Development of Renewable Polyester Resins for Coil Coatings Based on 2,5-Furandicarboxylic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Resin Synthesis
2.3. Resin Characterization
2.3.1. Non-Volatile Matter Determination
2.3.2. Acid Value Determination
2.3.3. Viscosity Measurement
2.3.4. Hydroxyl Value Determination
2.3.5. Color Measurement
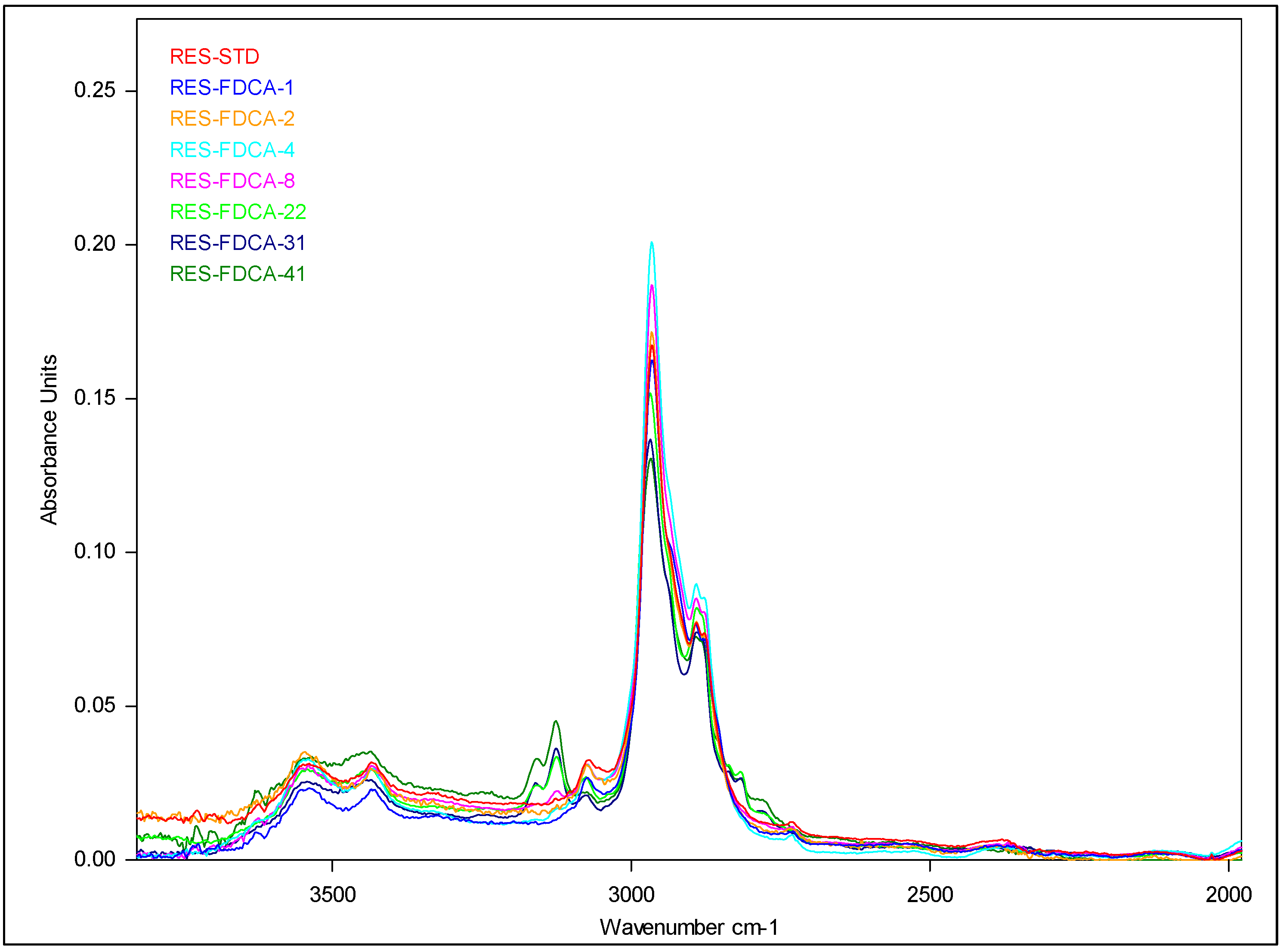
2.3.6. FTIR Measurements
2.3.7. Glass Transition Temperature (Tg)
2.3.8. Gel Permeation Chromatography (GPC)
2.4. Validation of Resins in Coatings
2.4.1. Viscosity Determination
2.4.2. Density Determination
2.4.3. Thickness of the Film
2.4.4. Gloss of the Topcoat
2.4.5. MEK Test
2.4.6. Adhesion of the Coating
2.4.7. T-Bend Test
2.4.8. Reverse Impact
2.4.9. Pencil Hardness
3. Results and Discussion
3.1. Properties of the Resins
3.2. Properties of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Sustainability and Carbon Certification. Available online: https://www.iscc-system.org/ (accessed on 19 November 2024).
