Surface Modification of Bioactive Glasses by Femtosecond and CO2 Lasers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Laser Surface Texturing
2.2. Surface Characterization
3. Results and Discussion
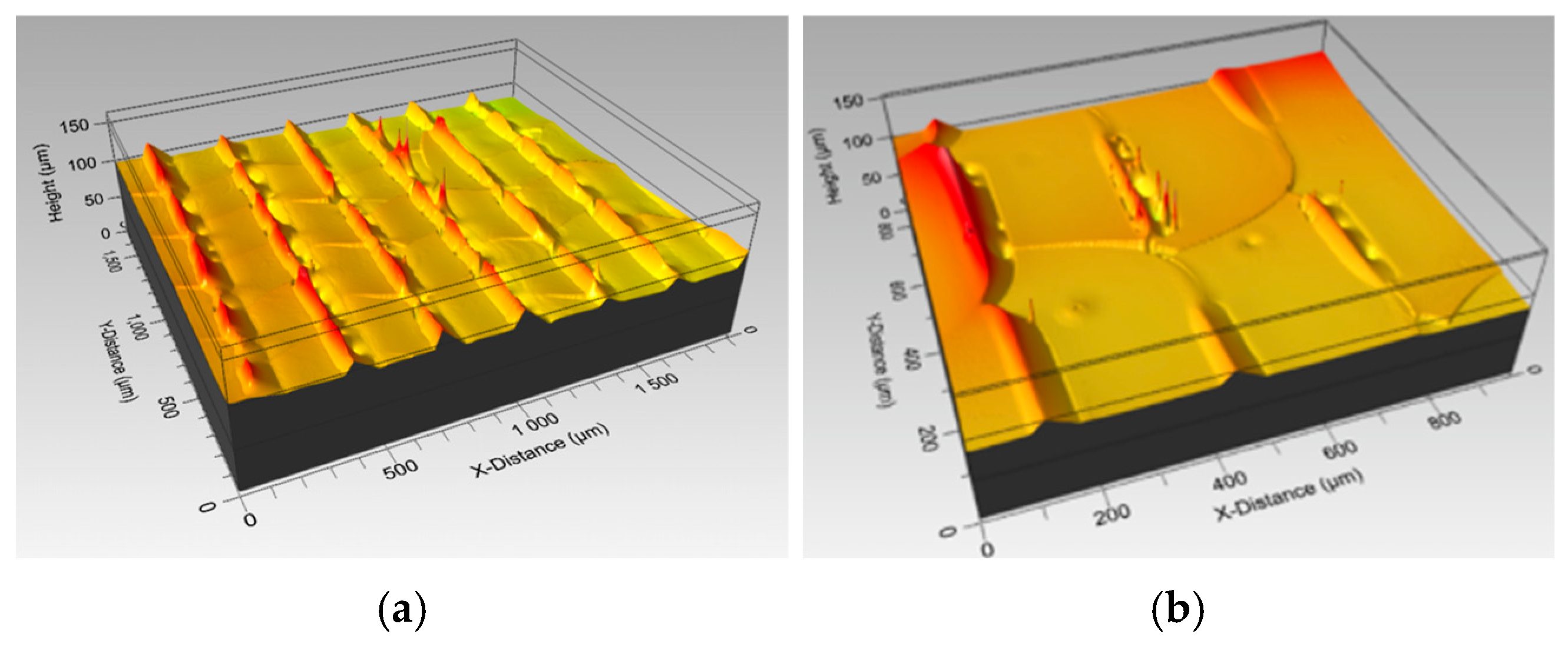
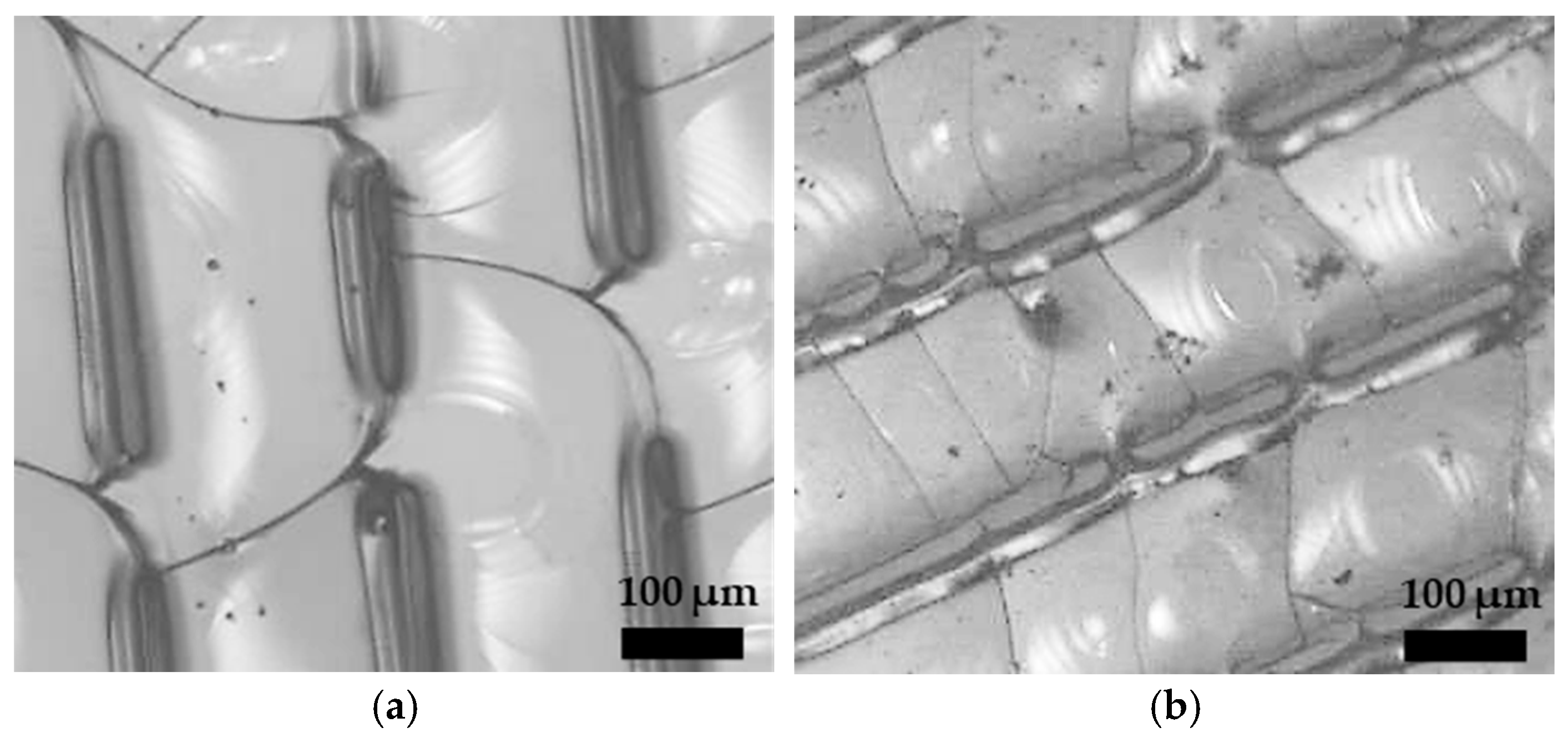
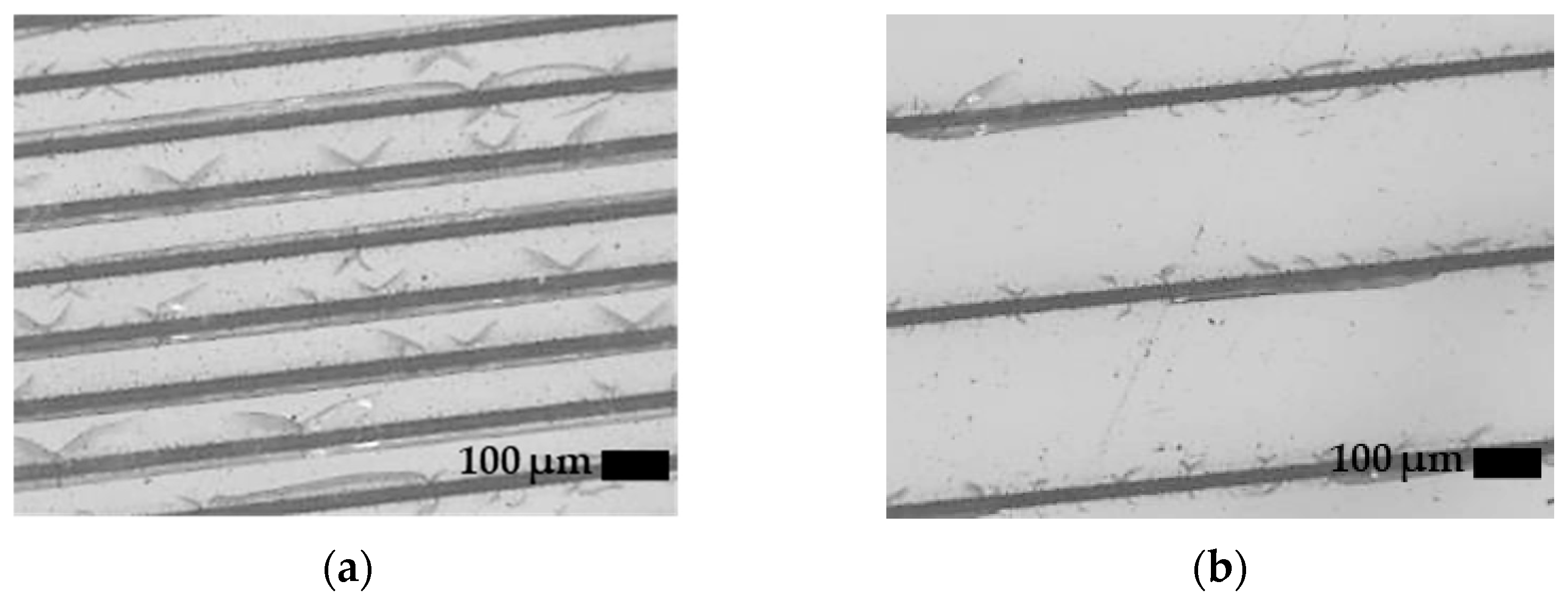
3.1. CO2 Laser Surface Modification. Surface Roughness Dependence on Processing Parameters and Topography
3.2. Femtosecond Laser Surface Modification. Surface Roughness Dependence on Pass Number and Topography
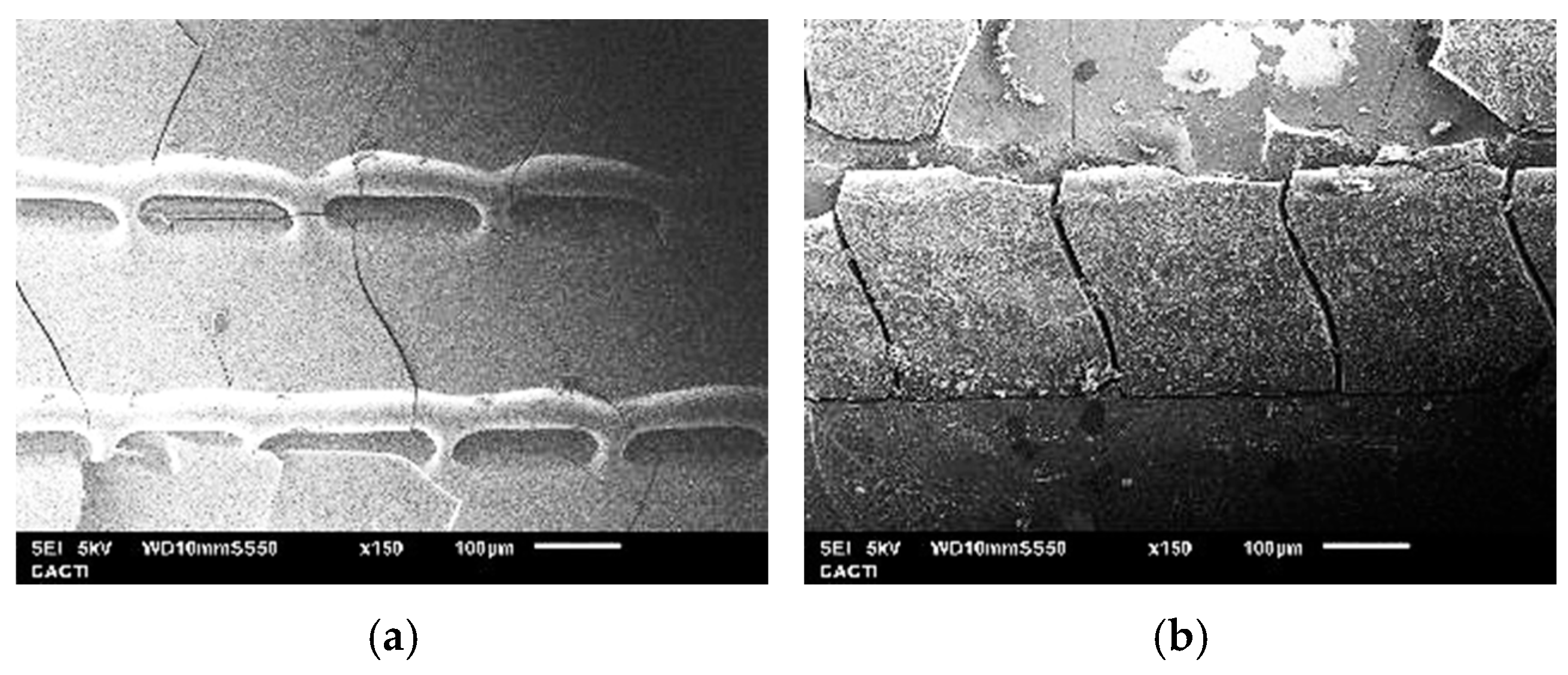
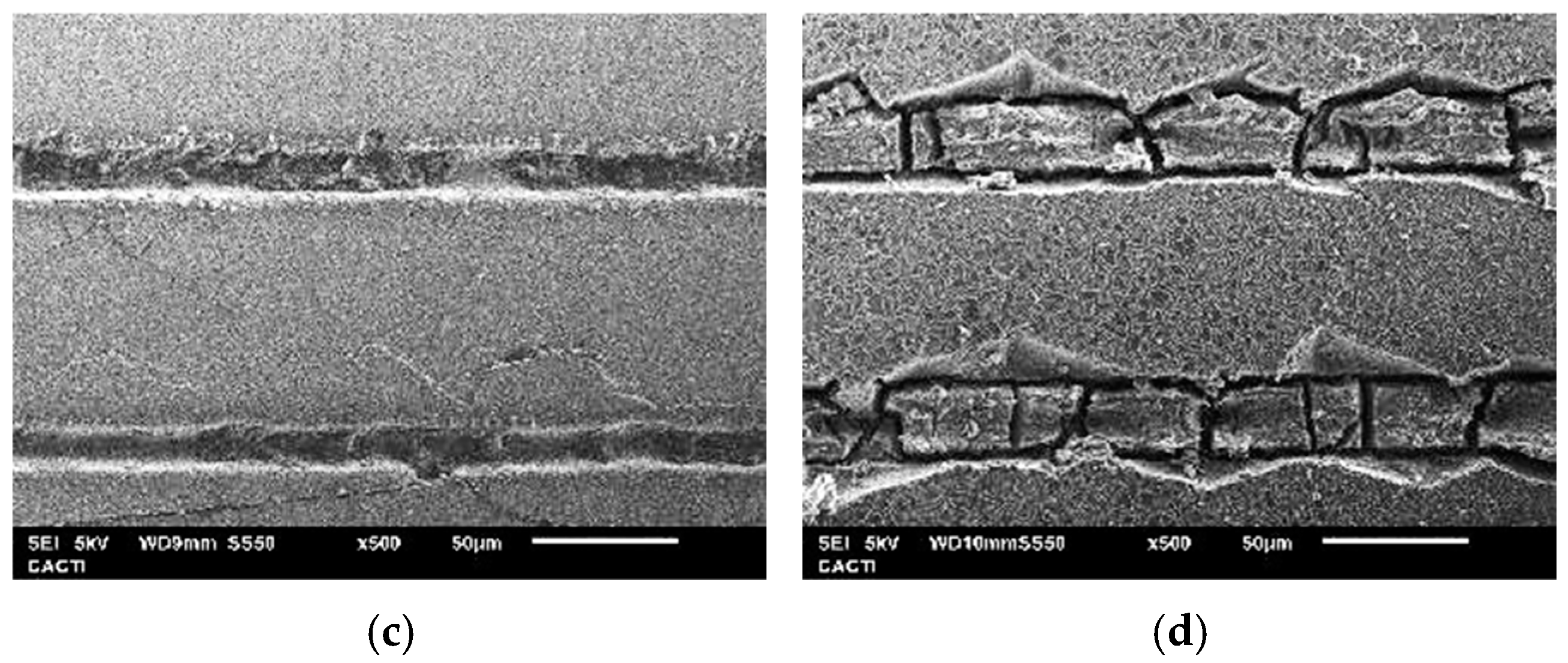
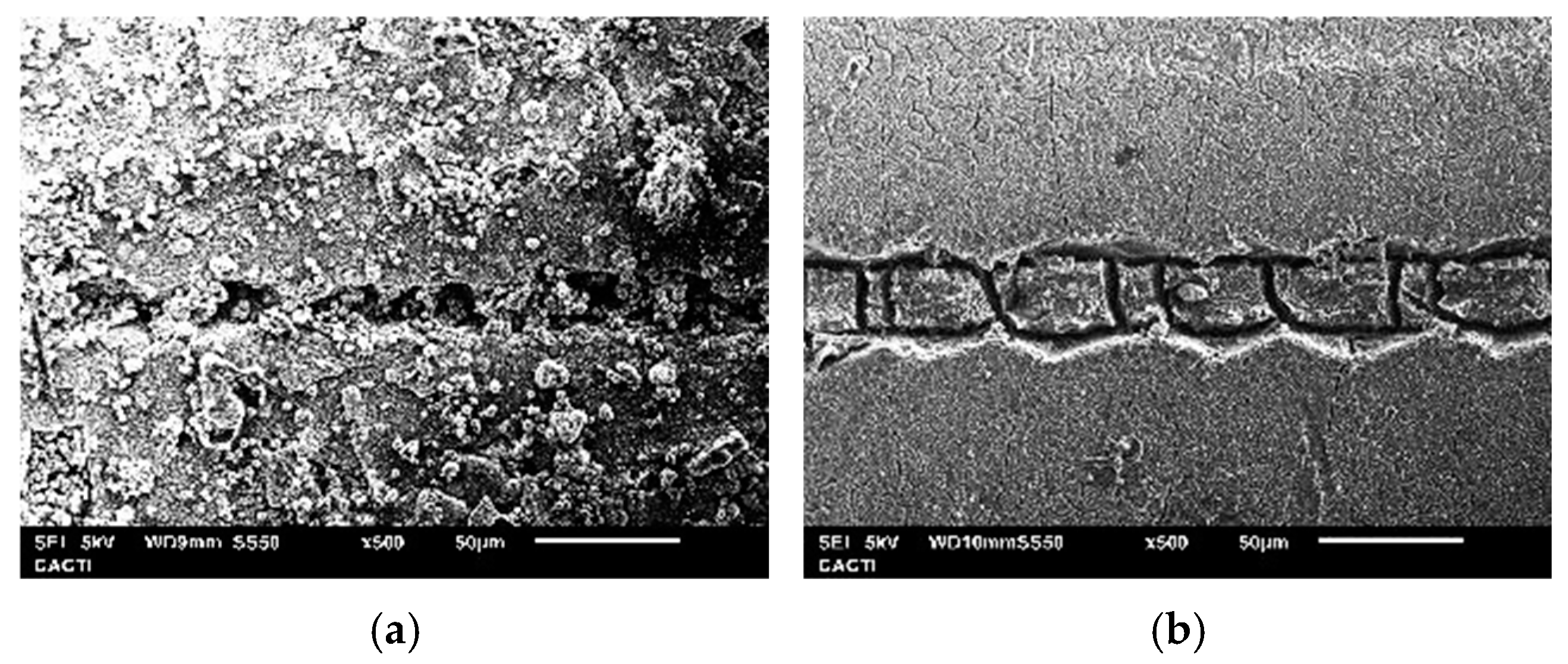
3.3. Surface Hardness and Fracture Toughness
3.4. Contact Angle
3.5. Roughness Evolution During Immersion in Tris-HCl Buffer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, T.K.; Mittinty, M.M.; March, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Woolf, A.D.; Haile, L.M.; Hagins, H.; et al. Global, Regional, and National Burden of Other Musculoskeletal Disorders, 1990–2020, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e670–e682. [Google Scholar] [CrossRef] [PubMed]
