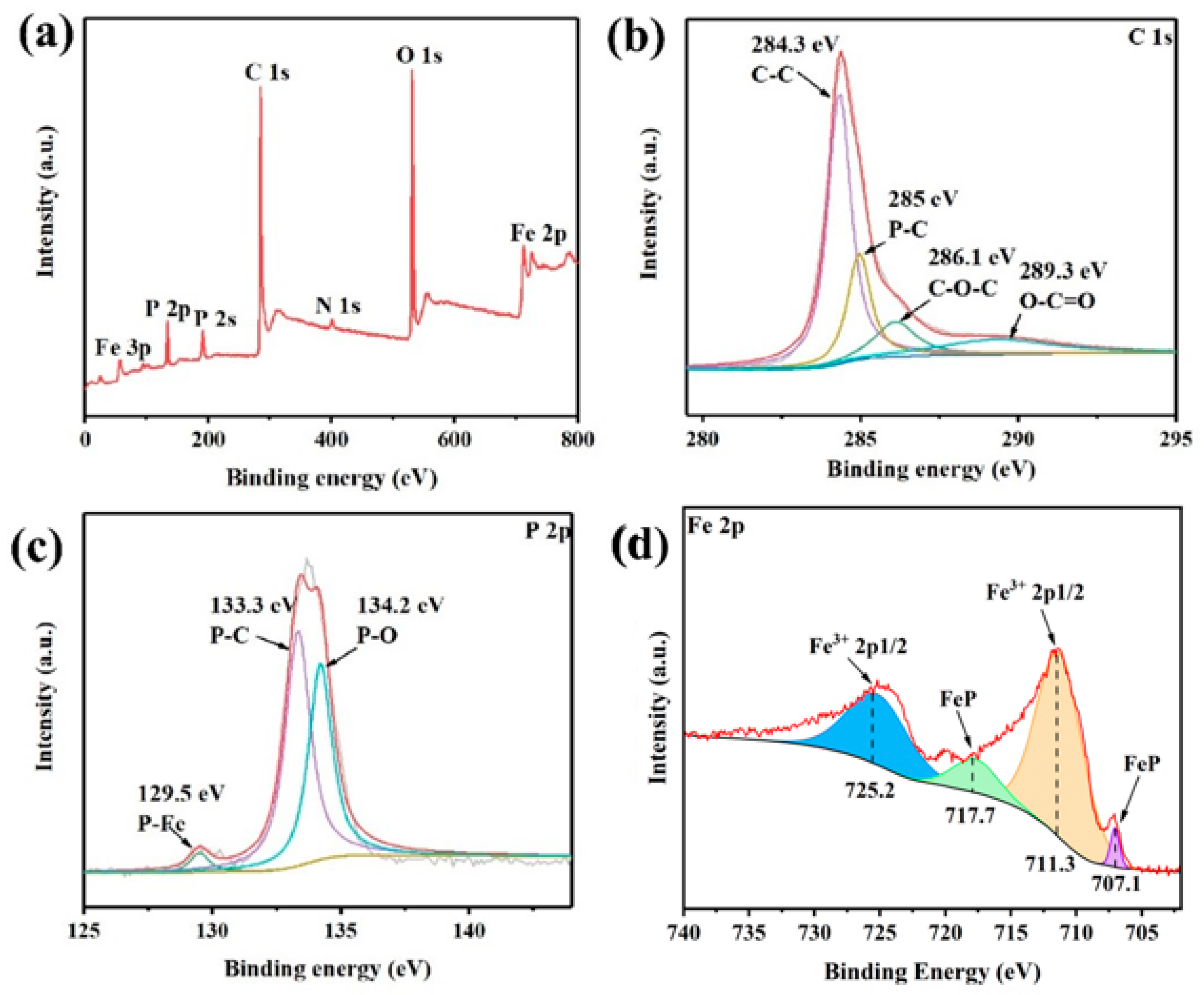
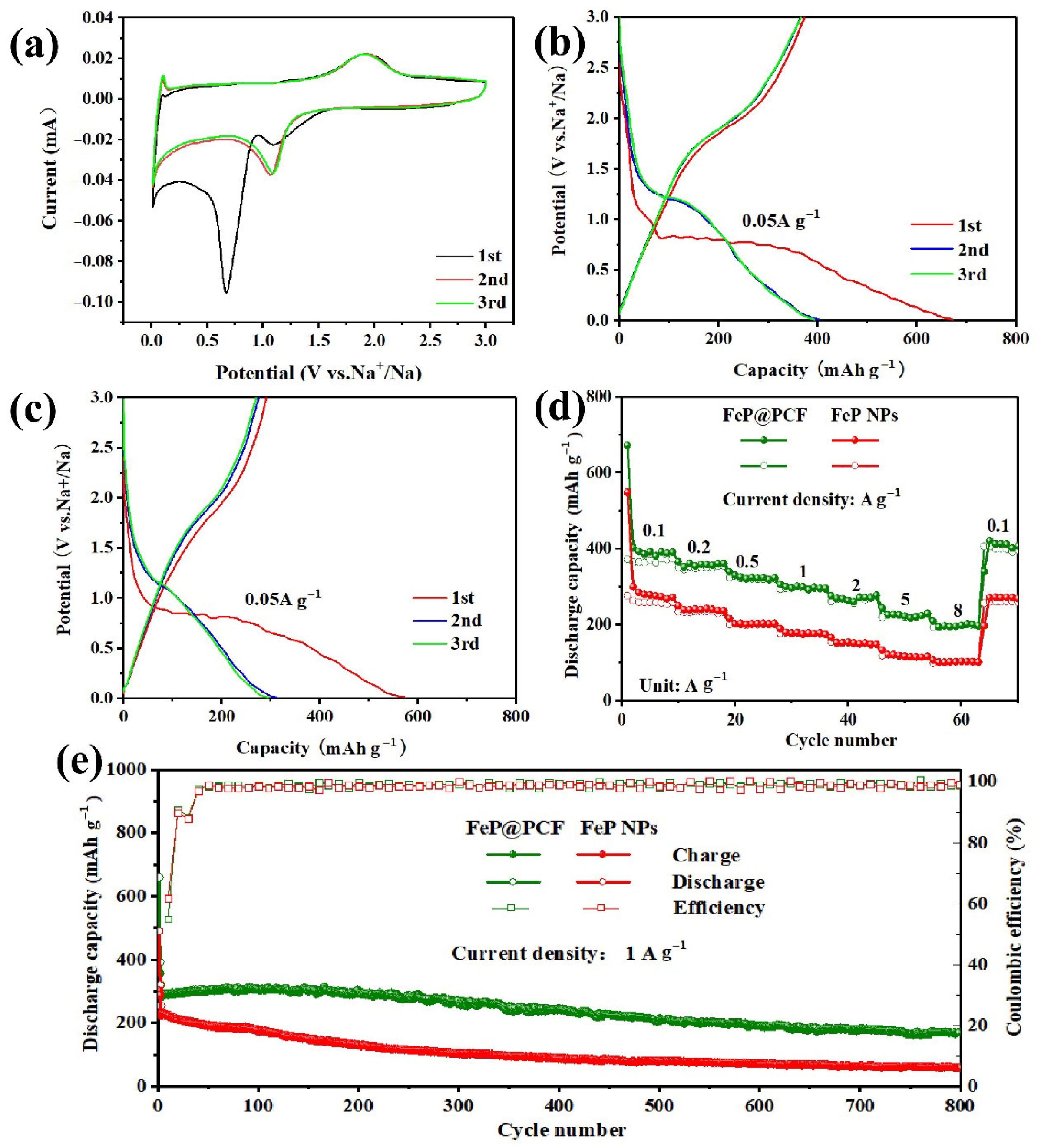
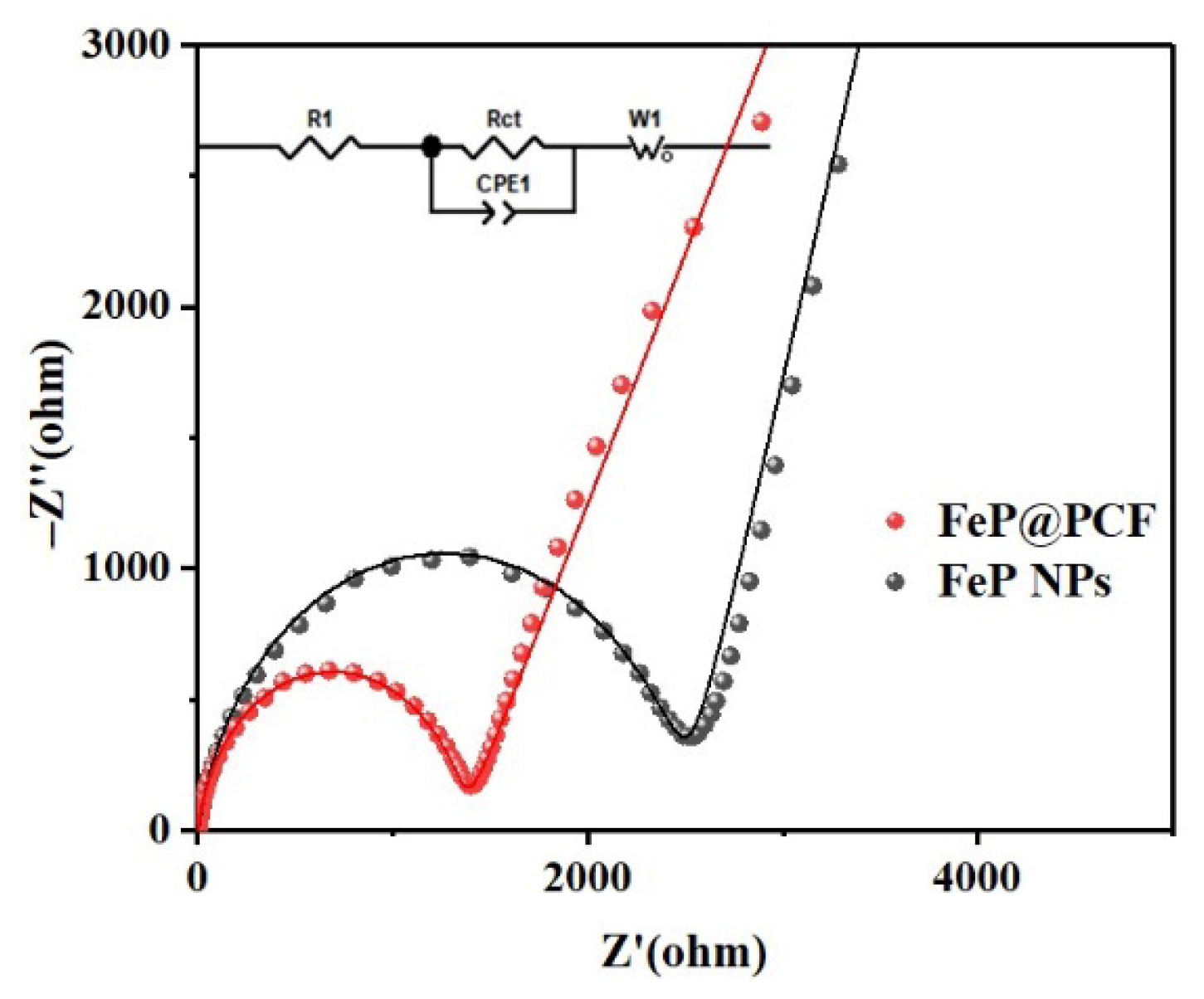
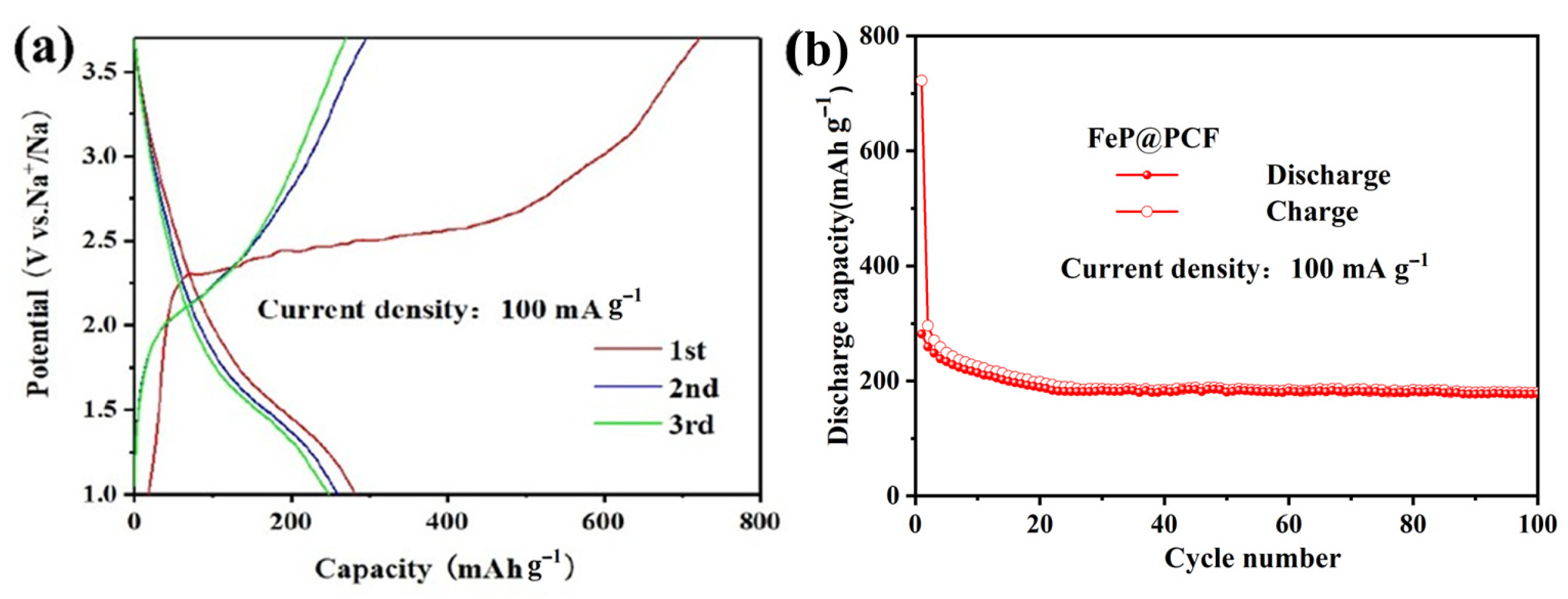
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.L.; Ma, C.R.; Zhang, W.M.; He, Y.S.; Li, X.J.; Yuan, X.Z. Controlled design for integration of FeP into 3D carbon frameworks for superior Na storage. Chem. Eng. J. 2022, 429, 132271. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.R.; Kong, F.; Guo, J.Y.; Shu, H.B.; Dai, J. Metallic penta-Graphene/penta-BN2 heterostructure with high specific capacity: A novel application platform for Li/Na-ion batteries. J. Alloys Compd. 2022, 901, 163538. [Google Scholar] [CrossRef]
- Nam, K.-H.; Hwa, Y.; Park, C.-M. Zinc Phosphides as Outstanding Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2020, 12, 15053–15062. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.R.; Wu, Y.H.; Xia, Y.J.; Yang, T.L.; Hu, Z.Y.; Chen, Q.; Yue, G.H. Tubular nanocarbon/SnS nanosheets/amorphous carbon composites with enhanced sodium ion storage performance. J. Mater. Sci. 2022, 57, 6308–6319. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, N.; Xu, B. Recent Advances in Heterostructured Carbon Materials as Anodes for Sodium-Ion Batteries. Small Struct. 2021, 2, 2100132. [Google Scholar] [CrossRef]
- Wang, X.T.; Yu, H.Y.; Liang, H.J.; Gu, Z.Y.; Nie, P.; Wang, H.Y.; Guo, J.Z.; Ang, E.H.; Wu, X.L. Waste utilization of crab shell: 3D hierarchical porous carbon towards high-performance Na/Li storage. New J. Chem. 2021, 45, 19439–19445. [Google Scholar] [CrossRef]
- Xiao, Z.D.; Gao, L.; Li, S.H. Engineering Heterostructured Fe-Co-P Arrays for Robust Sodium Storage. Materials 2024, 17, 1616. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.M.; Jamil, M.; Tabish, M.; Khoja, A.H.; Li, A.; Wang, D.K.; Song, H.H. Facile Synthesis of FeP-Decorated Heteroatomic-Doped Onion-like Carbon Nanospheres for Pseudocapacitance Enhanced Sodium Storage. ACS Appl. Energy Mater. 2023, 6, 9885–9896. [Google Scholar] [CrossRef]
