Effect of Holding Temperature on Wear and Corrosion Resistance of Rare Earth Oxide Thermally Diffused Zinc Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Wear Test at Room Temperature
2.3. Corrosion Resistance Test
3. Results and Discussion
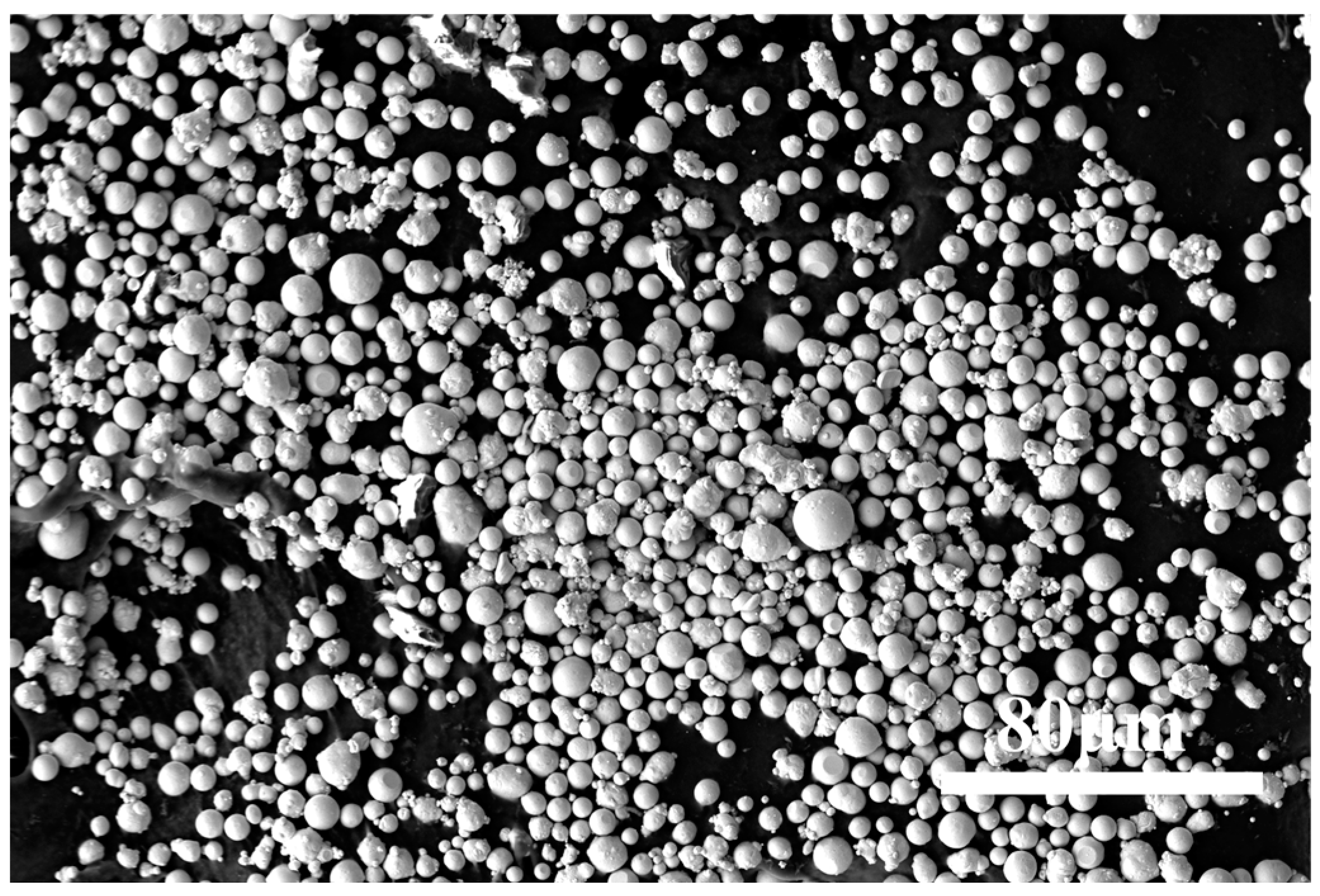
