Synthesis of LTA Zeolite from Beach Sand: A Solution for CO2 Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Materials
- A solution containing 40 g of H2O and 0.506 g NaOH was prepared, followed by its division into two fractions of equal volumes, V1 and V2.
- To V1, 3.860 g of sodium aluminate was added and stirred until complete homogenization.
- To V2, 7.471 g of H2O, 3.079 g of NaOH, and 2.615 g of SiO2 were added and stirred until complete homogenization.
- The V1 solution was poured into V2 and stirred for 30 min. The synthesized gel had the following molar composition: 2 SiO2: 1 Al2O3: 3.2 Na2O: 128 H2O.
- The formed gel was transferred to Teflon autoclaves and subjected to static crystallization at 100 °C for 1, 2, 3, and 4 h.
- Finally, the LTA zeolites obtained were dried in an oven at 60 °C for 12 h. The second stage consisted of the synthesis of zeolites with MPI silica, which followed the same steps described previously, but with variations in the weight of sodium aluminate, 3.740 g, and silica, 2.762 g, to maintain the molar proportions. The LTA zeolite synthesis flowchart is described in Figure 1. Table 1 contains the names of the synthesized zeolites.
2.3. Characterization of Materials
2.4. CO2 Capture
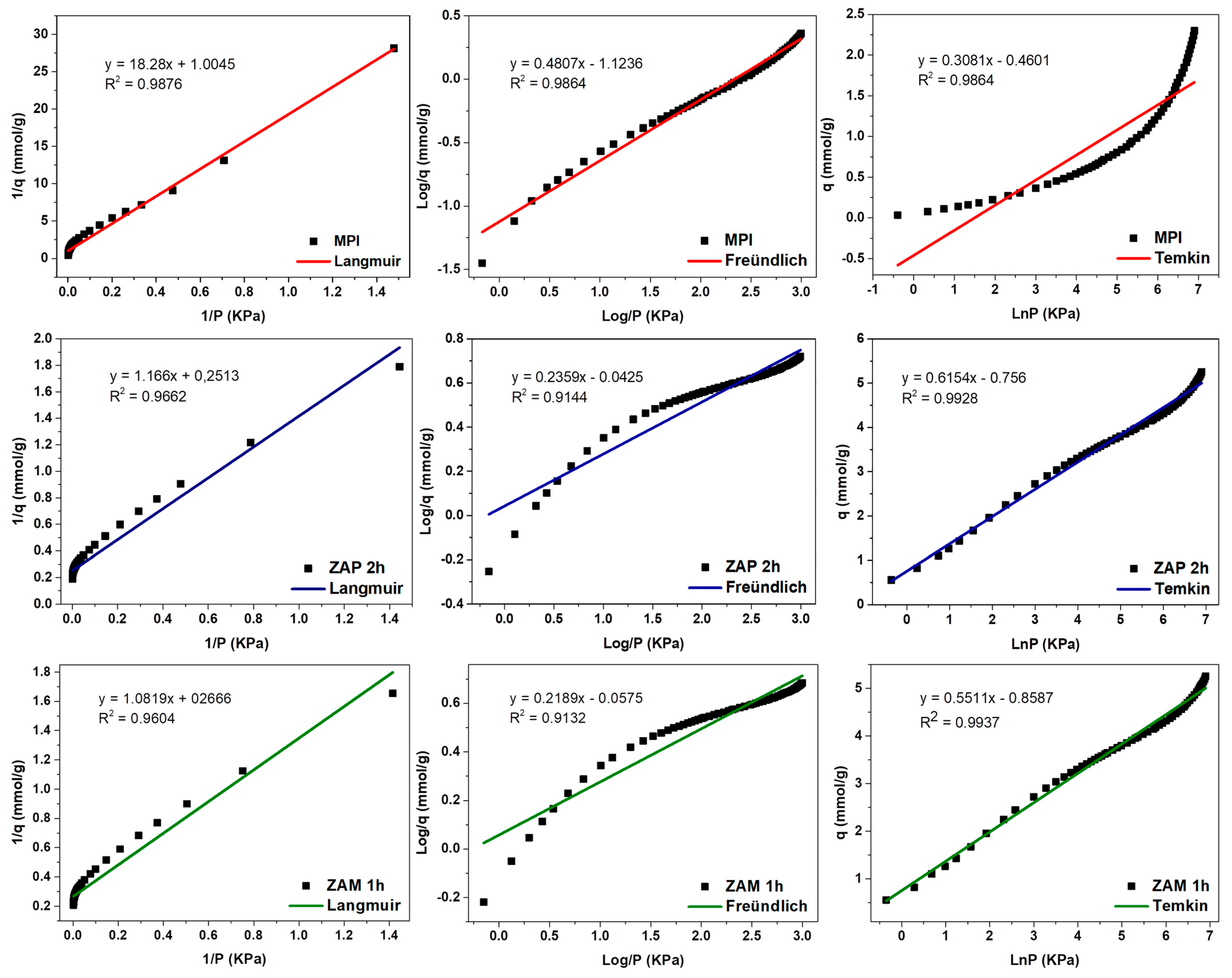
2.5. Mathematical Models
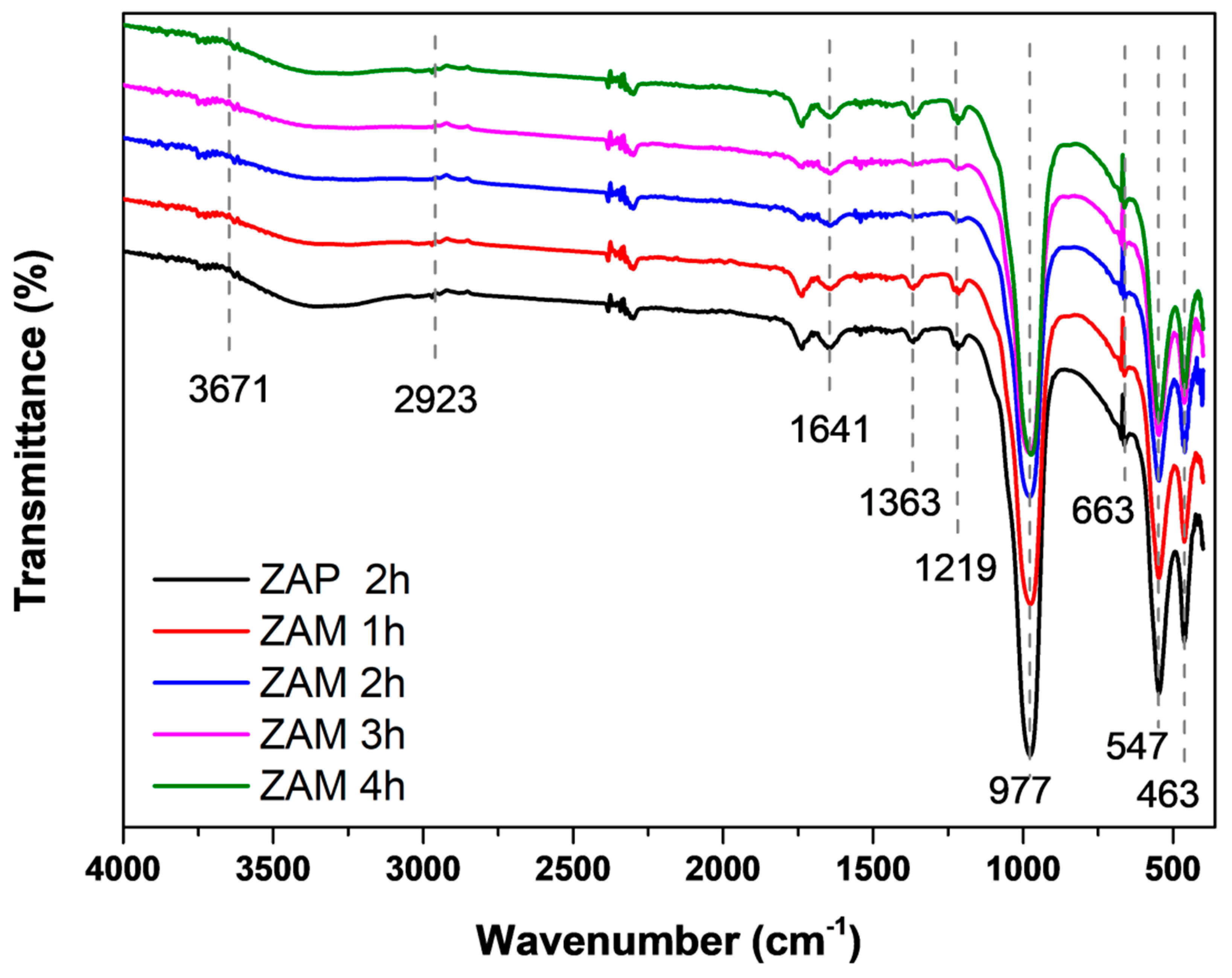
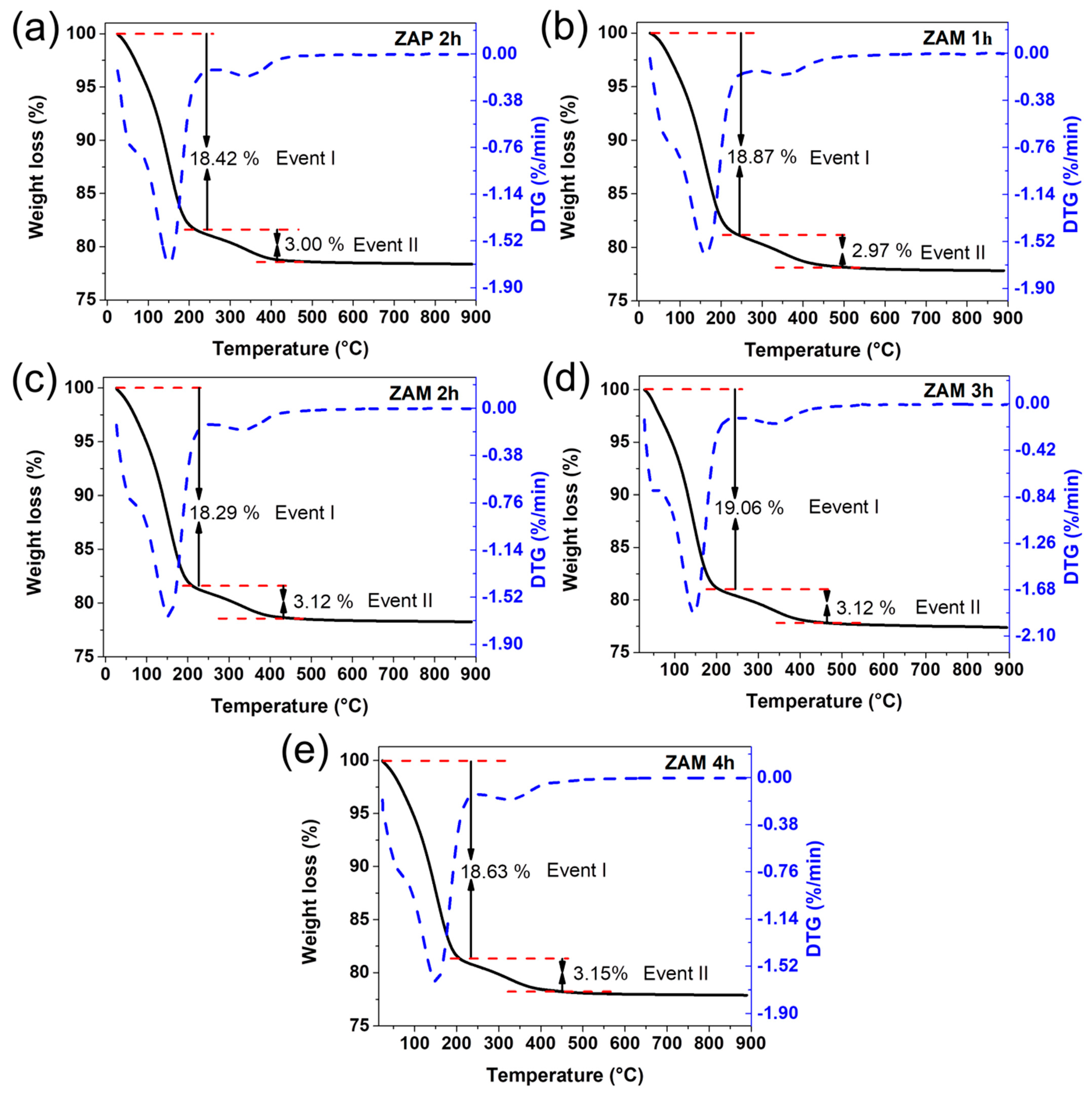
3. Results and Discussion
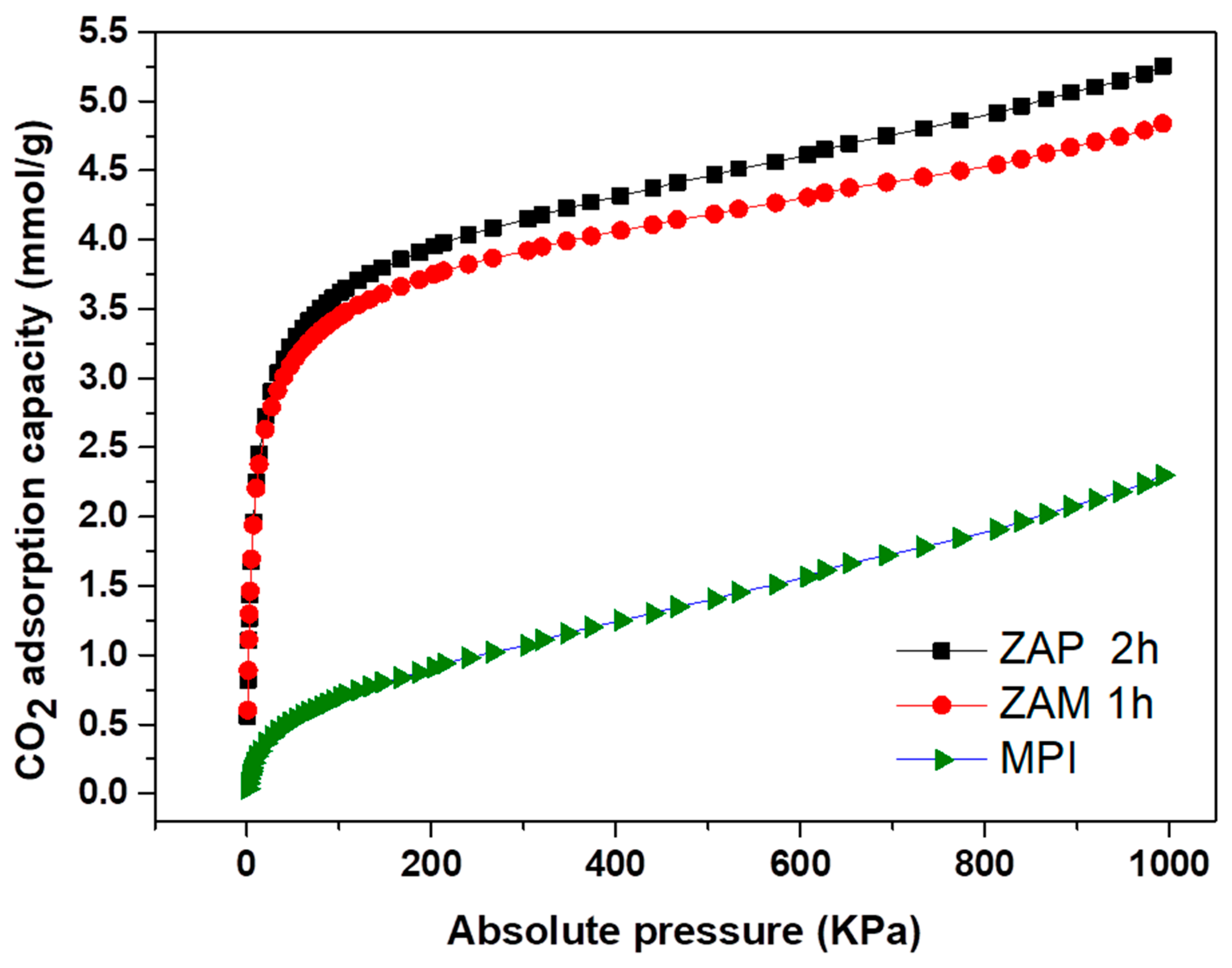
Application of Zeolites in CO2 Capture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madhu, J.; Ramakrishnan, V.M.; Palanichamy, P.; Sathanam, A.; Natarajan, M.; Ponnaian, P.; Brindhadevi, K.; Pugazhendhi, A.; Velauthapillai, D. Rubik’s cube shaped organic template free hydrothermal synthesis and characterization of zeolite NaA for CO2 adsorption. Fuel 2022, 317, 123492. [Google Scholar] [CrossRef]