- Li, W.J.; Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. A new, cheap, and productive FeP anode material for sodium-ion batteries. Chem. Commun. 2015, 51, 3682–3685. [Google Scholar] [CrossRef]
- Li, H.J.; Wang, X.M.; Zhao, Z.X.; Pathak, R.A.E.; Hao, S.Y.; Qiu, X.M.; Qiao, Q.Q. Microstructure controlled synthesis of Ni, N-codoped CoP/carbon fiber hybrids with improving reaction kinetics for superior sodium storage. J. Mater. Sci. Technol. 2022, 99, 184–192. [Google Scholar] [CrossRef]
- Shi, S.S.; Sun, C.L.; Yin, X.P.; Shen, L.Y.; Shi, Q.H.; Zhao, K.N.; Zhao, Y.F.; Zhang, J.J. FeP Quantum Dots Confined in Carbon-Nanotube-Grafted P-Doped Carbon Octahedra for High-Rate Sodium Storage and Full-Cell Applications. Adv. Funct. Mater. 2020, 30, 1909283. [Google Scholar] [CrossRef]
- Ren, L.B.; Huyan, Y.; Cao, Y.J.; Luo, Z.X.; Zhang, Y.; Wang, J.G. Revealing the electrolyte-enhancement effect of FeP/carbon nanosheets for superior sodium storage. Electrochim. Acta 2023, 467, 143077. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.W.; Lee, K.S.; Hwang, S.J.; Piao, Y. A densely packed air-stable free-standing film with FeP nanoparticles@C@P-doped reduced graphene oxide for sodium-ion batteries. Nanoscale 2023, 15, 14155–14164. [Google Scholar] [CrossRef]
- Zhan, L.; Song, X.H.; Deng, W.; Wei, T.Y.; Huang, L.; Wei, X.L.; Wang, C.X. Facile approach to prepare FeP/P/C nanofiber heterostructure via electrospinning as highly performance self-supporting anode for Li/Na ion batteries. Electrochim. Acta 2022, 403, 139682. [Google Scholar] [CrossRef]