- Kooy, C.E.V.W.; Jakobsen, R.B.; Fenstad, A.M.; Persson, A.; Visnes, H.; Engebretsen, L.; Ekås, G.R. Major Increase in Incidence of Pediatric ACL Reconstructions From 2005 to 2021: A Study from the Norwegian Knee Ligament Register. Am. J. Sports Med. 2023, 51, 2891. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, Allograft and Bone Substitutes in Reconstructive Orthopedic Surgery. Aging Clin. Exp. Res. 2013, 25, 101–103. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Greenspan, D.C. Glass and Medicine: The Larry Hench Story. Int. J. Appl. Glass Sci. 2016, 7, 134–138. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, D.; Kumar, P.; Yadav, A.; Nirala, G.; Yadav, S. Bioactive Glass for Biomedical Application: An Overview. In Defects Engineering in Electroceramics for Energy Applications; Springer: Singapore, 2024; pp. 305–327. [Google Scholar] [CrossRef]
- Jain, S.; Raghavendra, G.; Naik, R.H.; Daloji, L.; Azeem, P.A. Exploring the Versatility of Phosphate-Based Bioactive Glass for Biomedical Applications. In Recent Advances in Mechanical Engineering, Volume 1 (ICMech-REC 2023); Springer: Singapore, 2024; pp. 673–685. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive Calcium Phosphate–Based Glasses and Ceramics and Their Biomedical Applications: A Review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aguilar, C. Porous Phosphate-Based Bioactive Glass/β-TCP Scaffold for Tooth Remineralization. PLoS ONE 2023, 18, e0284885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kedia, S.; Singh, A.K.; Basak, C.B.; Chauhan, A.K.; Basu, S.; Sinha, S. Morphology and Structural Studies of Laser Treated 45S5 Bioactive Glass. J. Non Cryst. Solids 2016, 440, 43–48. [Google Scholar] [CrossRef]
- Szada-Borzyszkowska, M.; Kacalak, W.; Szada-Borzyszkowski, W.; Borkowski, P.J.; Laskowska, D.; Szafraniec, F. Topography of Textured Surfaces Using an Abrasive-Water Jet Technology. Arch. Civ. Mech. Eng. 2024, 24, 226. [Google Scholar] [CrossRef]
- Maximov, M.; Maximov, O.C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive Glass—An Extensive Study of the Preparation and Coating Methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Taye, M.B.; Ningsih, H.S.; Shih, S.J. Exploring the Advancements in Surface-Modified Bioactive Glass: Enhancing Antibacterial Activity, Promoting Angiogenesis, and Modulating Bioactivity. J. Nanopart. Res. 2024, 26, 28. [Google Scholar] [CrossRef]
