Citric Acid Cross-Linked Gelatin/Pectin Coatings Increase Shelf Life of Ripe Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
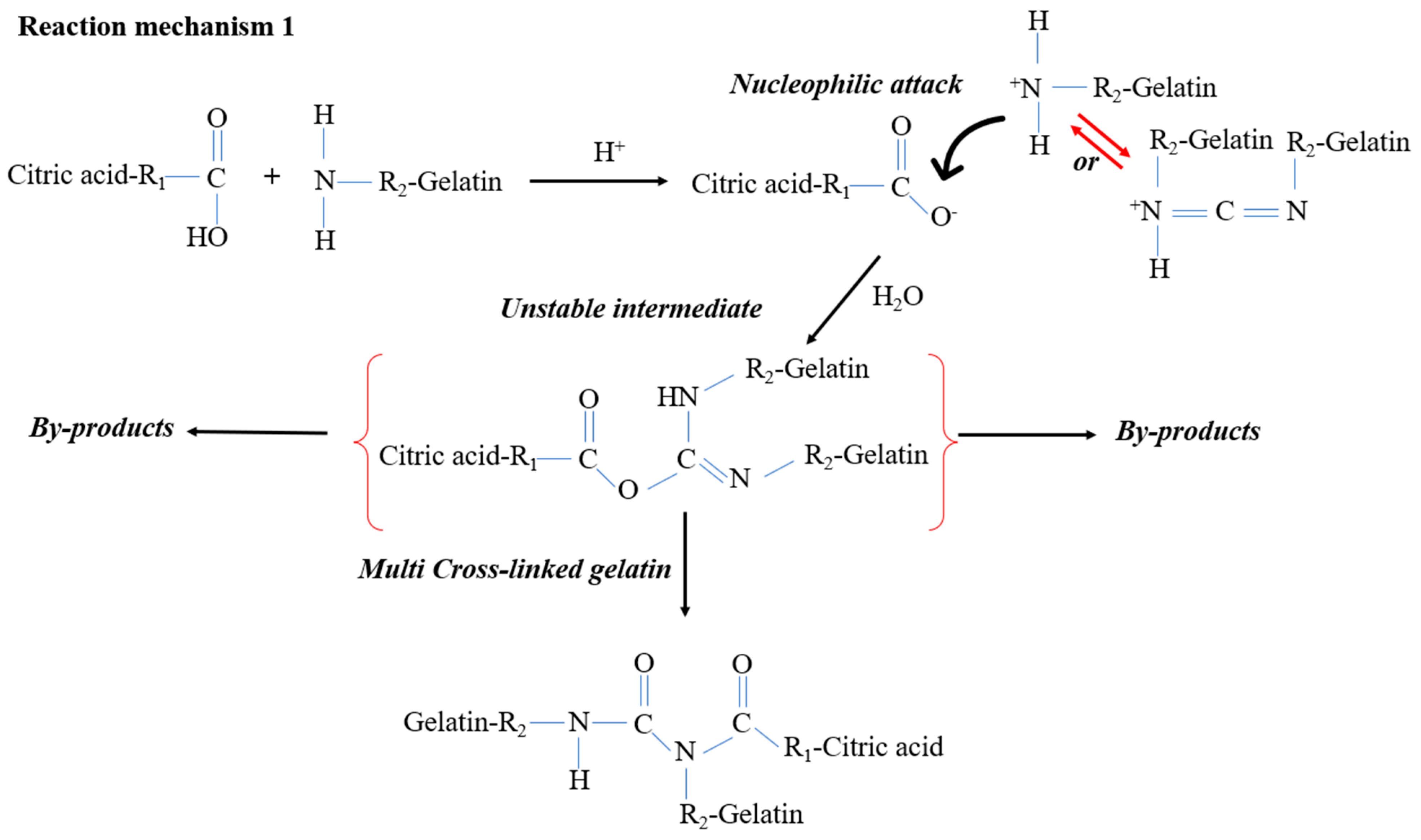
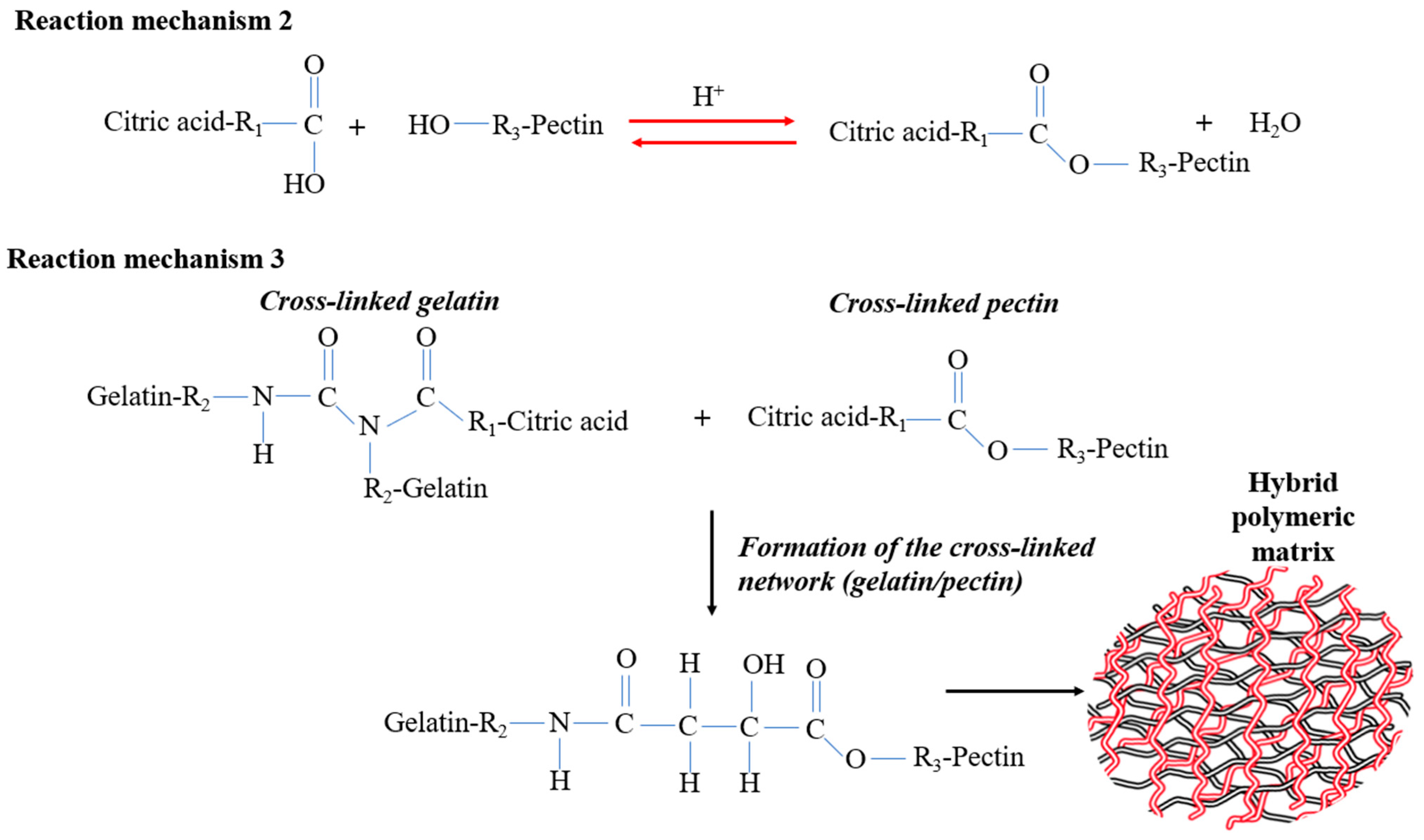
2.2. Preparation of Gelatin/Pectin Cross-Linked with Citric Acid
2.3. Characterization of Cross-Linked Gelatin/Pectin
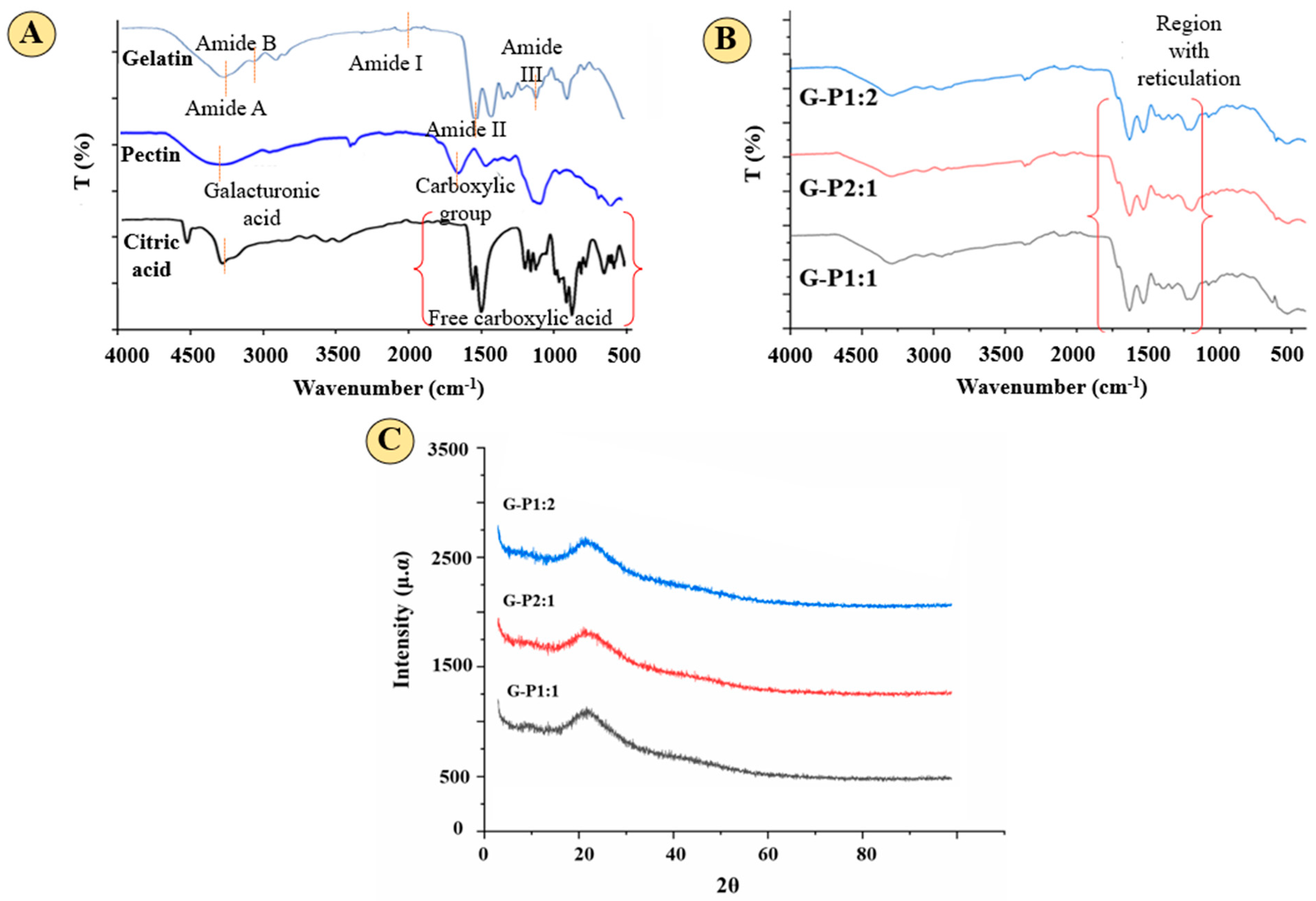
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. X-Ray Diffraction
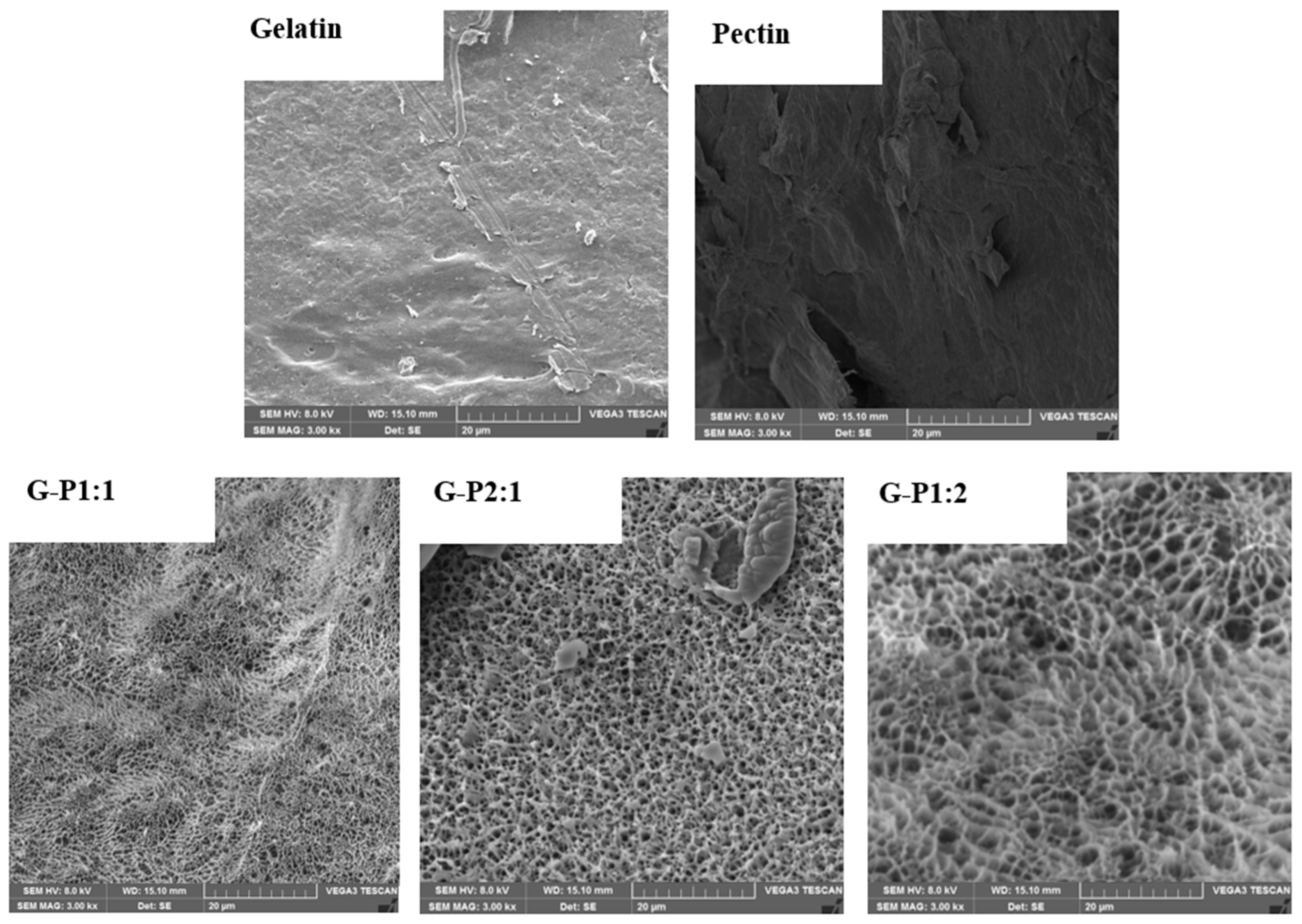
2.3.3. Micromorphology
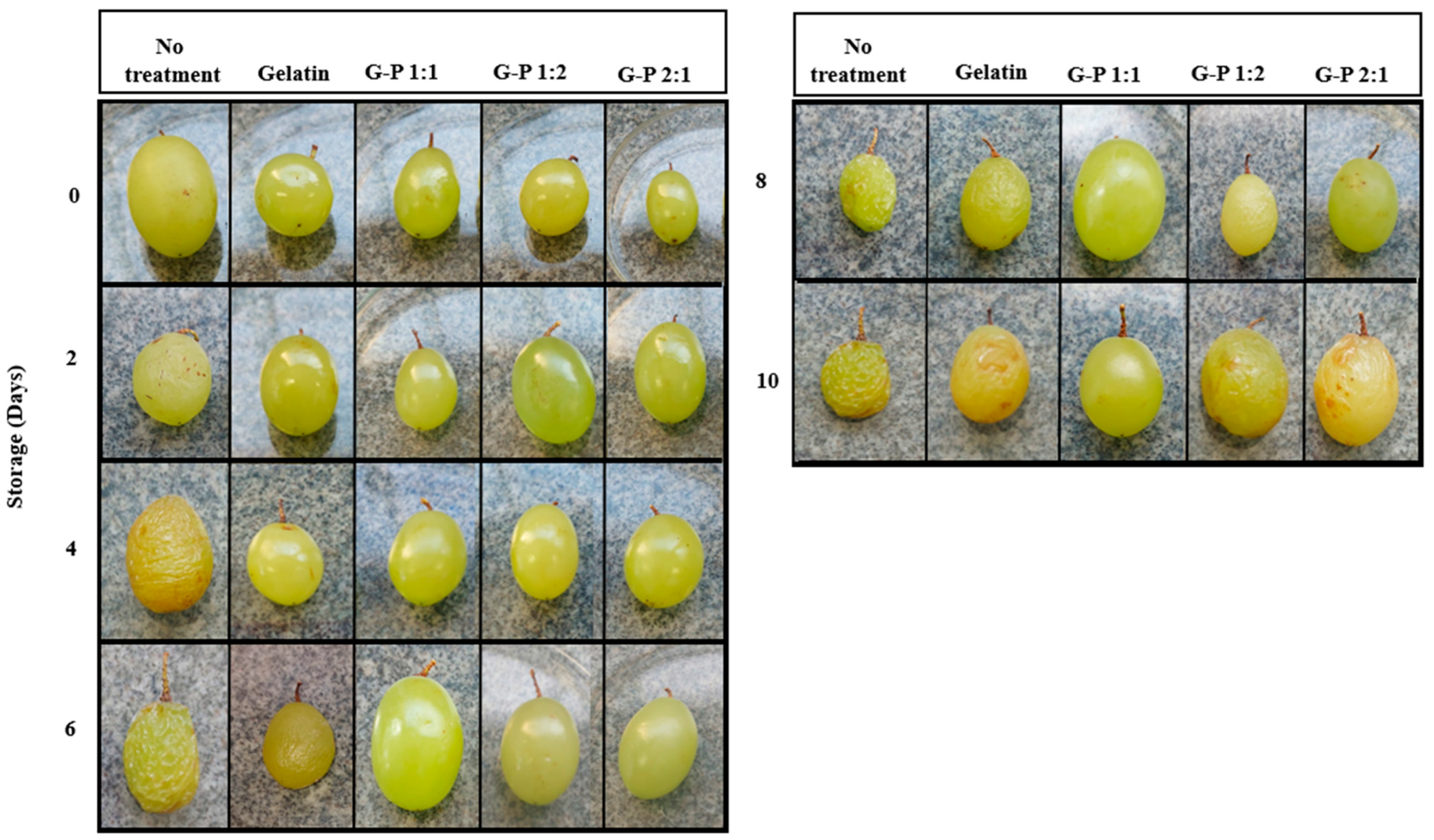
2.4. Preparation and Application of Cross-Linked Coatings on Ripe Grapes
2.5. Shelf-Life Study of Ripe Grapes
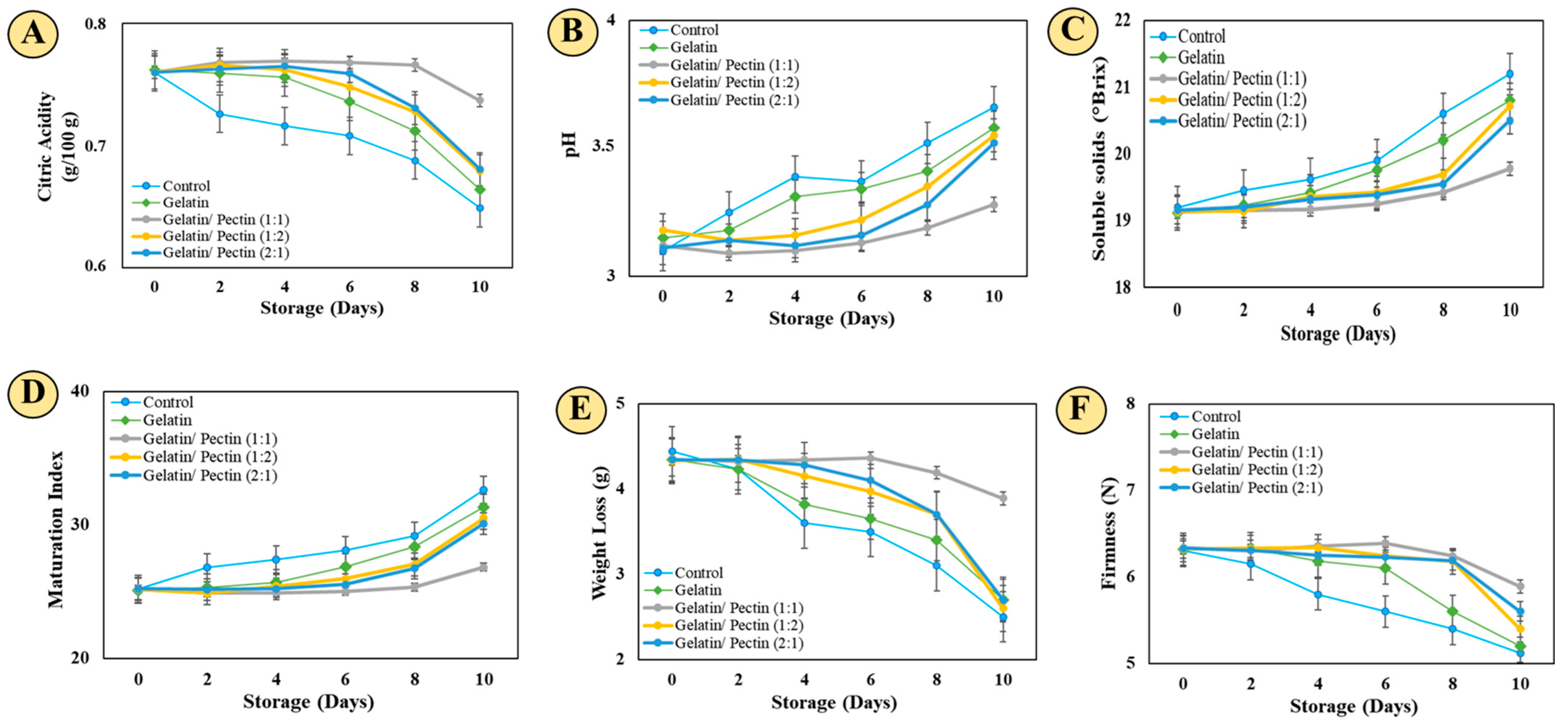
2.5.1. Titratable Acidity and pH
2.5.2. Soluble Solids and Maturation Index
2.5.3. Loss of Mass and Firmness
2.5.4. Thickness
2.6. Statistical Analysis
3. Results and Discussion
3.1. Reticulation Analysis
3.2. Shelf Life Study Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| G-P1:1 | Gelatin/pectin (m/m) in a 1:1 ratio. |
| G-P1:2 | Gelatin/pectin (m/m) in a 1:2 ratio. |
| G-P2:1 | Gelatin/pectin (m/m) in a 2:1 ratio. |
References