- Zhao, L.P.; Li, J.; Peng, B.; Wang, G.R.; Yu, L.; Guo, Y.M.; Shi, L.; Zhang, G.Q. Universal Synthesis of Transition-Metal Phosphide/Carbon Hybrid Nanosheets for Stable Sodium Ion Storage and Full-Cell Application. ChemElectroChem 2022, 9, e202200519. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Y.X.; Wu, B.; Zeng, P.; Xiang, C.; Zhao, C.K.; Sofer, Z.; Chen, M.F.; Wang, X.Y. A heterogeneous FeP-CoP electrocatalyst for expediting sulfur redox in high-specific-energy lithium-sulfur batteries. Electrochim. Acta 2021, 397, 139275. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.S.; Chen, X.H.; Song, H.H. Achieving Ultrafast and Stable Na-Ion Storage in FeSe2 Nanorods/Graphene Anodes by Controlling the Surface Oxide. ACS Appl. Mater. Interfaces 2018, 10, 22841–22850. [Google Scholar] [CrossRef]
- Han, F.; Zhang, C.Z.; Yang, J.X.; Ma, G.Z.; He, K.J.; Li, X.K. Well-dispersed and porous FeP@C nanoplates with stable and ultrafast lithium storage performance through conversion reaction mechanism. J. Mater. Chem. A 2016, 4, 12781–12789. [Google Scholar] [CrossRef]
- Lin, C.; Hu, R.Z.; Liu, J.; Yang, L.C.; Liu, J.W.; Ouyang, L.Z.; Zhu, M. A nanorod FeP@phosphorus-doped carbon composite for high-performance lithium -ion batteries. J. Alloys Compd. 2018, 763, 296–304. [Google Scholar] [CrossRef]
- Tan, Q.W.; Zhao, W.; Han, K.; Li, P.; Wang, W.; He, D.L.; Liu, Z.W.; Yu, Q.Y.; Qin, M.L.; Qu, X.H. The multi-yolk/shell structure of FeP@foam-like graphenic scaffolds: Strong P-C bonds and electrolyte- and binder-optimization boost potassium storage. J. Mater. Chem. A 2019, 7, 15673–15682. [Google Scholar] [CrossRef]
- Yang, F.H.; Goo, H.; Hao, J.N.; Zhang, S.L.; Li, P.; Liu, Y.Q.; Chen, J.; Guo, Z.P. Yolk-Shell Structured FeP@C Nanoboxes as Advanced Anode Materials for Rechargeable Lithium-/Potassium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1808291. [Google Scholar] [CrossRef]