3.1. Effect of Different Holding Temperature on Morphology of Thermally Diffused Zinc Coatings
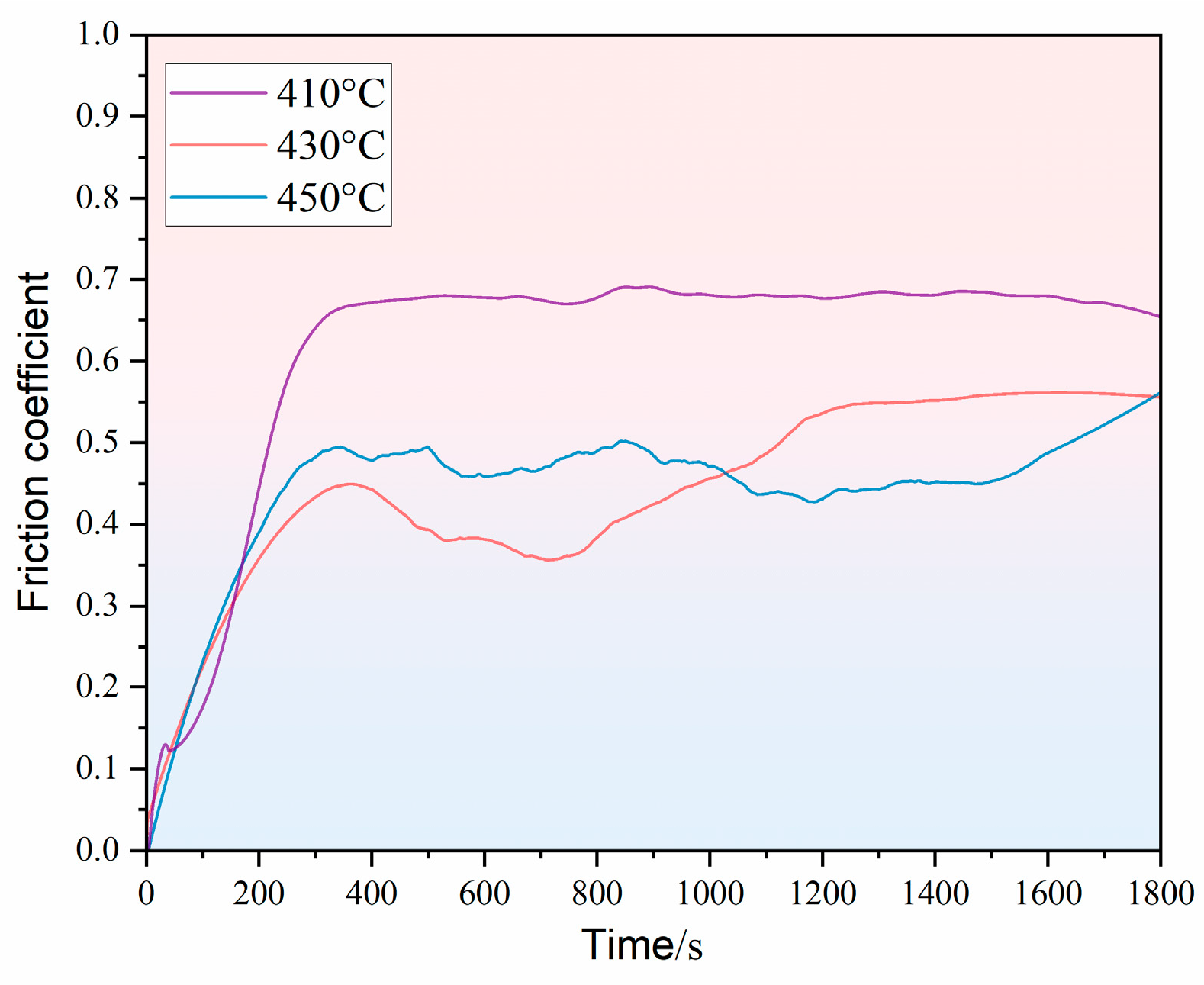
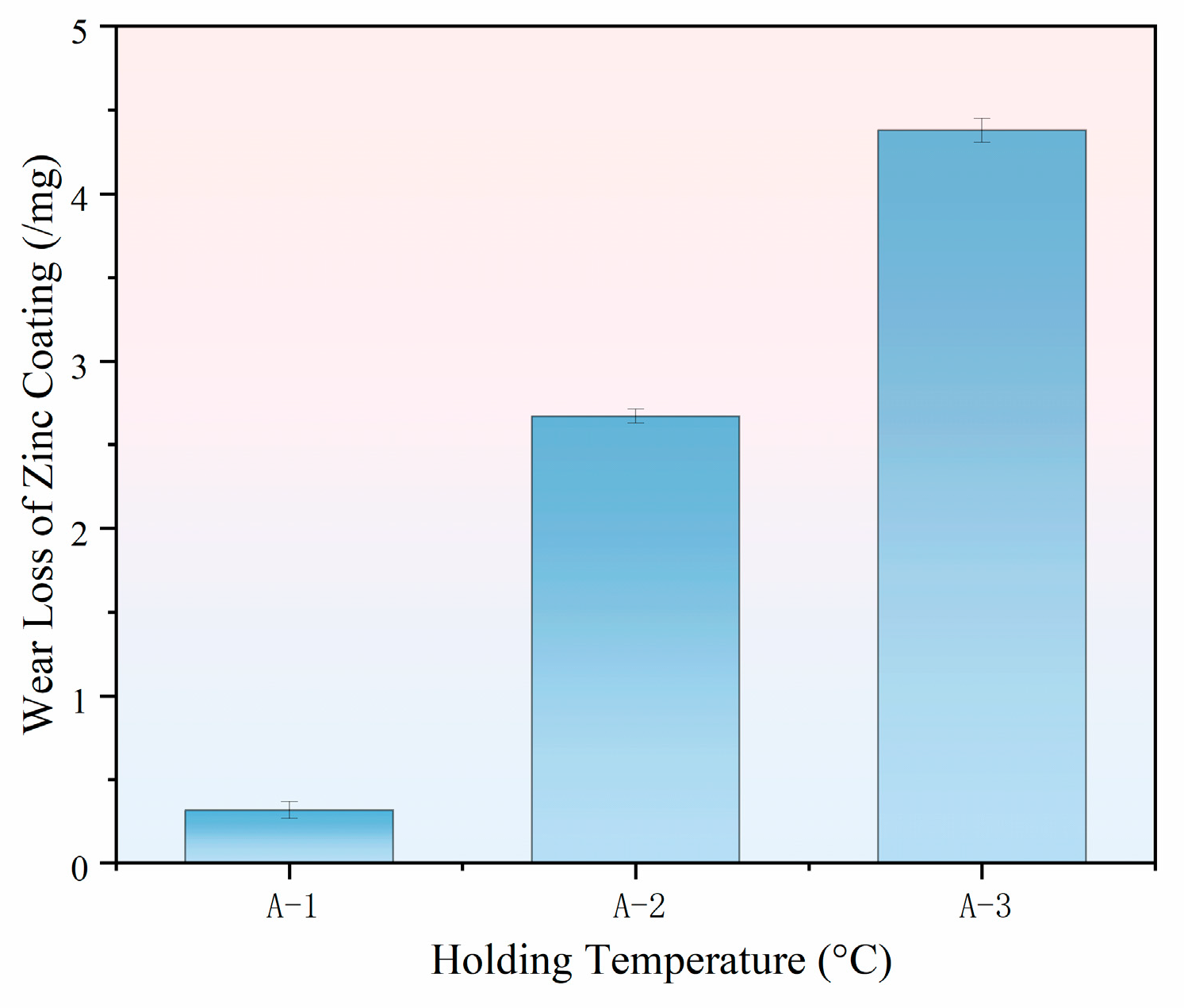
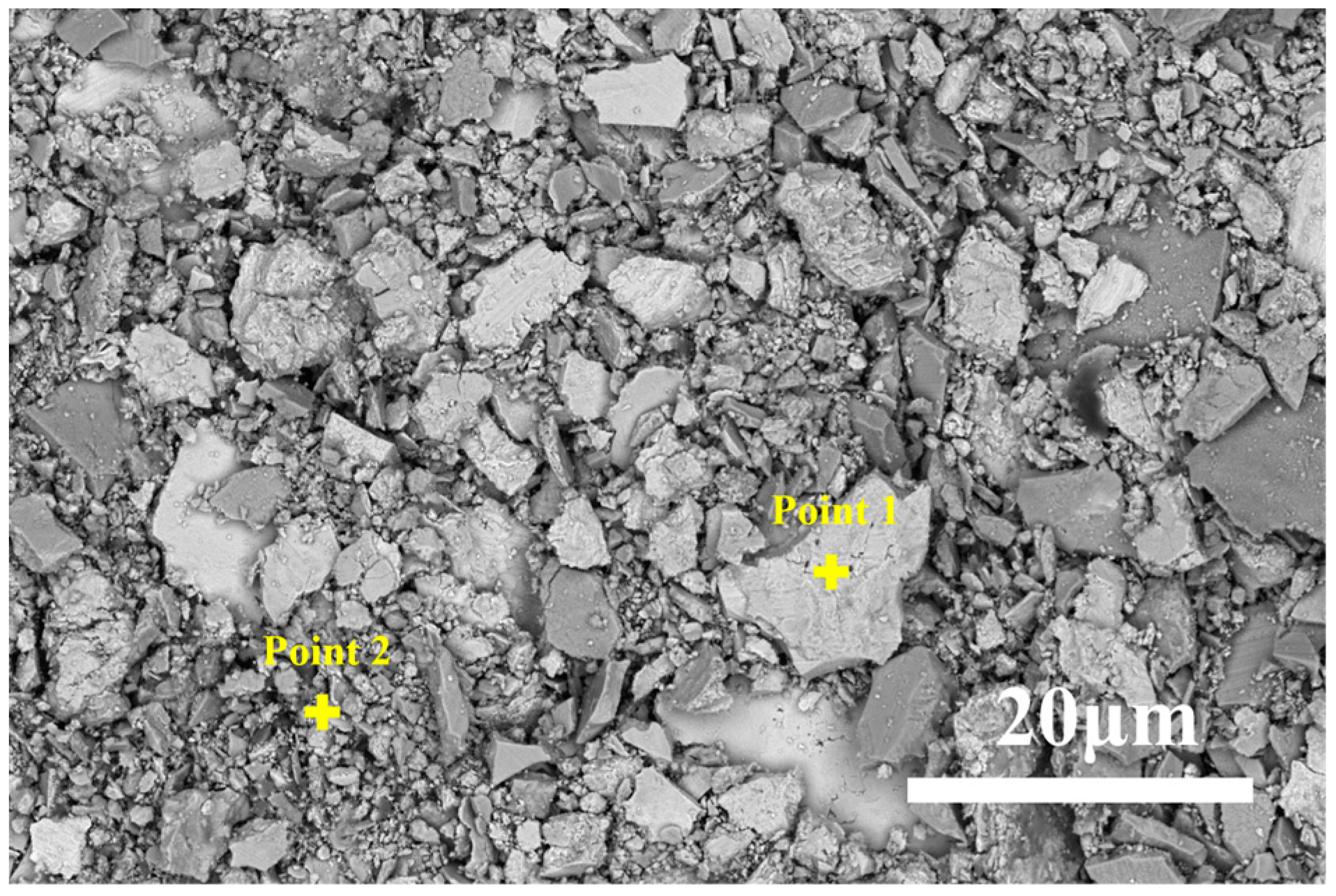
3.2. Effect of Different Holding Temperatures on the