- Liu, W.; Fu, W.; Zhang, L.; Zhu, L. Preparation of modified atmosphere and moisture packaging membranes by blending quaternized polyether ether ketone with polyvinylidene fluoride and its application in grape packaging. Food Packag. Shelf Life 2024, 45, 101342. [Google Scholar] [CrossRef]
- Muhammed, A.P.; Thangarasu, S.; Manoharan, R.K.; Oh, T.H. Ex-situ fabrication of engineered green network of multifaceted bacterial cellulose film with enhanced antimicrobial properties for post-harvest preservation of table grapes. Food Packag. Shelf Life 2024, 43, 101284. [Google Scholar] [CrossRef]
- da Silva, D.J.; de Oliveira, M.M.; Wang, S.H.; Carastan, D.J.; Rosa, D.S. Designing antimicrobial polypropylene films with grape pomace extract for food packaging. Food Packag. Shelf Life 2022, 34, 100929. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Ma, W.; He, R.; Rong, Y.; Sarker, S.; Liu, Q.; Tian, F. A novel active packaging film containing citronella oil: Preparation, characterization, antimicrobial activity and application in grape preservation. Food Packag. Shelf Life 2023, 40, 101168. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Vertuccio, L.; Vittoria, V.; Pace, B.; Cefola, M.; Quintieri, L.; Bernardo, P.; Clarizia, G. Active packaging for table grapes: Evaluation of antimicrobial performances of packaging for shelf life of the grapes under thermal stress. Food Packag. Shelf Life 2020, 25, 100545. [Google Scholar] [CrossRef]