- Boyanov, S.; Bernardi, J.; Gillot, F.; Dupont, L.; Womes, M.; Tarascon, J.M.; Monconduit, L.; Doublet, M.L. FeP: Another attractive anode for the Li-ion battery enlisting a reversible two-step insertion/conversion process. Chem. Mater. 2006, 18, 3531–3538. [Google Scholar] [CrossRef]
- Xu, X.J.; Liu, J.; Liu, Z.B.; Wang, Z.S.; Hu, R.Z.; Liu, J.W.; Ouyang, L.Z.; Zhu, M. FeP@C Nanotube Arrays Grown on Carbon Fabric as a Low Potential and Freestanding Anode for High-Performance Li-Ion Batteries. Small 2018, 14, 1613–6810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.P.; Zhang, Z.; Hao, S.J.; Zhang, B.W.; Zhao, P.F.; Yu, J.; Cai, J.X.; Huang, Y.Z.; Yang, Z.Y. Multi-channel FeP@C octahedra anchored on reduced graphene oxide nanosheet with efficient performance for lithium-ion batteries. Carbon 2018, 139, 477–485. [Google Scholar] [CrossRef]
- Ju, Z.C.; Li, P.Z.; Ma, G.Y.; Xing, Z.; Zhuang, Q.C.; Qian, Y.T. Few layer nitrogen-doped graphene with highly reversible potassium storage. Energy Storage Mater. 2018, 11, 38–46. [Google Scholar] [CrossRef]
- Wang, X.; Sun, P.P.; Qin, J.W.; Wang, J.Q.; Xiao, Y.; Cao, M.H. A three-dimensional porous MoP@C hybrid as a high-capacity, long-cycle life anode material for lithium-ion batteries. Nanoscale 2016, 8, 10330–10338. [Google Scholar] [CrossRef]
- Cook, J.B.; Kim, H.S.; Yan, Y.; Ko, J.S.; Robbennolt, S.; Dunn, B.; Tolbert, S.H. Mesoporous MoS2 as a Transition Metal Dichalcogenide Exhibiting Pseudocapacitive Li and Na-Ion Charge Storage. Adv. Energy Mater. 2016, 6, 1501937. [Google Scholar] [CrossRef]
- Zou, G.Q.; Hou, H.S.; Foster, C.W.; Banks, C.E.; Guo, T.X.; Jiang, Y.L.; Zhang, Y.; Ji, X.B. Advanced Hierarchical Vesicular Carbon Co-Doped with S, P, N for High-Rate Sodium Storage. Adv. Sci. 2018, 5, 1800241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Ming, J.; Zhao, W.L.; Dong, X.C.; Hedhili, M.N.; Costa, P.M.F.J.; Alshareef, H.N. Graphitic Nanocarbon with Engineered Defects for High-Performance Potassium-Ion Battery Anodes. Adv. Funct. Mater. 2019, 29, 1903641. [Google Scholar] [CrossRef]