Wear Resistance of Thermally Diffused Zinc Coatings
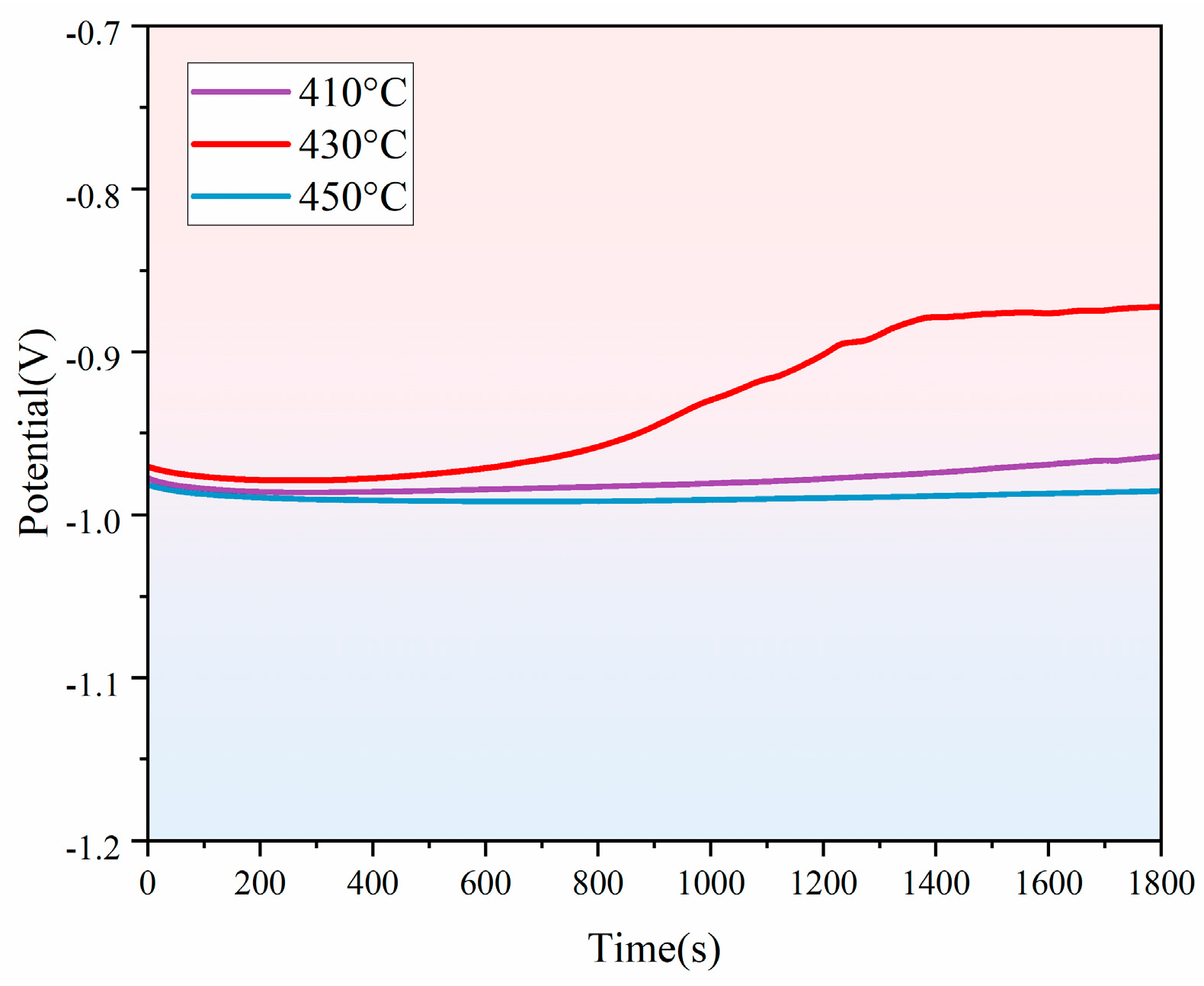
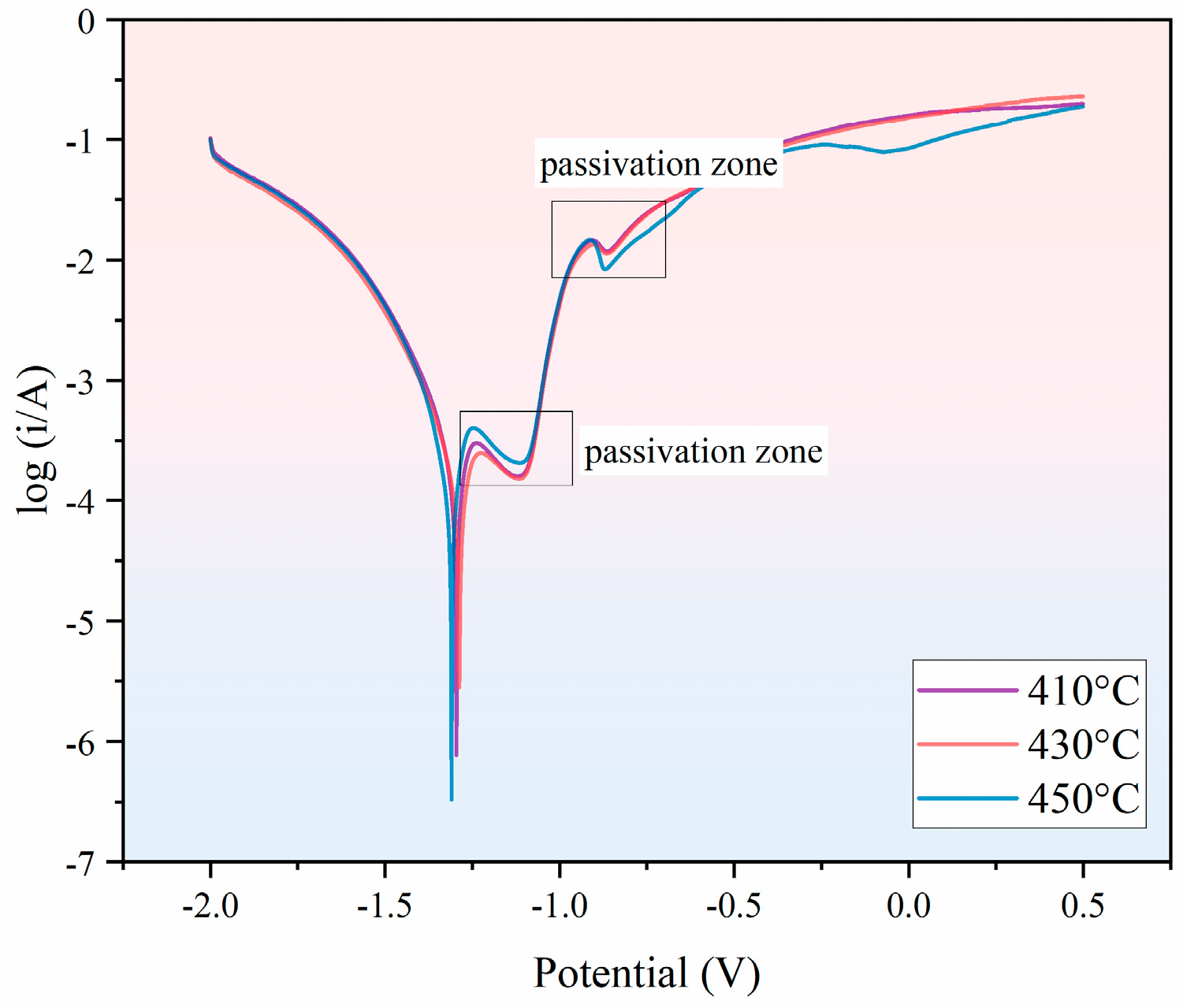
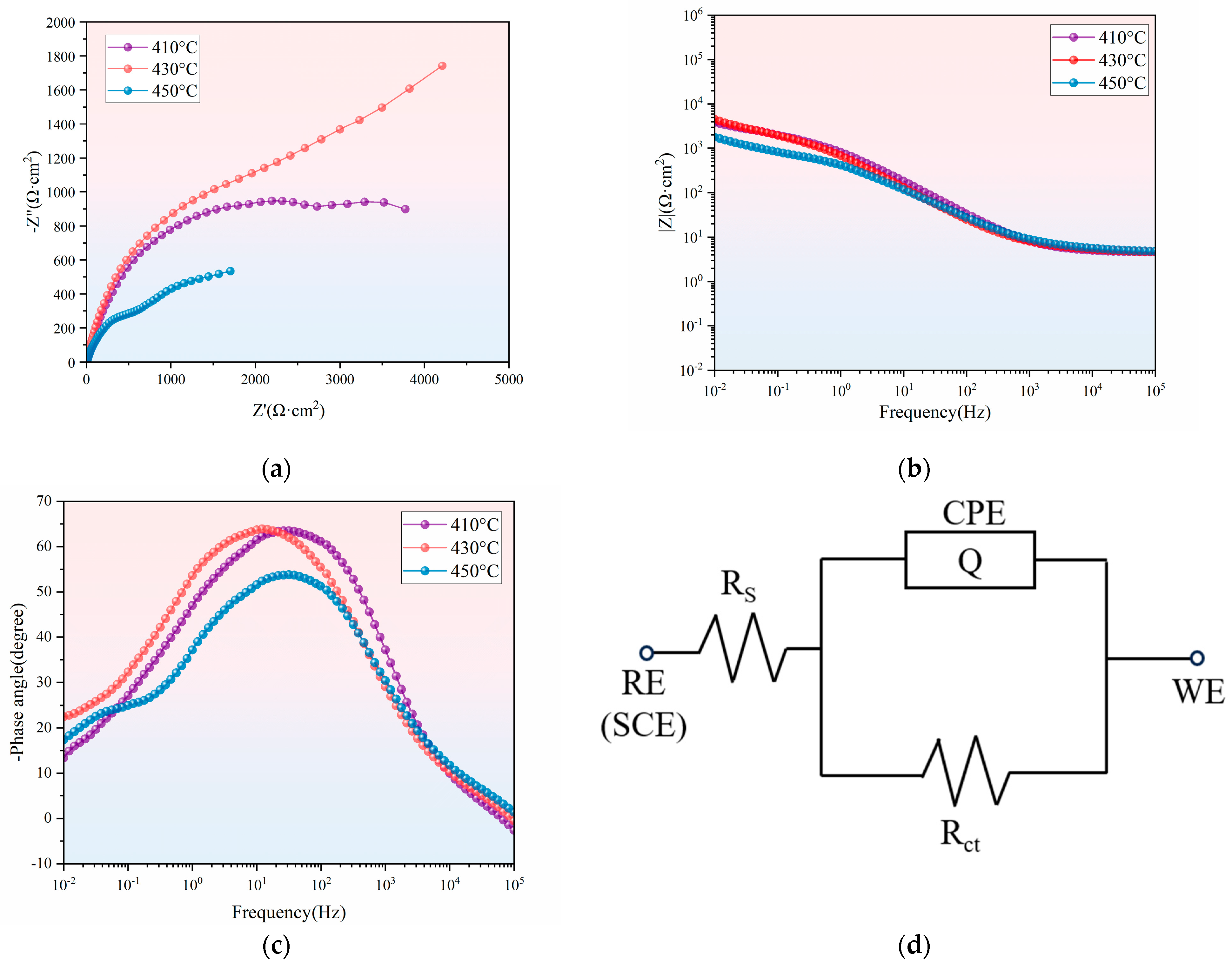
3.3. Effect of Different Holding Temperatures on the Corrosion Resistance of Thermally Diffused Zinc Coatings
4. Conclusions
- As the holding temperature increases, the diffused zinc and iron atoms gain energy, facilitating the formation of the thermally diffused zinc coating and resulting in an increase in its thickness. At a holding temperature of 450 °C, the zinc coating reaches a maximum thickness of 60 μm. Although thicker zinc coatings can be achieved at temperatures 450 °C, doing so adversely affects surface topography, friction resistance, and corrosion resistance. Therefore, it is recommended to maintain the holding temperature below 450 °C.