- Lou, L.; Chen, H.; Zhang, L. Biodegradable gelatin/pectin films containing cellulose nanofibers and biguanide polymers: Characterization and application in sweet cherry packaging. Int. J. Biol. Macromol. 2024, 274, 133530. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Kang, Y.R.; Chang, Y.H. Physicochemical and in vitro digestion properties of gelatin/low-methoxyl pectin synbiotic microgels co-encapsulating Lacticaseibacillus casei and pectic oligosaccharides via double-crosslinking with transglutaminase and calcium ions. Food Hydrocoll. 2023, 142, 108757. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Menezes, M.D.L.L.R.; da Rocha Pires, N.; da Cunha, P.L.R.; de Freitas Rosa, M.; de Souza, B.W.S.; de Andrade Feitosa, J.P.; de Souza, M.D.S.M. Effect of tannic acid as crosslinking agent on fish skin gelatin-silver nanocomposite film. Food Packag. Shelf Life 2019, 19, 7–15. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and high methyl pectin coacervates crosslinked with tannic acid: The characterization, rheological properties, and application for peppermint oil microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y.; Li, M.; Zhang, W.; Li, Q.; Tang, K. Investigation on the tunable effect of oxidized konjac glucomannan with different molecular weight on gelatin-based composite hydrogels. Int. J. Biol. Macromol. 2021, 168, 233–241. [Google Scholar] [CrossRef]
- Ding, F.; Wu, R.; Huang, X.; Shi, J.; Zou, X. Anthocyanin loaded composite gelatin films crosslinked with oxidized alginate for monitoring spoilage of flesh foods. Food Packag. Shelf Life 2024, 42, 101255. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Goyanes, S.; Garrigós, M.C.; Jiménez, A. Antioxidant water-resistant fish gelatin nanofibers: A comparative analysis of fructose and citric acid crosslinking and investigation of chlorogenic acid release kinetics. Food Hydrocoll. 2024, 150, 109696. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Norouzi, R.; Ramezani, S.; Ghorbani, M. Novel electrospun nanofibers based on gelatin/oxidized xanthan gum containing propolis reinforced by Schiff base cross-linking for food packaging. Food Chem. 2023, 416, 135806. [Google Scholar] [CrossRef]
- Zand-Rajabi, H.; Madadlou, A. Citric acid cross-linking of heat-set whey protein hydrogel influences its textural attributes and caffeine uptake and release behaviour. Int. Dairy J. 2016, 61, 142–147. [Google Scholar] [CrossRef]