- Kargozar, S.; Kermani, F.; Beidokhti, S.M.; Hamzehlou, S.; Verné, E.; Ferraris, S.; Baino, F. Functionalization and Surface Modifications of Bioactive Glasses (BGs): Tailoring of the Biological Response Working on the Outermost Surface Layer. Materials 2019, 12, 3696. [Google Scholar] [CrossRef]
- Jonušauskas, L.; Martínez Vázquez, R.; Bragheri, F.; Paiè, P. 3D Manufacturing of Glass Microstructures Using Femtosecond Laser. Micromachines 2021, 12, 499. [Google Scholar] [CrossRef]
- Ferraris, S.; Vitale-Brovarone, C.; Bretcanu, O.; Cassinelli, C.; Vernè, E. Surface Functionalization of 3D Glass–Ceramic Porous Scaffolds for Enhanced Mineralization in vitro. Appl. Surf. Sci. 2013, 271, 412–420. [Google Scholar] [CrossRef]
- Shivakoti, I.; Kibria, G.; Cep, R.; Pradhan, B.B.; Sharma, A. Laser Surface Texturing for Biomedical Applications: A Review. Coatings 2021, 11, 124. [Google Scholar] [CrossRef]
- Pani, R.; Roy, S.; Behera, R.R.; Chakraborty, S.; Das, D.; Mahanta, K. Laser Texturing for Biomedical Application: A State of Art. AIP Conf. Proc. 2024, 3007, 100026. [Google Scholar] [CrossRef]
- Shaikh, S.; Kedia, S.; Singh, A.K.; Sharma, K.; Sinha, S. Surface Treatment of 45S5 Bioglass Using Femtosecond Laser to Achieve Superior Growth of Hydroxyapatite. J. Laser Appl. 2017, 29, 022004. [Google Scholar] [CrossRef]