- As the holding temperature increases, both the average friction coefficient and the wear loss of the thermally diffused zinc coating rise. The primary wear mechanism transitions from abrasive wear to fatigue wear and adhesive wear, accompanied by progressively more severe oxidative wear. Notably, the thermally diffused zinc coating produced at 410 °C exhibits the lowest wear loss at room temperature, indicating its optimal wear resistance.
- As the holding temperature increases, the corrosion current density of the thermally diffused zinc coating exhibits an initial increase, followed by a decrease, and then a further increase, leading to a corresponding trend in its corrosion resistance. The thermally diffused zinc coating produced at 430 °C exhibits the fewest cracks at room temperature, indicating its optimal corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Z.; Wu, C.; Liu, X.; Huang, F. Numerical Simulation of the Dynamic Responses of Electric Power Fitting of Transmission Lines after Ice Shedding. Shock Vib. 2022, 2022, 3675271. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X. Characteristics of Icing without Icicle on Insulators under Natural Conditions. Cold Reg. Sci. Technol. 2020, 180, 103153. [Google Scholar] [CrossRef]
- Nikiforov, E.P. Raising the Reliability of Overhead Transmission Lines under the Action of Atmospheric Loads. Power Technol. Eng. 2004, 38, 49–53. [Google Scholar] [CrossRef]
- Wang, X.; D’Avella, S.; Liang, Z.; Zhang, B.; Wu, J.; Zscherpel, U.; Tripicchio, P.; Yu, X. On the Effect of the Attention Mechanism for Automatic Welding Defects Detection Based on Deep Learning. Expert. Syst. Appl. 2025, 268, 126386. [Google Scholar] [CrossRef]
- Gengbiao, C.; Hanxiao, W.; Lairong, Y. Design, Modeling and Validation of a Low-Cost Linkage-Spring Telescopic Rod-Slide Underactuated Adaptive Robotic Hand. Bioinspir. Biomim. 2025, 20, 016026. [Google Scholar] [CrossRef]
- Huang, J. Study on Tribological Properties of 35CrMo Steel after Laser Textured. Optik 2021, 226, 165437. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.; Long, J. Rietveld-Based Quantitative Phase Analysis of Sherardizing Coatings. Trans. Indian. Inst. Met. 2020, 73, 2947–2954. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, C.Y.; Yu, J.Y.; Huang, L.; Wei, J.; Zhang, J. Microstructure Evolution of a Zn-Al Coating Co-Deposited on Low-Carbon Steel by Pack Cementation. J. Alloys Compd. 2017, 699, 1012–1021. [Google Scholar] [CrossRef]
- Shih, H.C.; Hsu, J.W.; Sun, C.N.; Chung, S.C. The Lifetime Assessment of Hot-Dip 5% Al–Zn Coatings in Chloride Environments. Surf. Coat. Technol. 2002, 150, 70–75. [Google Scholar] [CrossRef]
- Chen, J.; Bao, W.; Chen, H.; Ding, N.; Yang, X.; Yu, B.; Hong, T.; Cai, Z.; Xie, G. Dual Phase Reinforced CuCrZr Alloy: Synergistic Improvement of Mechanical Properties and Corrosion Resistance via Metallic Glass and Rare Earth Oxides. Mater. Des. 2025, 251, 113686. [Google Scholar] [CrossRef]
- Liang, S.; Li, Y.; Zhang, Y.; Zhou, M.; Liu, S.; Li, X.; Geng, Y.; Tian, B.; Jia, Y.; Liu, Y.; et al. Mechanical and Electrical Properties of Cu30Cr0.2Zr Composites Enhanced by CeO2/GO. J. Alloys Compd. 2023, 934, 167759. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, C.; Liu, X.; Xie, H.; Wu, Y.; Li, J. Effect of CeO2 Addition on Pack Cementation Zinc Coatings on Mg-4Y-4Al Magnesium Alloy. Mater. Res. Express 2019, 6, 026417. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Tong, D.; Ren, H.; Du, J.; Zhao, Y.; Cheng, Y.; Lin, P.; Zhao, C.; Lv, Y. Study on the Alloy Coatings Properties of the New Rare Earth Yttrium Zinc Aluminum Magnesium. IOP Conf. Ser. Earth Environ. Sci. 2020, 453, 012075. [Google Scholar] [CrossRef]
- Hosking, N.C.; Ström, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion Resistance of Zinc–Magnesium Coated Steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, B.R.; Oh, S.-Y.; Yun, D.W.; Hwang, J.K.; Oh, M.-S.; Kim, S.J. Mechanistic Study on the Cut-Edge Corrosion Behaviors of Zn-Al-Mg Alloy Coated Steel Sheets in Chloride Containing Environments. Corros. Sci. 2019, 160, 108170. [Google Scholar] [CrossRef]
- Vontorová, J.; Mohyla, P. Use of GDOES Method for Evaluation of the Quality and Thickness of Hot Dip Galvanised Coating. Trans. IMF 2018, 96, 313–318. [Google Scholar] [CrossRef]