- Hou, H.S.; Shao, L.D.; Zhang, Y.; Zou, G.Q.; Chen, J.; Ji, X.B. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 2198–3844. [Google Scholar] [CrossRef]
- He, H.N.; Huang, D.; Tang, Y.G.; Wang, Q.; Ji, X.B.; Wang, H.Y.; Guo, Z.P. Tuning nitrogen species in three-dimensional porous carbon via phosphorus doping for ultra-fast potassium storage. Nano Energy 2019, 57, 728–736. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.J.; Wu, Z.G.; Cui, G.W.; Xie, F.Y.; Guo, X.D.; Sun, X.P. FeP nanorod arrays on carbon cloth: A high-performance anode for sodium-ion batteries. Chem. Commun. 2018, 54, 9341–9344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zhang, L.Y.; Ge, X.L.; Li, C.X.; Dong, S.H.; Wang, C.X.; Yin, L.W. Core-shell structured CoP/FeP porous microcubes interconnected by reduced graphene oxide as high performance anodes for sodium ion batteries. Nano Energy 2017, 32, 494–502. [Google Scholar] [CrossRef]
- Miao, X.G.; Yin, R.Y.; Ge, X.L.; Li, Z.Q.; Yin, L.W. Ni2P@Carbon Core-Shell Nanoparticle-Arched 3D Interconnected Graphene Aerogel Architectures as Anodes for High-Performance Sodium-Ion Batteries. Small 2017, 13, 1702138. [Google Scholar] [CrossRef]
- Bai, Z.C.; Ju, Z.C.; Guo, C.L.; Qian, Y.T.; Tang, B.; Xiong, S.L. Direct large-scale synthesis of 3D hierarchical mesoporous NiO microspheres as high-performance anode materials for lithium ion batteries. Nanoscale 2014, 6, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.R.; Li, W.J.; Chou, S.L.; Wang, J.Z.; Liu, H.K. Ball-milled FeP/graphite as a low-cost anode material for the sodium-ion battery. RSC Adv. 2015, 5, 80536–80541. [Google Scholar] [CrossRef]