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethyfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Q.L.; Xie, W.; Peng, L.; He, L.; He, Z.; Chowdhury, S.P.; Christensen, R.; Ni, Y. An eco-friendly method to get a bio-based dicarboxylic acid monomer 2,5-furandicarboxylic acid and its application in the synthesis of poly(hexylene 2,5-furandicarboxylate) (PHF). Polymers 2019, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent advances in catalytic conversion of biomass to 2,5-furandicarboxylic acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Marshall, A.; Jiang, B.; Gauvin, R.M.; Thomas, C.M. 2,5-furandicarboxylic acid: An intriguing precursor for monomer and polymer synthesis. Molecules 2022, 27, 4071. [Google Scholar] [CrossRef]
- Robert, T.; Eschig, S.; Sangermano, M.; Ocepek, M. Biobased aromatic building blocks for coating applications. Curr. Opin. Green Sustain. 2024, 49, 100962. [Google Scholar] [CrossRef]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A perspective on PEF synthesis, properties, and end-life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Haas, V.; Wenger, J.; Ranacher, L.; Guigo, N.; Sousa, A.F.; Stern, T. Developing future visions for bio-plastics substituting PET—A backcasting approach. Sustain. Prod. Consum. 2022, 31, 370–383. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Walkowiak, K.; Paszkiewicz, S. Modifications of furan-based polyesters with the use of rigid diols. Polymers 2024, 16, 2064. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Teng, N.; Dai, X.; Shen, X.; Wang, S.; Liu, X.; Zhu, J. 2,5-furandicarboxylic acid- and itaconic- acid-derived fully biobased unsaturated polyesters and their cross-linked networks. Ind. Eng. Chem. Res. 2017, 56, 2650–2657. [Google Scholar] [CrossRef]
- Gubbels, E.; Jasinska-Walc, L.; Koning, C.E. Synthesis and characterization of novel renewable polyesters based on 2,5-furandicarboxylic acid and 2,3-butanediol. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 890–898. [Google Scholar] [CrossRef]
- Lomelí-Rodríguez, M.; Corpas-Martínez, J.R.; Willis, S.; Mulholland, R.; Lopez-Sanchez, J.A. Synthesis and characterization of renewable polyester coil coatings from biomass-derived isosorbide, FDCA, 1,5-pentadiol, succinic acid and 1,3-propanediol. Polymers 2018, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the properties of furandicarboxylic acid-based polyesters with copolymerization: A review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.J.I.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957. [Google Scholar] [CrossRef]
- Janesch, J.; Bacher, M.; Padhi, S.; Rosenau, T.; Gindl-Altmutter, W.; Hansmann, C. Biobased alkyd resins from plant oil and furan-2,5-dicarboxylic acid. ACS Sustain. Chem. Eng. 2023, 11, 17625–17632. [Google Scholar] [CrossRef]
- Kamran, M.; Davidson, M.G.; de Vos, S.; Tsanaktsis, V.; Yeniad, B. Synthesis and characterisation of polyamides based on 2,5-furandicarboxylic acid as a sustainable building block for engineering plastics. Polym. Chem. 2022, 13, 3433. [Google Scholar] [CrossRef]
- Deng, J.; Liu, X.; Li, C.; Jiang, Y.; Zhu, J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). RSC Adv. 2015, 5, 15930. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased heat resistant epoxy resin with extremely high biomass content from 2,5-furancarboxylic acid and eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- Nameer, S.; Larsen, D.B.; Duus, J.Ø.; Daugaard, A.E.; Johansson, M. Biobased cationically polymerizable epoxy thermosets from furan and fatty acid derivatives. ACS Sustain. Chem. Eng. 2018, 6, 9442–9450. [Google Scholar] [CrossRef]