- Ferguson, D. Approaching Zero Discharge: In Plant Evaluation of Zinc Thermal Diffusion Coating Technology, Phase I. Clean. Technol. Environ. Policy 2006, 8, 198–202. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Papazoglou, M.; Tsipas, S.; Pavlidou, E.; Skolianos, S.; Stergioudis, G.; Vourlias, G. The Effect of Al and Cr Additions on Pack Cementation Zinc Coatings. Appl. Surf. Sci. 2010, 256, 3618–3623. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S. Effect of Deposition Time on Thickness and Corrosion Behavior of Zn-Fe Coating. J. Shanghai Jiaotong Univ. (Sci.) 2019, 24, 395–401. [Google Scholar] [CrossRef]
- Biryukov, A.I.; Galin, R.G.; Zakharyevich, D.A.; Wassilkowska, A.V.; Batmanova, T.V. The Effect of the Chemical Composition of Intermetallic Phases on the Corrosion of Thermal Diffusion Zinc Coatings. Surf. Coat. Technol. 2019, 372, 166–172. [Google Scholar] [CrossRef]
- Jedrzejczyk, D.; Skotnicki, W. Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings. Materials 2021, 14, 1655. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Su, X.; Peng, H.; Liu, Y.; Wu, C.; Tu, H. Al-Zn-Cr Diffusion Process Aided by Mechanical Energy and Microstructure of Alloying Layer. China Surf. Eng. 2016, 29, 44–51. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Le, L.J.; Shen, W. Microstructure and Corrosion Resistance of Zn-Mg Alloy Coating Prepared by Mechanical Energy Aided Diffusing Method. Corros. Prot. 2022, 43, 53–57. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, Y.; Sheng, Z.; Xing, W.; Wei, S. Anticorrosion and Wear Properties of Mechanical Energy Assisted Zn-Al Coating. China Surf. Eng. 2020, 33, 65–74. [Google Scholar] [CrossRef]
- Kania, H.; Sipa, J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained on High-Strength Grade 10.9 Bolts Using a New Thermal Diffusion Process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Li, X.; Tong, Y.; Hu, Y.; Hu, H.; Chang, B.; Ju, J. Effect of Hot Isostatic Pressing on Microstructure and Properties of High Chromium K648 Superalloy Manufacturing by Extreme High-Speed Laser Metal Deposition. J. Mater. Res. Technol. 2024, 28, 3951–3959. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Liu, Y.; Fang, R.; Guo, L. Research Progress of Formation Mechanism and Influencing Factors of Sherardizing Coating on Steel. Surf. Technol. 2020, 49, 141–150. [Google Scholar] [CrossRef]
- Xing, X.; Wang, H.; Lu, P.; Han, Z. Influence of Rare Earths on Electrochemical Corrosion and Wear Resistance of RE–Cr/Ti Pack Coatings on Cemented 304 Stainless Steel. Surf. Coat. Technol. 2016, 291, 151–160. [Google Scholar] [CrossRef]
- Marder, A.R. The Metallurgy of Zinc-Coated Steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Le, L.; Zhao, X.; Gao, K. Organizational structure and corrosion properties of Zn-Ni alloy infiltration layer. China Surf. Eng. 2022, 35, 135–143. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Chaliampalias, D.; Pavlidou, E.; Stergioudis, G.; Polychroniadis, E.K.; Tsipas, D. Zinc Deposition with Pack Cementation on Low Carbon Steel Substrates. J. Alloys Compd. 2006, 416, 125–130. [Google Scholar] [CrossRef]
- Reumont, G.; Vogt, J.B.; Iost, A.; Foct, J. The Effects of an Fe–Zn Intermetallic-Containing Coating on the Stress Corrosion Cracking Behavior of a Hot-Dip Galvanized Steel. Surf. Coat. Technol. 2001, 139, 265–271. [Google Scholar] [CrossRef]
- Chung, P.P.; Esfahani, M.; Wang, J.; Cook, P.; Durandet, Y. Effects of Heat Treatment on Microstructure Evolution and Corrosion Performance of Mechanically Plated Zinc Coatings. Surf. Coat. Technol. 2019, 377, 124916. [Google Scholar] [CrossRef]
- Chung, P.P.; Wang, J.; Durandet, Y. Effects of Heat Treatment and Plating Sequence on Microstructure Evolution and Corrosion Properties of Mechanically Plated Zinc-Tin Coatings. Met. Mater. Trans. A 2023, 54, 791–807. [Google Scholar] [CrossRef]
- Stott, F.H. High-Temperature Sliding Wear of Metals. Tribol. Int. 2002, 35, 489–495. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Wang, G.; Duan, X.; Zhao, P.; Yang, H. Microstructure and friction and wear properties of laser-prepared Ni60-WC-Cu coatings on copper alloy surfaces. Chin. J. Nonferrous Met. 2024, 34, 1252–1267. [Google Scholar] [CrossRef]