- Cavallo, M.; Dosa, M.; Porcaro, N.G.; Bonino, F.; Piumetti, M.; Crocellà, V. Shaped natural and synthetic zeolites for CO2 capture in a wide temperature range. J. CO2 Util. 2023, 67, 102335. [Google Scholar] [CrossRef]
- Tão, Z.; Tian, Y.; Hanif, A.; Chan, V.; Gu, Q.; Shang, J. Metal cation-exchanged LTA zeolites for CO2/N2 and CO2/CH4 separation: The roles of gas-framework and gas-cation interactions. Carbon Capture Sci. Technol. 2023, 8, 100126. [Google Scholar] [CrossRef]
- Hasan, H.F.; Al-Sudani, F.T.; Albayati, T.M.; Salih, I.K.; Hharah, H.N.; Majdi, H.S.; Saady, N.M.C.; Zendehboudi, S.; Amari, A.; Gheni, S.A. Solid adsorbent material: A review on trends of post-combustion CO2 capture. Process Saf. Environ. Prot. 2024, 182, 975–988. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Liang, D.; Chen, Q.; Zhang, R.; Xie, Q.; Zhu, M.; Liu, G.; Wang, Y.; Song, Y.; Zhang, Z.; Xie, F.; et al. Investigating the role of sodium persulfate as a hydroxyl radical initiator in Catalyzing zeolite synthesis from fly ash. Chem. Eng. J. 2025, 507, 160714. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100564. [Google Scholar] [CrossRef]
- Tsiotsias, A.; Georgiadis, A.G.; Charision, N.D.; Goula, M.A. CO2 Physisorption over an Industrial Molecular Sieve Zeolite: An Experimental and Theoretical Approach. Materials 2023, 16, 6656. [Google Scholar] [CrossRef]
- Lee, C.H.; Hyeon, D.H.; Jung, H.; Chung, W.; Jo, D.H.; Shin, D.K.; Kim, S.H. Effects of pore structure and PEI impregnation on carbon dioxide adsorption by ZSM-5 zeolites. J. Ind. Eng. Chem. 2015, 23, 251–256. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Wahedi, Y.A. Synthesis of hierarchical porous Zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261. [Google Scholar] [CrossRef]
- Elimbinzi, E.; Nyandoro, S.S.; Mubofu, E.B.; Manayil, J.; Lee, A.F.; Wilson, K. Valorization of rice husk silica waste: Organo-amine functionalized castor oil templated mesoporous silicas for biofuels synthesis. Microporous Mesoporous Mater. 2020, 294, 109868. [Google Scholar] [CrossRef]