- Zhu, H.J.; Liu, T.T.; Peng, L.; Yao, W.T.; Kang, F.Y.; Shu, J.; Yang, C. A compact Bi2WO6 microflowers anode for potassium-ion storage: Taming a sequential phase evolution toward stable electrochemical cycling. Nano Energy 2021, 82, 105784. [Google Scholar] [CrossRef]
- Hou, B.H.; Wang, Y.Y.; Ning, Q.L.; Fan, C.Y.; Xi, X.T.; Yang, X.; Wang, J.W.; Zhang, J.P.; Wang, X.L.; Wu, X.L. An FeP@ C nanoarray vertically grown on graphene nanosheets: An ultrastable Li-ion battery anode with pseudocapacitance- boosted electrochemical kinetics. Nanoscale 2019, 11, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.H.; Wang, Y.Y.; Ning, Q.L.; Liang, H.J.; Yang, X.; Wang, J.W.; Liu, M.K.; Zhang, J.P.; Wang, X.; Wu, X.L. Dual-Carbon Enhanced FeP Nanorods Vertically Grown on Carbon Nanotubes with Pseudocapacitance-Boosted Electrochemical Kinetics for Superior Lithium Storage. Adv. Electron. Mater. 2019, 5, 1900006. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Yang, S.; Wang, F.X.; Zhuang, X.D.; Müllen, K.; Feng, X.L. Vertically Aligned MoS2 Nanosheets Patterned on Electrochemically Exfoliated Graphene for High-Performance Lithium and Sodium Storage. Adv. Energy Mater. 2018, 8, 1702254. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.Y.; Zhang, Z.; Zhang, Y.; Peng, J.; Zhang, Y.; Wu, H. Tailoring yolk-shell FeP@carbon nanoboxes with engineered void space for pseudocapacitance-boosted lithium storage. Inorg. Chem. Front. 2018, 5, 2605–2614. [Google Scholar] [CrossRef]
- Hao, S.Y.; Li, H.J.; Zhao, Z.X.; Wang, X.M. Pseudocapacitance-Enhanced Anode of CoP@C Particles Embedded in Graphene Aerogel toward Ultralong Cycling Stability Sodium-Ion Batteries. Chemelectrochem 2019, 6, 5712–5720. [Google Scholar] [CrossRef]