- García González, M.N.; Börjesson, P.; Levi, M.; Turri, S. Development and life cycle assessment of polyester binders containing 2,5-furandicarboxylic acid and their polyurethane coatings. J. Polym. Environ. 2018, 26, 3626–3637. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Malitowski, N.M.; Zamboulis, A.; Friebel, S.; Bikiaris, D.; Robert, T. Influence of bio-based 2,5-furandicarboxylic acid on the properties of water-borne polyurethane dispersions. React. Funct. Polym. 2023, 190, 105622. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, X.; Liu, J.; Wang, J.; Liu, X. Synthesis of a fire-retardant and high Tg biobased polyester from 2,5-furandicarboxylic acid. Polym. J. 2022, 54, 995–1008. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z. Synthesis and characterization of in-situ prepared nanocomposites based on poly(propylene 2,5-furan dicarboxylate) and aluminosilicate clays. Polymers 2018, 10, 937. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Fei, X.; Gao, R.; Liu, F.; Fan, L.; Zhu, J.; Liu, X. Synthesis of high thermal-resistant poly(ester-ether) elastomers from bio-based 2,5-furandicarboxylic acid. ACS Sustain. Chem. Eng. 2022, 10, 13595–13606. [Google Scholar] [CrossRef]
- Yi, J.; Dai, Y.; Li, Y.; Wu, Y.; Jiang, M.; Zhou, G. Biobased polyester network from 2,5-furandicarboxylic acid and5,5’-((dodecylazanedyl)bis(methylene))bis(furan-5,2-diyl))dimethanol: Shape memory, adhesiveness, self-healing, and recyclability. ACS Appl. Polym. Mater. 2023, 5, 8250–8259. [Google Scholar] [CrossRef]
- Van Strien, N.; Niskanen, J.; Berghuis, A.; Pohler, H.; Rautiainen, S. Production of 2,5-furandicarboxylic acid methyl esters from pectin-based aldaric acid: From laboratory to bench scale. ChemSusChem 2024, 17, e202300732. [Google Scholar] [CrossRef]
- Annatelli, M.; Sánchez-Velandia, J.E.; Mazzi, G.; Pandeirada, S.V.; Giannakoudakis, D.; Rautiainen, S.; Esposito, A.; Thiyagarajan, S.; Richel, A.; Triantafyllidis, K.S.; et al. Beyond 2,5-furandicarboxylic acid: Status quo, environmental assessment, and blind spots of furanic monomers for bio-based polymers. Green Chem. 2024, 26, 8894. [Google Scholar] [CrossRef]
- Caretto, A.; Passoni, V.; Brenna, N.; Sitta, M.; Ogliosi, L.; Catel, G.; Turri, S.; Griffini, G. Fully biobased polyester based on an isosorbide monomer for coatings applications. ACS Sustain. Chem. Eng. 2018, 6, 14125–14134. [Google Scholar] [CrossRef]
- SIST EN ISO 3251:2019; Paints, Varnishes and Plastics—Determination of Non-Volatile-Matter Content. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2019.
- SIST EN ISO 2114:2002; Plastics (Polyester Resins) and Paints and Varnishes (Binders)—Determination of Partial Acid Value and Total Acid Value. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2002.
- SIST EN ISO 3219:2021; Plastics—Polymers/Resins in the Liquid State or as Emulsions or Dispersions—Determination of Viscosity Using a Rotational Viscometer with Defined Shear Rate. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2021.
- SIST EN ISO 4629-1:2016; Binders for Paints and Varnishes—Determination of Hydroxyl Value—Titrimetric Method. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2016.
- SIST EN ISO 6271:2016; Clear Liquids—Estimation of Colour by the Platinum-Cobalt Scale. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2016.
- DIN 53211:1974; Prüfung von Anstrichstoffen: Bestimmung der Auslaufzeit mit dem DIN-Becher 4. DIN German Institute for Standardization: Berlin, Germany, 1974.
- SIST EN ISO 2811-1:2016; Determination of Density—Part 1: Pycnometer Method. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2016.