- Schille, J.; Schneider, L.; Mauersberger, S.; Szokup, S.; Höhn, S.; Pötschke, J.; Reiß, F.; Leidich, E.; Löschner, U. High-Rate Laser Surface Texturing for Advanced Tribological Functionality. Lubricants 2020, 8, 33. [Google Scholar] [CrossRef]
- Korovessis, P.G.; Deligianni, D.D. Role of Surface Roughness of Titanium versus Hydroxyapatite on Human Bone Marrow Cells Response. J. Spinal Disord. Tech. 2002, 15, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of Surface Roughness of Hydroxyapatite on Human Bone Marrow Cell Adhesion, Proliferation, Differentiation and Detachment Strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Mehmood, S.; Liu, X.; Liu, Y.; Wang, G.; Pei, R. Decoding Bone-Inspired and Cell-Instructive Cues of Scaffolds for Bone Tissue Engineering. Eng. Reg. 2024, 5, 21–44. [Google Scholar] [CrossRef]
- Saiz, E.; Boccaccini, A.R.; Chevalier, J.; Peroglio, M. Editorial on Bioceramics for Bone Repair. J. Eur. Ceram. Soc. 2018, 38, 821–822. [Google Scholar] [CrossRef]
- Elgayar, I.; Aliev, A.E.; Boccaccini, A.R.; Hill, R.G. Structural Analysis of Bioactive Glasses. J. Non Cryst. Solids 2005, 351, 173–183. [Google Scholar] [CrossRef]
- ISO 25178-2; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Anstis, G.R.; Chantikul, P.; Lawn, B.R.; Marshall, D.B. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: I, Direct Crack Measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Miyoshi, T.; Sagawa, N.; Sassa, T. Study on fracture toughness evaluation for structural ceramics. Trans. Jpn. Soc. Mech. Eng. A 1985, 51, 2489–2497. [Google Scholar] [CrossRef]
- Quinn, G.D.; Bradt, R.C. On the Vickers indentation fracture toughness test. J. Am. Ceram. Soc. 2007, 90, 673–680. [Google Scholar] [CrossRef]
- Del Val, J.; López-Cancelos, R.; Riveiro, A.; Badaoui, A.; Lusquiños, F.; Quintero, F.; Comesaña, R.; Boutinguiza, M.; Pou, J. On the fabrication of bioactive glass implants for bone regeneration by laser assisted rapid prototyping based on laser cladding. Ceram. Int. 2016, 42, 2021–2035. [Google Scholar] [CrossRef]
- ISO 10993-14; Biological Evaluation of Medical Devices—Part 14: Identification and Quantification of Degradation Products from Ceramics. International Organization for Standardization (ISO): Geneva, Switzerland, 2001.