- Shi, J.R.; Wang, Y.P.; Su, Q.; Cheng, F.Y.; Kong, X.Z.; Lin, J.D.; Zhu, T.; Liang, S.Q.; Pan, A.Q. N-S co-doped C@SnS nanoflakes/graphene composite as advanced anode for sodium-ion batteries. Chem. Eng. J. 2018, 353, 606–614. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Xiong, X.Y.; Ding, S.P.; Liu, X.F.; Jiang, Q.Q.; Hu, J.C. In-situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures. Appl. Catal. B-Environ. 2018, 220, 570–580. [Google Scholar] [CrossRef]
- Wang, B.B.; Wang, G.; Wang, H.; Bai, J.T. Hierarchically Porous Carbon Nanofibers Encapsulating Carbon-Coated Mini Hollow FeP Nanoparticles for High Performance Lithium and Sodium Ion Batteries. ChemNanoMat 2018, 4, 924–935. [Google Scholar] [CrossRef]
- Shi, S.S.; Li, Z.; Shen, L.Y.; Yin, X.P.; Liu, Y.M.; Chang, G.L.; Wang, J.; Xu, S.M.; Zhang, J.J.; Zhao, Y.F. Electrospun free-standing FeP@NPC film for flexible sodium ion batteries with remarkable cycling stability. Energy Storage Mater. 2020, 29, 78–83. [Google Scholar] [CrossRef]
- Lim, Y.V.; Huang, S.Z.; Zhang, Y.M.; Kong, D.Z.; Wang, Y.; Guo, L.; Zhang, J.; Shi, Y.M.; Chen, T.P.; Ang, L.K.; et al. Bifunctional porous iron phosphide/carbon nanostructure enabled high-performance sodium-ion battery and hydrogen evolution reaction. Energy Storage Mater. 2018, 15, 98–107. [Google Scholar]
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational Design of Three Dimensional Graphene Encapsulated with Hollow FeP@Carbon Nanocomposite as Outstanding Anode Material for Lithium Ion and Sodium Ion Batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhao, H.L.; Du, Z.H.; Zhao, L.N.; Wang, J.; Zhang, Z.J. Iron phosphide@N-doped carbon nanosheets with open-framework structure as an ultralong lifespan and outstanding rate performance electrode material for sodium-ion batteries. J. Power Sources 2020, 465, 228253. [Google Scholar] [CrossRef]