- de Brito, R.S.; Costa, M.J.A.; Barbosa, J.R.; Santos Filho, A.F.; Farias, F.D.S.; Lourenço, L.D.F.H. K-Nearest Neighbor algorithm for selection of new gelatin-carboxymethylcellulose films with TiO2 nanoparticles and propolis extract with antioxidant and light barrier activity. Food Packag. Shelf Life 2023, 38, 101119. [Google Scholar] [CrossRef]
- da Silva, A.C.; Barbosa, J.R.; da Silva Araújo, C.; Batista, J.T.; Neves, E.M.; Cardoso, D.N.; Joele, M.R.; Lourenço, L.D. A new edible coating of fish gelatin incorporated into açaí oil to increase the post-harvest shelf life of tomatoes. Food Chem. 2024, 438, 138047. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; de la Caba, K. The effect of cross-linking with citric acid on the properties of agar/fish gelatin films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Amelia, S.T.; Adiningsih, S.N.; Widiyastuti, W.; Nurtono, T.; Setyawan, H.; Panatarani, C.; Praseptiangga, D.; Nazir, N.; Syamani, F.A. Novel cross-linking of toxic-free biopolymers for cellulose-gelatin films from avocado seed waste. Bioresour. Technol. Rep. 2024, 25, 101725. [Google Scholar] [CrossRef]