- Jal, P.K.; Sudarshan, M.; Saha, A.; Patel, S.; Mishra, B.K. Synthesis and characterization of nanosilica prepared by precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 173–178. [Google Scholar] [CrossRef]
- Sapawe, N.; Osman, N.S.; Zakaria, M.Z.; Shahab, S.A.; Fikry, S.M.; Aris, A.M. Synthesis of green silica from agricultural waste by sol-gel method. Mater. Today Proc. 2018, 5, 21861–21866. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Silva, E.; Andrade, J.C.; Silva, J.A.; Urbina, M.; Nascimento, P.F.; Carvalho, F.; Ruiz, J.A. Low-cost mesoporous adsorbents amines-impregnated for CO2 capture. Adsorption 2015, 8, 597–609. [Google Scholar] [CrossRef]
- Sales, R.V.; Moura, H.O.M.A.; Câmara, A.B.F.; Rodríguez-Castellón, E.; Silva, J.A.B.; Pergher, S.B.C.; Campos, L.M.A.; Urbina, M.M.; Bicudo, T.C.; Carvalho, L.S. Assessment of ag nanoparticles interaction over low-cost mesoporous silica in deep desulfurization of diesel. Catalysts 2019, 651, 651. [Google Scholar] [CrossRef]
- Sales, R.V.; Moura, H.O.M.A.; Silva, S.R.B.; Souza, M.A.F.; Campos, L.M.A.; Rodríguez-Castellón, E.; Carvalho, L.S. Experimental and theoretical study of adsorptive interactions in diesel fuel desulfurization over Ag/MCM-41 adsorbent. Adsorption 2020, 26, 189–201. [Google Scholar] [CrossRef]
- Kwon, D.; Numan, M.; Kim, J.; Yilmaz, M.; Park, S.; Ihee, H.; Jo, C. Tailoring the CO2 selective adsorption properties of MOR zeolites by post functionalization. J. CO2 Util. 2022, 62, 102064. [Google Scholar] [CrossRef]
- Thompso, R.V.; Huber, M.J. Analysis of the growth of molecular sieve zeolite NaA in a batch precipitation system. J. Cryst. Growth 1982, 56, 711–722. [Google Scholar] [CrossRef]
- Bieseki, L.; Penha, F.G.; Pergher, S.B.C. Zeolite a synthesis employing a brazilian coal ash as the silicon and aluminum source and its applications in adsorption and pigment formulation. Mater. Res. 2013, 16, 38–43. [Google Scholar] [CrossRef]