- Ma, C.; Fu, Z.; Deng, C.; Liao, X.; He, Y.; Ma, Z.; Xiong, H. Carbon-coated FeP nanoparticles anchored on carbon nanotube networks as an anode for long-life sodium-ion storage. Chem. Commun. 2018, 54, 11348–11351. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.; Jang, M.; Hwang, T.; Hwang, S.J.; Piao, Y. An expanded sandwichlike heterostructure with thin FeP nanosheets@graphene via charge-driven self-assembly as high-performance anodes for sodium ion battery. Nanoscale 2022, 14, 6184–6194. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Dai, S.; Shen, Y.; Wang, M. High-rate and stable iron phosphide nanorods anode for sodium-ion battery. Electrochim. Acta 2019, 314, 142–150. [Google Scholar] [CrossRef]
- Jiang, M.; Ren, L.; Hou, Z.; Hua, W.; Lei, D.; Cao, Y.; Zhang, Y.; Wang, J.-G. A superior sodium-ion battery based on tubular Prussian blue cathode and its derived phosphide anode. J. Power Sources 2023, 554, 232334. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Lin, S.; Xia, Y.; Zhou, Z.; Li, J.; Yue, G. Facile Synthesis of Iron Phosphide Nanoparticles in 3D Porous Carbon Framework as Superior Anodes for Sodium-Ion Batteries. Coatings 2025, 15, 85. https://doi.org/10.3390/coatings15010085
Yan J, Lin S, Xia Y, Zhou Z, Li J, Yue G. Facile Synthesis of Iron Phosphide Nanoparticles in 3D Porous Carbon Framework as Superior Anodes for Sodium-Ion Batteries. Coatings. 2025; 15(1):85. https://doi.org/10.3390/coatings15010085
Chicago/Turabian StyleYan, Jian, Sheng Lin, Yongji Xia, Zhidong Zhou, Jintang Li, and Guanghui Yue. 2025. "Facile Synthesis of Iron Phosphide Nanoparticles in 3D Porous Carbon Framework as Superior Anodes for Sodium-Ion Batteries" Coatings 15, no. 1: 85. https://doi.org/10.3390/coatings15010085
APA StyleYan, J., Lin, S., Xia, Y., Zhou, Z., Li, J., & Yue, G. (2025). Facile Synthesis of Iron Phosphide Nanoparticles in 3D Porous Carbon Framework as Superior Anodes for Sodium-Ion Batteries. Coatings, 15(1), 85. https://doi.org/10.3390/coatings15010085