- Moreno-Ricardo, M.A.; Gómez-Contreras, P.; González-Delgado, Á.D.; Hernández-Fernández, J.; Ortega-Toro, R. Development of films based on chitosan, gelatin and collagen extracted from bocachico scales (Prochilodus magdalenae). Heliyon 2024, 10, e25194. [Google Scholar] [CrossRef]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, M.; Seon, H.; Choi, S.; Kim, H.J.; Kim, S. Tailoring dual cross-linked polymer-ionic liquid composites by blending co-crystallizable polymers for stretchable electronics. RSC Adv. 2024, 14, 36022–36030. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, S.; Nagao, M.; Matsumoto, H.; Miura, Y. Effects of the cross-linked structures of polymer gels containing iron porphyrins on photoreduction of carbon dioxide. RSC Appl. Polym. 2024, 2, 1026–1031. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Cheng, J.; Yao, K.; Shi, L.; Zhou, X. Non-destructive estimation for Kyoho grape shelf-life using Vis/NIR hyperspectral imaging and deep learning algorithm. Infrared Phys. Technol. 2024, 142, 105532. [Google Scholar] [CrossRef]
- Bodana, V.; Swer, T.L.; Kumar, N.; Singh, A.; Samtiya, M.; Sari, T.P.; Babar, O.A. Development and characterization of pomegranate peel extract-functionalized jackfruit seed starch-based edible films and coatings for prolonging the shelf life of white grapes. Int. J. Biol. Macromol. 2024, 254, 127234. [Google Scholar] [CrossRef]
- Hadimani, S.; Supriya, D.; Roopa, K.; Soujanya, S.K.; Rakshata, V.; Netravati, A.; Akshayakumar, V.; De Britto, S.; Jogaiah, S. Biodegradable hybrid biopolymer film based on carboxy methyl cellulose and selenium nanoparticles with antifungal properties to enhance grapes shelf life. Int. J. Biol. Macromol. 2023, 237, 124076. [Google Scholar] [CrossRef]
- Fu, B.; Mei, S.; Su, X.; Chen, H.; Zhu, J.; Zheng, Z.; Lin, H.; Dai, C.; Luque, R.; Yang, D.-P. Integrating waste fish scale-derived gelatin and chitosan into edible nanocomposite film for perishable fruits. Int. J. Biol. Macromol. 2021, 191, 1164–1174. [Google Scholar] [CrossRef]
- Singh, H. Modification of food proteins by covalent crosslinking. Trends Food Sci. Technol. 1991, 2, 196–200. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, N.; Zhao, X.H. The non-covalent interaction between two polyphenols and caseinate as affected by two types of enzymatic protein crosslinking. Food Chem. 2021, 364, 130375. [Google Scholar] [CrossRef] [PubMed]
| Sample | Thickness (mm) |