- Milošev, I.; Žerjav, G.; Calderon Moreno, J.M.; Popa, M. Electrochemical Properties, Chemical Composition and Thickness of Passive Film Formed on Novel Ti–20Nb–10Zr–5Ta Alloy. Electrochim. Acta 2013, 99, 176–189. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, J.; Zhang, Y.; Wan, Y. Preparation and Corrosion Resistance of Nb2N Coating on TC4 Ti-alloy. J. Chin. Soc. Corros. Prot. 2019, 39, 313–318. [Google Scholar] [CrossRef]
- Ge, T.; Chen, L.; Gu, P.; Ren, X.; Chen, X. Microstructure and Corrosion Resistance of TiC/Inconel 625 Composite Coatings by Extreme High Speed Laser Cladding. Opt. Laser Technol. 2022, 150, 107919. [Google Scholar] [CrossRef]
- Dai, X.; Xie, M.; Zhou, S.; Wang, C.; Gu, M.; Yang, J.; Li, Z. Formation Mechanism and Improved Properties of Cu95Fe5 Homogeneous Immiscible Composite Coating by the Combination of Mechanical Alloying and Laser Cladding. J. Alloys Compd. 2018, 740, 194–202. [Google Scholar] [CrossRef]
- Yuan, W.; Li, R.; Chen, Z.; Gu, J.; Tian, Y. A Comparative Study on Microstructure and Properties of Traditional Laser Cladding and High-Speed Laser Cladding of Ni45 Alloy Coatings. Surf. Coat. Technol. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Liu, H.; Zhao, X.; Huang, G. Properties of Mechanically Deposited Zinc Coating After Heat Treatment. Mater. Prot. 2018, 51, 1–3. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Du, D.; Chang, B.; Liu, G.; Hu, Y.; Tong, Y.; Zhang, M.; Zhang, J.; Ju, J. Microstructure and Properties of K648 Superalloy Additively Manufactured by Extreme High-Speed Laser Metal Deposition. Trans. Nonferrous Met. Soc. China 2024, 34, 2192–2203. [Google Scholar] [CrossRef]





| Column | Holding Temperature (°C) | Thickness of Zinc Coating (μm) |
|---|---|---|
| A | 410 | 22 |
| B | 430 | 46 |
| C | 450 | 60 |
| Column | Holding Temperature (°C) | Wear Loss of Zinc Coating (/mg) |
|---|---|---|
| A-1 | 410 | 0.32 |
| A-2 | 430 | 2.67 |
| A-3 | 450 | 4.38 |
| Point | Fe | Zn | O | C |
|---|---|---|---|---|
| 1 | 9.59 | 80.44 | 1.84 | 8.13 |
| 2 | 7.01 | 70.45 | 7.84 | 14.70 |
| 3 | 10.36 | 59.31 | 23.23 | 7.10 |
| 4 | 10.83 | 70.19 | 9.26 | 9.72 |
| 5 | 15.89 | 43.23 | 27.59 | 13.29 |
| 6 | 18.70 | 69.97 | 2.08 | 9.25 |
| Point | Fe | Zn | O | C |
|---|---|---|---|---|
| 1 | 6.66 | 68.63 | 14.20 | 10.51 |
| 2 | 10.14 | 66.05 | 15.16 | 8.65 |
| Number | Holding Temperature (°C) | Corrosion Voltage (V) | Corrosion Current Density (A/cm2) |
|---|---|---|---|
| 1 | 410 | −1.295 | 1.900 × 10−4 |
| 2 | 430 | −1.287 | 1.529 × 10−4 |
| 3 | 450 | −1.309 | 2.071 × 10−4 |
| Our Experiment | Wang et al. [42] Experiment | ||
|---|---|---|---|
| Holding temperature (°C) | Corrosion current density (A/cm2) | Holding temperature (°C) | Corrosion current density (A/cm2) |
| 430 | 1.529 × 10−4 | Hot-dip galvanizing | 2.130 × 10−4 |
| Hot-dip galvanizing + Thermal diffusion galvanizing | 1.800 × 10−4 | ||
| Hot-dip galvanizing (Vacuum environment) | 2.600 × 10−4 | ||
| Holding Temperature (°C) | Rs (Ω·cm2) | CPE (S·Secn·cm−2) | n | Rct (Ω·cm2) | ∑χ2 |
|---|---|---|---|---|---|
| 410 | 4.462 | 2.833 × 10−4 | 0.730 | 3185 | 8.59 × 10−3 |
| 430 | 4.863 | 3.942 × 10−4 | 0.721 | 3936 | 7.46 × 10−3 |
| 450 | 4.656 | 6.545 × 10−4 | 0.631 | 1477 | 1.12 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Liu, W.; Wang, Z.; Xie, B.; Yi, Z.; Wang, Z.; Xiao, J.; Gu, J.; Wang, K. Effect of Holding Temperature on Wear and Corrosion Resistance of Rare Earth Oxide Thermally Diffused Zinc Coatings. Coatings 2025, 15, 290. https://doi.org/10.3390/coatings15030290
Chen R, Liu W, Wang Z, Xie B, Yi Z, Wang Z, Xiao J, Gu J, Wang K. Effect of Holding Temperature on Wear and Corrosion Resistance of Rare Earth Oxide Thermally Diffused Zinc Coatings. Coatings. 2025; 15(3):290. https://doi.org/10.3390/coatings15030290
Chicago/Turabian StyleChen, Ruolei, Wei Liu, Zeyang Wang, Biao Xie, Zeng Yi, Zhiyuan Wang, Jingwei Xiao, Jian Gu, and Kaiming Wang. 2025. "Effect of Holding Temperature on Wear and Corrosion Resistance of Rare Earth Oxide Thermally Diffused Zinc Coatings" Coatings 15, no. 3: 290. https://doi.org/10.3390/coatings15030290
APA StyleChen, R., Liu, W., Wang, Z., Xie, B., Yi, Z., Wang, Z., Xiao, J., Gu, J., & Wang, K. (2025). Effect of Holding Temperature on Wear and Corrosion Resistance of Rare Earth Oxide Thermally Diffused Zinc Coatings. Coatings, 15(3), 290. https://doi.org/10.3390/coatings15030290