- Comesaña, R.; Riveiro, A.; del Val, J.; Badaoui, A.; Penide, J.; Quintero, F.; Boutinguiza, M.; Lusquiños, F.; Pou, J. Laser Surface Modification of Structural Glass for Anti-Slip Applications. Ceram. Int. 2019, 45, 24734–24741. [Google Scholar] [CrossRef]
- Calvo-García, E.; Comesaña, R.; Riveiro, A.; del Val, J.; Badaoui, A.; Quintero, F.; Boutinguiza, M.; Lusquiños, F.; Pou, J. Experimental Study of Strength and Fracture Toughness of Laser-Blasted Glass. Procedia Struct. Integr. 2022, 42, 251–258. [Google Scholar] [CrossRef]
- Bajda, S.; Cholewa-Kowalska, K.; Krzyzanowski, M.; Dziadek, M.; Kopyscianski, M.; Liu, Y.; Rai, A. Laser-Directed Energy Deposition of Bioactive Glass on Ti-6Al-7Nb Titanium Alloy Substrate with Highly Refined Grain Structure. Surf. Coat. Technol. 2024, 485, 130904. [Google Scholar] [CrossRef]
- Bajda, S.; Liu, Y.; Tosi, R.; Cholewa-Kowalska, K.; Krzyzanowski, M.; Dziadek, M.; Kopyscianski, M.; Dymek, S.; Polyakov, A.V.; Semenova, I.P.; et al. Laser Cladding of Bioactive Glass Coating on Pure Titanium Substrate with Highly Refined Grain Structure. J. Mech. Behav. Biomed. Mater. 2021, 119, 104519. [Google Scholar] [CrossRef]
- Comesaña, R.; del Val, J.; Quintero, F.; Riveiro, A.; Arias-González, F.; Boutinguiza, M.; Lusquiños, F.; Pou, J. Laser Cladding and Laser Direct Glass Deposition of Bioactive Glass and Glass-Ceramics. In Bioactive Glasses and Glass-Ceramics: Fundamentals and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2022; pp. 311–340. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Qu, S.; Dong, S. Plastic Residual Strains under Femtosecond Laser Microexplosion Initiated High Pressure inside Quartz. Int. J. Nanomanuf. 2013, 9, 10–18. [Google Scholar] [CrossRef]
- Locker, S.; Sundaram, S.K. Ultrafast Modification of Oxide Glass Surface Hardness. Appl. Phys. B 2019, 125, 225. [Google Scholar] [CrossRef]
- Alqurashi, T.; Alnufaili, M.; Hassan, M.U.; Aloufi, S.; Yetisen, A.K.; Butt, H. Laser Inscription of Microfluidic Devices for Biological Assays. ACS Appl. Mater. Interfaces 2019, 11, 12253–12260. [Google Scholar] [CrossRef]
- Kostal, E.; Stroj, S.; Kasemann, S.; Matylitsky, V.; Domke, M. Fabrication of Biomimetic Fog-Collecting Superhydrophilic-Superhydrophobic Surface Micropatterns Using Femtosecond Lasers. Langmuir 2018, 34, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Comesaña, R.; Lusquiños, F.; Del Val, J.; López-Álvarez, M.; Quintero, F.; Riveiro, A.; Boutinguiza, M.; De Carlos, A.; Jones, J.R.; Hill, R.G.; et al. Three-Dimensional Bioactive Glass Implants Fabricated by Rapid Prototyping Based on CO2 Laser Cladding. Acta Biomater. 2011, 7, 3476–3487. [Google Scholar] [CrossRef]
- Westhauser, F.; Hohenbild, F.; Arango-Ospina, M.; Schmitz, S.I.; Wilkesmann, S.; Hupa, L.; Moghaddam, A.; Boccaccini, A.R. Bioactive Glass (BG) ICIE16 Shows Promising Osteogenic Properties Compared to Crystallized 45S5-BG. Int. J. Mol. Sci. 2020, 21, 1639. [Google Scholar] [CrossRef]
- Groh, D.; Döhler, F.; Brauer, D.S. Bioactive Glasses with Improved Processing. Part 1. Thermal Properties, Ion Release and Apatite Formation. Acta Biomater. 2014, 10, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Q.; de Pablos-Martín, A.; Contreras Jaimes, A.T.; Scheffler, F.; Wagner, T.; Brauer, D.S.; Boccaccini, A.R. Comparison of Microstructure, Sintering Behavior, and Biological Response of Sol-Gel and Melt-Derived 13–93 Bioactive Glass Scaffolds. Open Ceram. 2023, 15, 100407. [Google Scholar] [CrossRef]