|---|---|
| Control | 0.0 |
| Gelatin | 0.141 ± 0.002 a |
| G-P1:1 | 0.201 ± 0.003 b |
| G-P1:2 | 0.312 ± 0.004 c |
| G-P2:1 | 0.311 ± 0.012 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, J.R.; Gonçalves, M.A.; da Silva Martins, L.H.; de Fátima Henriques Lourenço, L. Citric Acid Cross-Linked Gelatin/Pectin Coatings Increase Shelf Life of Ripe Grapes. Coatings 2025, 15, 129. https://doi.org/10.3390/coatings15020129
Barbosa JR, Gonçalves MA, da Silva Martins LH, de Fátima Henriques Lourenço L. Citric Acid Cross-Linked Gelatin/Pectin Coatings Increase Shelf Life of Ripe Grapes. Coatings. 2025; 15(2):129. https://doi.org/10.3390/coatings15020129
Chicago/Turabian StyleBarbosa, Jhonatas Rodrigues, Magally Araújo Gonçalves, Luiza Helena da Silva Martins, and Lúcia de Fátima Henriques Lourenço. 2025. "Citric Acid Cross-Linked Gelatin/Pectin Coatings Increase Shelf Life of Ripe Grapes" Coatings 15, no. 2: 129. https://doi.org/10.3390/coatings15020129
APA StyleBarbosa, J. R., Gonçalves, M. A., da Silva Martins, L. H., & de Fátima Henriques Lourenço, L. (2025). Citric Acid Cross-Linked Gelatin/Pectin Coatings Increase Shelf Life of Ripe Grapes. Coatings, 15(2), 129. https://doi.org/10.3390/coatings15020129