- Bergaoui, M.; Nakhli, A.; Benguerba, Y.; Khalfaoui, M.; Erto, A.; Soetaredjo, F.E.; Ismadji, S.; Ernst, B. Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J. Mol. Liq. 2018, 272, 697–707. [Google Scholar] [CrossRef]
- Khosrowshahi, M.S.; Mashhadimoslem, H.; Emrooz, H.B.M.; Ghaemi, A.; Hosseini, M.S. Green self-activating synthesis system for porous carbons: Celery biomass wastes as a typical case for CO2 uptake with kinetic, equilibrium and thermodynamic studies. Diam. Relat. Mater. 2022, 127, 109204. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Tang, M.; Hao, X.; Liu, J.; Zhang, G.; Zhang, Y. Preparation of N, O co-doped carbon nanotubes and activated carbon composites with hierarchical porous structure for CO2 adsorption by coal pyrolysis. Fuel 2023, 333, 126465. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.M.; Fernández, J.M. Mitigation of CO2 emissions by hydrotalcites of Mg3Al-CO3 at 0 °C and high pressure. Appl. Clay Sci. 2021, 202, 105950. [Google Scholar] [CrossRef]
- Wang, X.; Ji, G.; Zhu, G.; Song, C.; Zhang, H.; Gao, C. Surface hydroxylation of SBA-15 via alkaline for e ffi cient amidoxime-functionalization and enhanced uranium adsorption. Sep. Purif. Technol. 2019, 209, 623–635. [Google Scholar] [CrossRef]
- Erdogan, F.O. Freundlich, Langmuir, Temkin, DR and Harkins-Jura Isotherm Studies on the Adsorption of CO2 on Various Porous Adsorbents. Int. J. Chem. React. Eng. 2018, 17, 20180134. [Google Scholar] [CrossRef]
- Feyzbar-Khalkhali-Nejad, F.; Hassani, E.; Rashti, A.; Oh, T.S. Adsorption-based CO removal: Principles and materials. J. Environ. Chem. Eng. 2021, 9, 105317. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Domin, A.; Mohamedali, M.; Benneker, A.M.; Mahinpey, N. CO2 capture using in-situ polymerized amines into pore-expanded-SBA-15: Performance evaluation, kinetics, and adsorption isotherms. Fuel 2022, 333, 126401. [Google Scholar] [CrossRef]