- SIST EN 13523-1:2010; Coil Coated Metals—Test Methods—Part 1: Film Thickness. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2010.
- SIST EN 13523-2:2002; Coil Coated Metals—Test Methods—Part 2: Specular Gloss. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2002.
- SIST EN 13523-11:2011; Coil Coated Metals—Test Methods—Part 11: Resistance to Solvents (Rubbing Test). Slovenian Institute for Standardization: Ljubljana, Slovenia, 2011.
- SIST EN 13523-6:2003; Coil Coated Metals—Test Methods—Part 6: Adhesion After Indentation (Cupping Test). Slovenian Institute for Standardization: Ljubljana, Slovenia, 2003.
- SIST EN 13523-7:2002; Coil Coated Metals—Test Methods—Part 7: Resistance to Cracking on Bending (T-Bend Test). Slovenian Institute for Standardization: Ljubljana, Slovenia, 2002.
- SIST EN 13523-5:2002; Coil Coated Metals—Test Methods—Part 5: Resistance to Rapid Deformation (Impact Test). Slovenian Institute for Standardization: Ljubljana, Slovenia, 2002.
- SIST EN 13523-4:2002; Coil Coated Metals—Test Methods—Part 4: Pencil Hardness. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2002.
- Gruter, G.J.M.; Sipos, L.; Dam, M.A. Accelerating research into bio-based FDCA-polyesters by small scale parallel film reactors. Comb. Chem. High Throughput Screen. 2012, 15, 180–188. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M. Exploring the Application of 2,5-Furandicarboxylic Acid as a Monomer in High Performance Polymers: Synthesis, Characterization, and Properties. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2015. [Google Scholar]
- Silvianti, F.; Maniar, D.; Agostinho, B.; de Leeuw, T.C.; Pelras, T.; Dijkstra, L.; Woortman, A.J.J.; van Dijken, J.; Thiyagarajan, S.; Sousa, A.F.; et al. Unraveling the impact of isomerism on encymatic polymerization of furanic polyesters. Adv. Sustain. Syst. 2024, 8, 2300542. [Google Scholar] [CrossRef]





| RES-STD | RES-FDCA-1 | RES-FDCA-2 | RES-FDCA-4 | RES-FDCA-8 | RES-FDCA-22 | RES-FDCA-31 | RES-FDCA-41 | |
|---|---|---|---|---|---|---|---|---|
| Renewable content C-14 (%) | 0 | 1 | 2 | 4 | 8 | 22 | 31 | 41 |
| Renewable content according to ISCC BMB (%) | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Total renewable content on polymer (%) | 24 | 25 | 26 | 28 | 32 | 46 | 55 | 65 |
| Total renewable content on final resin (%) | 14 | 15 | 16 | 17 | 19 | 28 | 33 | 39 |
| Requirements | RES-STD | RES-FDCA-1 | RES-FDCA-2 | RES-FDCA-4 | RES-FDCA-8 | |
|---|---|---|---|---|---|---|
| Non-volatile matter (%) | 59–61 | 59.5 | 60.1 | 59.8 | 59.6 | 59.7 |
| Acid value (mg KOH/g) | ≤5 | 2.5 | 3.5 | 2.5 | 3.1 | 4.0 |
| Hydroxyl value (mg KOH/g) | 20–28 | 24.7 | 24.6 | 23.8 | 23.7 | 24.9 |
| Viscosity Brookfield @200 °C (mPa·s) | 1900–2400 | 1900 | 1976 | 2055 | 2014 | 2108 |
| Viscosity @23 °C (mPa·s) | 5500–7500 | 6200 | 5950 | 6150 | 6350 | 7250 |
| Color (Gardner) | ≤3 | 0.1 | 2.2 | 3.4 | 4.5 | 4.9 |
| Tg (°C) | n/a | 42.4 | 39.4 | 36.1 | 38.8 | 35.8 |
| Mn (g/mol) | n/a | 6022 | 5266 | 5733 | 5740 | 5946 |