- Liang, W.; Wu, X.; Dong, Y.; Shao, R.; Chen, X.; Zhou, P.; Xu, F. In Vivo Behavior of Bioactive Glass-Based Composites in Animal Models for Bone Regeneration. Biomater. Sci. 2021, 9, 1924–1944. [Google Scholar] [CrossRef]
- Gough, J.E.; Notingher, I.; Hench, L.L. Osteoblast Attachment and Mineralized Nodule Formation on Rough and Smooth 45S5 Bioactive Glass Monoliths. J. Biomed. Mater. Res. A 2004, 68A, 640–650. [Google Scholar] [CrossRef] [PubMed]








| Composition (wt%) | ||
|---|---|---|
| Oxide | ICIE16 | 45S5 |
| SiO2 | 48.0 | 45 |
| CaO | 32.9 | 24.5 |
| P2O5 | 2.5 | 6.0 |
| K2O | 10.0 | - |
| Na2O | 6.6 | 24.5 |
| NC | 2.04 | 1.90 |
| Laser Source | ||
|---|---|---|
| Parameters (Units) | CO2 | fs Yb |
| Wavelength (nm) | 10,600 | 532 |
| Power (W) | 6–25 | 25 |
| Scanning speed (mm/s) | 40–500 | 100 |
| Lattice spacing (μm) | 30–500 | 100 and 300 |
| Number of Passes | 1.0 | 1–50 |
| Focal distance (mm) | 280.0 | 9.5 |
| Steps | Procedures (Materials, Quantities, and Task) |
|---|---|
| 1 | Prepare 800 mL of distilled water in an appropriate container. |
| 2 | Add 121.14 g of Tris base (molecular weight 121.14 g/mol) to the solution. |
| 3 | Adjust the solution to the desired pH using HCl (pH ≈ 7.4). |
| 4 | Add distilled water until the total volume reaches 1 L. |
| Specimen | Hardness (HV0.3) | KIc (MPa·m1/2) Miyoshi [29] | KIc (MPa·m1/2) Anstis [28] | Fracture Surface Energy (J/m2) | Contact Angle (°) |
|---|---|---|---|---|---|
| 45S5 Ref. | 524 ± 16 | 0.55 ± 0.10 | 0.49 ± 0.09 | 0.72 ± 0.24 | 39.9 ± 8.9 |
| ICIE16 Ref. | 562 ± 11 | 0.58 ±0.07 | 0.52 ± 0.06 | 0.80 ± 0.18 | 36.0 ± 7.6 |
| CO2 45S5 | 573 ± 39 | 0.54 ± 0.22 | 0.48 ± 0.20 | 0.71 ± 0.61 | 63.2 ± 8.1 |
| CO2 ICIE16 | 561 ± 35 | 0.54 ± 0.17 | 0.49 ± 0.15 | 0.76 ± 0.49 | 59.1 ± 6.4 |
| fs 45S5 | 464 ± 37 | 0.56 ± 0.03 | 0.50 ± 0.03 | 0.73 ± 0.09 | 37.4 ± 2.0 |
| fs ICIE16 | 482 ± 21 | 0.54 ± 0.16 | 0.45 ± 0.07 | 0.60 ± 0.20 | 38.7 ± 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Quintas, M.; Gago-Vidal, B.; Calvo-García, E.; Sajjad, H.; Riveiro, A.; Comesaña, R.; Pou, J. Surface Modification of Bioactive Glasses by Femtosecond and CO2 Lasers. Coatings 2025, 15, 195. https://doi.org/10.3390/coatings15020195
González-Quintas M, Gago-Vidal B, Calvo-García E, Sajjad H, Riveiro A, Comesaña R, Pou J. Surface Modification of Bioactive Glasses by Femtosecond and CO2 Lasers. Coatings. 2025; 15(2):195. https://doi.org/10.3390/coatings15020195
Chicago/Turabian StyleGonzález-Quintas, Mario, Bruno Gago-Vidal, Erik Calvo-García, Hamza Sajjad, Antonio Riveiro, Rafael Comesaña, and Juan Pou. 2025. "Surface Modification of Bioactive Glasses by Femtosecond and CO2 Lasers" Coatings 15, no. 2: 195. https://doi.org/10.3390/coatings15020195
APA StyleGonzález-Quintas, M., Gago-Vidal, B., Calvo-García, E., Sajjad, H., Riveiro, A., Comesaña, R., & Pou, J. (2025). Surface Modification of Bioactive Glasses by Femtosecond and CO2 Lasers. Coatings, 15(2), 195. https://doi.org/10.3390/coatings15020195