- Rigo, R.T.; Pergher, S.B.C.; Petkowicz, D.I.; SantoS, J.H.Z. A new procedure for a zeolite synthesis from natural clays. Química Nova 2009, 32, 21–25. [Google Scholar] [CrossRef]
- Saifuddin, M.; Bae, J.; Kim, K.S. Role of Fe, Na and Al in Fe-Zeolite-A for adsorption and desorption of phosphate from aqueous solution. Water Res. 2019, 158, 246–256. [Google Scholar] [CrossRef]
- Liu, B.; Oh, S.C.; Chen, H.; Liu, D. The effect of oxidation of ethane to oxygenates on Pt- and Zn-containing LTA zeolites with tunable selectivity. J. Energy Chem. 2019, 30, 42–48. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Li, C.J. Cost-effective and facile one step synthesis of ZSM-5 from silica fume waste with the aid of metakaolin and its NOx removal performance. Powder Technol. 2020, 367, 558–567. [Google Scholar] [CrossRef]
- Liguori, B.; Aprea, P.; Roviello, G.; Ferone, C. Self-supporting zeolites by Geopolymer Gel Conversion (GGC). Microporous Mesoporous Mater. 2019, 286, 125–132. [Google Scholar] [CrossRef]
- Kannangara, I.; Jayawardhana, Y.; Munasinghe, E.; Rajapakse, A.; Bandara, A.; Weerasooriya, R.; Jayarathna, L. Synthesis and characterization of nano zeolite-A with aid of sodium dodecyl sulfate (SDS) as particle size-controlling agent. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124427. [Google Scholar] [CrossRef]
- Asrori, M.R.; Santoso, A.; Sumari, S. Initial defect product on immiscible mixture of palm oil: Ethanol by amphiphilic chitosan/Zeolite LTA as optimization of microemulsion fuel. Ind. Crops Prod. 2022, 180, 114727. [Google Scholar] [CrossRef]
- Madhu, J.; Ramakrishnan, V.M.; Sathanam, A.; Natarajan, M.; Palanichamy, B.; Velauthapillai, D.; Chi, N.T.L.; Pugazhendhi, A. Comparison of three different structures of zeolites prepared by template-free hydrothermal method and its CO2 adsorption properties. Environ. Res. 2022, 2014, 113949. [Google Scholar] [CrossRef] [PubMed]