| Mw (g/mol) | n/a | 11,704 | 11,478 | 11,875 | 11,644 | 11,621 |
| PDI | n/a | 1.944 | 2.180 | 2.071 | 2.029 | 1.954 |
| COAT-STD | COAT-FDCA-1 | COAT-FDCA-2 | COAT-FDCA-4 | COAT-FDCA-8 | COAT-FDCA-22 | COAT-FDCA-31 | |
|---|---|---|---|---|---|---|---|
| Viscosity DIN4 20 °C (s) | 79 | 80 | 81 | 81 | 80 | 80 | 79 |
| Density 20 °C (g/cm3) | 1.217 | 1.217 | 1.208 | 1.207 | 1.209 | 1.237 | 1.233 |
| Non-volatile matter (%) | 53.7 | 53.3 | 52.0 | 51.9 | 51.7 | 51.7 | 50.8 |
| COAT-STD | COAT-FDCA-1 | COAT-FDCA-2 | COAT-FDCA-4 | COAT-FDCA-8 | COAT-FDCA-22 | COAT-FDCA-31 | |
|---|---|---|---|---|---|---|---|
| Film thickness primer (µm) | 6 | 6 | 7 | 7 | 7 | 7 | 7 |
| Film thickness topcoat (µm) | 18 | 20 | 19 | 20 | 20 | 20 | 18 |
| Gloss 60° topcoat (%) | 39 | 41 | 40 | 40 | 39 | 40 | 39 |
| Adhesion topcoat (Gt) | 2 | 1 | 1 | 0 | 0 | 0 | 0 |
| T-bend adhesion primer (T) | 2 | 0.5 | 1 | 0.5 | 1.5 | 1 | 1.5 |
| T-bend adhesion topcoat (T) | 1.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1.5 |
| T-bend cracking topcoat (T) | 1.5 | 2 | 1 | 1 | 1.5 | 2 | 2 |
| MEK test topcoat (DR) | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Reverse impact topcoat (J) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Pencil hardness topcoat | H/2H | H/2H | H/2H | H/2H | H/2H | H/2H | H/2H |
| Requirements | RES-STD | RES-FDCA-22 | RES-FDCA-31 | RES-FDCA-41 | |
|---|---|---|---|---|---|
| Non-volatile matter (%) | 59–61 | 59.5 | 59.5 | 60.1 | 59.0 |
| Acid value (mg KOH/g) | ≤5 | 2.5 | 4.6 | 3.4 | 3.6 |
| Hydroxyl value (mg KOH/g) | 20–28 | 24.7 | 24.9 | 22.7 | 22.0 |
| Viscosity Brookfield (mPa·s) | 1900–2400 | 1900 | 1924 | 2318 | 2329 |
| Viscosity 23 °C (mPa·s) | 5500–7500 | 6200 | 6100 | 6100 | 7500 |
| Color (Gardner) | ≤3 | 0.1 | 3.8 | 3.0 | 2.3 |
| Tg (°C) | n/a | 42.4 | 38.9 | 32.2 | 37.8 |
| Mn (g/mol) | n/a | 6022 | 4974 | 4691 | 4362 |
| Mw (g/mol) | n/a | 11,704 | 10,046 | 9582 | 8620 |
| PDI | n/a | 1.944 | 2.020 | 2.043 | 1.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čuk, N.; Štular, D.; Ocepek, M.; Langerholc, J.; Venturini, P. Development of Renewable Polyester Resins for Coil Coatings Based on 2,5-Furandicarboxylic Acid. Coatings 2025, 15, 350. https://doi.org/10.3390/coatings15030350
Čuk N, Štular D, Ocepek M, Langerholc J, Venturini P. Development of Renewable Polyester Resins for Coil Coatings Based on 2,5-Furandicarboxylic Acid. Coatings. 2025; 15(3):350. https://doi.org/10.3390/coatings15030350
Chicago/Turabian StyleČuk, Nataša, Danaja Štular, Martin Ocepek, Jaka Langerholc, and Peter Venturini. 2025. "Development of Renewable Polyester Resins for Coil Coatings Based on 2,5-Furandicarboxylic Acid" Coatings 15, no. 3: 350. https://doi.org/10.3390/coatings15030350
APA StyleČuk, N., Štular, D., Ocepek, M., Langerholc, J., & Venturini, P. (2025). Development of Renewable Polyester Resins for Coil Coatings Based on 2,5-Furandicarboxylic Acid. Coatings, 15(3), 350. https://doi.org/10.3390/coatings15030350