- Amoni, B.C.; Freitas, A.D.L.; Bessa, R.A.; Oliveira, C.P.; Bastos-Neto, M.; Azevedo, D.C.S.; Lucena, S.M.P.; Sasaki, J.M.; Soares, J.B.; Soares, S.A.; et al. Effect of coal fly ash treatments on synthesis of high-quality zeolite A as a potential additive for warm mix asphalt. Mater. Chem. Phys. 2022, 275, 125197. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Bakker, B.; Pescarmona, P.P. Binderless zeolite LTA beads with hierarchical porosity for selective CO2 adsorption in biogas upgrading. Microporous Mesoporous Mater. 2022, 344, 112208. [Google Scholar] [CrossRef]
- Mokhtari, A.; Khatamian, M. Insights to the hydrothermal synthesis of highly crystalline aluminum-free Na[Co]ZSM-5 zeolites and their CO2 adsorption performance. J. Alloys Compd. 2021, 886, 161225. [Google Scholar] [CrossRef]
- Boer, D.G.; Čiliak, D.; Langerak, J.; Bakker, B.; Pescarmona, P.P. Sustainable Chemistry for Climate Action Binderless SAPO-34 beads for selective CO2 adsorption. Sustain. Chem. Clim. Action 2023, 2, 100026. [Google Scholar] [CrossRef]
- Liu, Q.; He, P.; Qian, X.; Fei, Z.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; Qiao, X.; Shi, Y. Enhanced CO2 Adsorption Performance on Hierarchical Porous ZSM-5 Zeolite. Energy Fuels 2017, 31, 13933–13941. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; Morales-Ospino, R.; Finocchio, E.; Busa, G.; Sapag, K.; Villarroel-Rocha, J.; Bastos-Neto, M.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Kaolinite-based zeolites synthesis and their application in CO2 capture processes. Fuel 2022, 320, 123953. [Google Scholar] [CrossRef]



| Nomenclature | Description |
|---|---|
| ZAP 2 h | LTA zeolite with Aerosil®200 silica in 2 h |
| ZAM 1 h | LTA zeolite with MPI silica in 1 h |
| ZAM 2 h | LTA zeolite with MPI silica in 2 h |
| ZAM 3 h | LTA zeolite with MPI silica in 3 h |
| ZAM 4 h | LTA zeolite with MPI silica in 4 h |
| Equations and Parameters of Mathematical Modeling | ||||
|---|---|---|---|---|
| Model | Equation | Parameter | Unit | |
| Langmuir | 1 qe, 2 qmax, 3 P e 4 KL | KPa/g, mmol/g and KPa and 1/KPa | (2) | |
| 3 P e 4 KL | KPa and 1/KPa | (3) | ||
| Freündlich | 1 qe, 3 P, 5 KF e 6 n | mmol/g, KPa (mmol/g)·(1/KPa)1/n and Const. | (4) | |
| Temkin | 1 qe, 3 P, 7 β e 8 Kt | mmol/g, Const. and 1/KPa | (5) | |
| Chemical Composition (%) | ZAP | ZAM 1 h | ZAM 2 h | ZAM 3 h | ZAM 4 h | MPI |
|---|---|---|---|---|---|---|
| SiO2 | 46.80 | 47.73 | 45.36 | 47.61 | 47.70 | 90.62 |
| Al2O3 | 37.13 | 35.89 | 35.48 | 35.34 | 35.69 | 2.25 |
| Na2O | 16.07 | 14.50 | 15.05 | 15.00 | 14.60 | 3.50 |
| MgO | - | 1.30 | 3.50 | 1.40 | 1.40 | 1.50 |
| Cl | - | 0.20 | 0.22 | 0.21 | 0.19 | 1.43 |
| Others | - | 0.38 | 0.39 | 0.44 | 0.42 | 0.7 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Si/Al | 1.26 | 1.32 | 1.27 | 1.34 | 1.33 | 40.27 |
| Adsorbent | Pressure (KPa) | Adsorbed Capacity (mmol/g) |
|---|---|---|
| ZAP 2 h | 0.69 | 0.55 |
| 1.27 | 0.82 | |
| 2.09 | 1.10 | |
| 2.67 | 1.26 | |
| 3.40 | 1.43 | |
| 4.72 | 1.67 | |
| 6.87 | 1.95 | |
| 10.09 | 2.24 | |
| 13.35 | 2.44 | |
| 20.04 | 2.72 | |
| 999.27 | 5.25 | |
| ZAM 1 h | 0.70 | 0.60 |
| 1.33 | 0.88 | |
| 1.98 | 1.11 | |
| 2.68 | 1.29 | |
| 3.44 | 1.46 | |
| 4.82 | 1.69 | |
| 6.84 | 1.93 | |
| 10.13 | 2.20 | |
| 13.22 | 2.37 | |
| 20.00 | 2.62 | |
| 992.85 | 4.83 | |
| MPI | 0.67 | 0.03 |
| 1.41 | 0.07 | |
| 2.10 | 0.10 | |
| 2.99 | 0.13 | |
| 3.82 | 0.16 | |
| 4.97 | 0.18 | |
| 6.94 | 0.22 | |
| 10.16 | 0.27 | |
| 13.64 | 0.30 | |
| 20.16 | 0.36 | |
| 992.75 | 2.29 |
| Adsorbent | Conditions | Adsorbed Capacity (mmol/g) | References |
|---|---|---|---|
| ZAP 2 h | 23 °C | 5.25 | This work |
| ZAM 1 h | 23 °C | 4.83 | This work |
| MPI | 23 °C | 2.29 | This work |
| zeolite LTA (Na-e CaLTA) | 25 °C | 3.10, 3.30 | [2] |
| zeolite LTA spherical (LTA-P1) | 25 °C | 4.48 | [37] |
| ZSM-5 (Na [Co] ZSM-5) | 25 °C | 5.33 | [38] |
| zeolite MOR | 25 °C | 3.20 | [17] |
| zeolite Y (ZY and PDY-7: hierarchical) | 25 °C | 4.50, 5.40 | [10] |
| SAPO-34 | 24 °C | 3.00, 3.40 | [39] |
| Coal | 0.00 °C, 25 °C | 5.00, 2.03 | [21] |
| Models | Parameters | |||
|---|---|---|---|---|
| MPI | ZAP 2 h | ZAM 1 h | ||
| Langmuir | qm (mmol/g) | 0.9955 | 3.9793 | 3.7509 |
| KL (1/KPa) | 0.0054 | 0.0215 | 0.2446 | |
| R2 | 0.9876 | 0.9662 | 0.9604 | |
| RL | 0.0024 | 0.0006 | 0.0005 | |
| Freündlich | KF (mmol/g)·(1/KPa)1/n | 0.0752 | 0.3458 | 0.8759 |
| 1/n | 0.4807 | 0.2359 | 0.2189 | |
| R2 | 0.9864 | 0.9144 | 0.9132 | |
| Temkin | KT (1/KPa) | 0.0224 | 0.2927 | 0.2105 |
| β | 0.3082 | 0.6157 | 0.5493 | |
| BT (KJ/mmol) | 7960.5 | 3985.4 | 4466.6 | |
| R2 | 0.8116 | 0.9928 | 0.9937 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Longe, C.; da Silva, A.; Câmara, A.B.F.; Bieseki, L.; de Carvalho, L.S.; Pergher, S.B.C.; de Mello, M.I.S. Synthesis of LTA Zeolite from Beach Sand: A Solution for CO2 Capture. Coatings 2025, 15, 334. https://doi.org/10.3390/coatings15030334
de Longe C, da Silva A, Câmara ABF, Bieseki L, de Carvalho LS, Pergher SBC, de Mello MIS. Synthesis of LTA Zeolite from Beach Sand: A Solution for CO2 Capture. Coatings. 2025; 15(3):334. https://doi.org/10.3390/coatings15030334
Chicago/Turabian Stylede Longe, Clenildo, Aryandson da Silva, Anne Beatriz Figueira Câmara, Lindiane Bieseki, Luciene Santos de Carvalho, Sibele Berenice Castellã Pergher, and Mariele Iara Soares de Mello. 2025. "Synthesis of LTA Zeolite from Beach Sand: A Solution for CO2 Capture" Coatings 15, no. 3: 334. https://doi.org/10.3390/coatings15030334
APA Stylede Longe, C., da Silva, A., Câmara, A. B. F., Bieseki, L., de Carvalho, L. S., Pergher, S. B. C., & de Mello, M. I. S. (2025). Synthesis of LTA Zeolite from Beach Sand: A Solution for CO2 Capture. Coatings, 15(3), 334. https://doi.org/10.3390/coatings15030334








