Toxicity, Irritation, and Allergy of Metal Implants: Historical Perspective and Modern Solutions
Abstract
:1. Introduction: Historical Overview
2. Motivation and Methodology
3. The Interactions of Metals Within a Biological Environment
4. Irritancy
5. Immune Response to Metals
6. Metal Toxicity
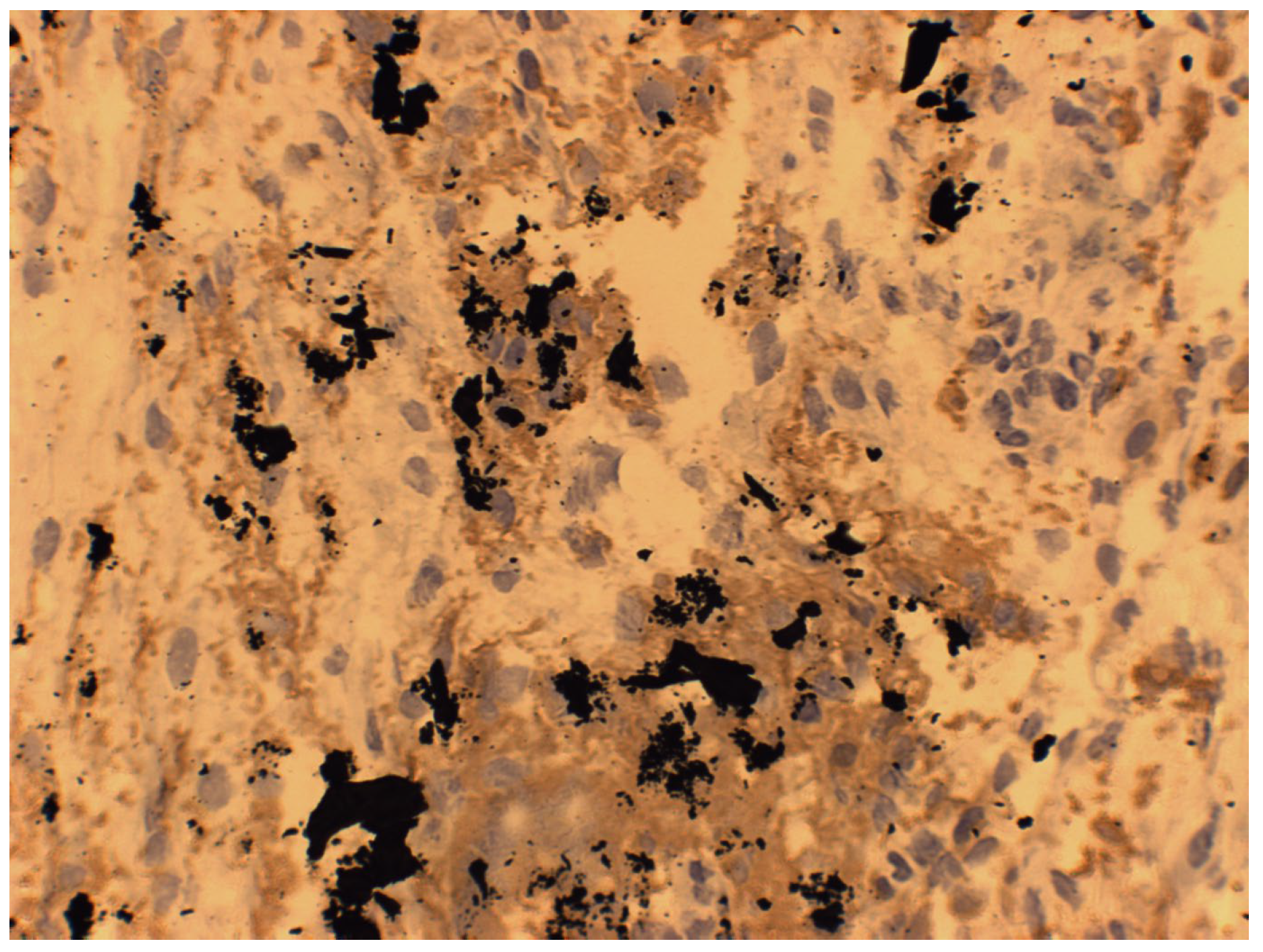
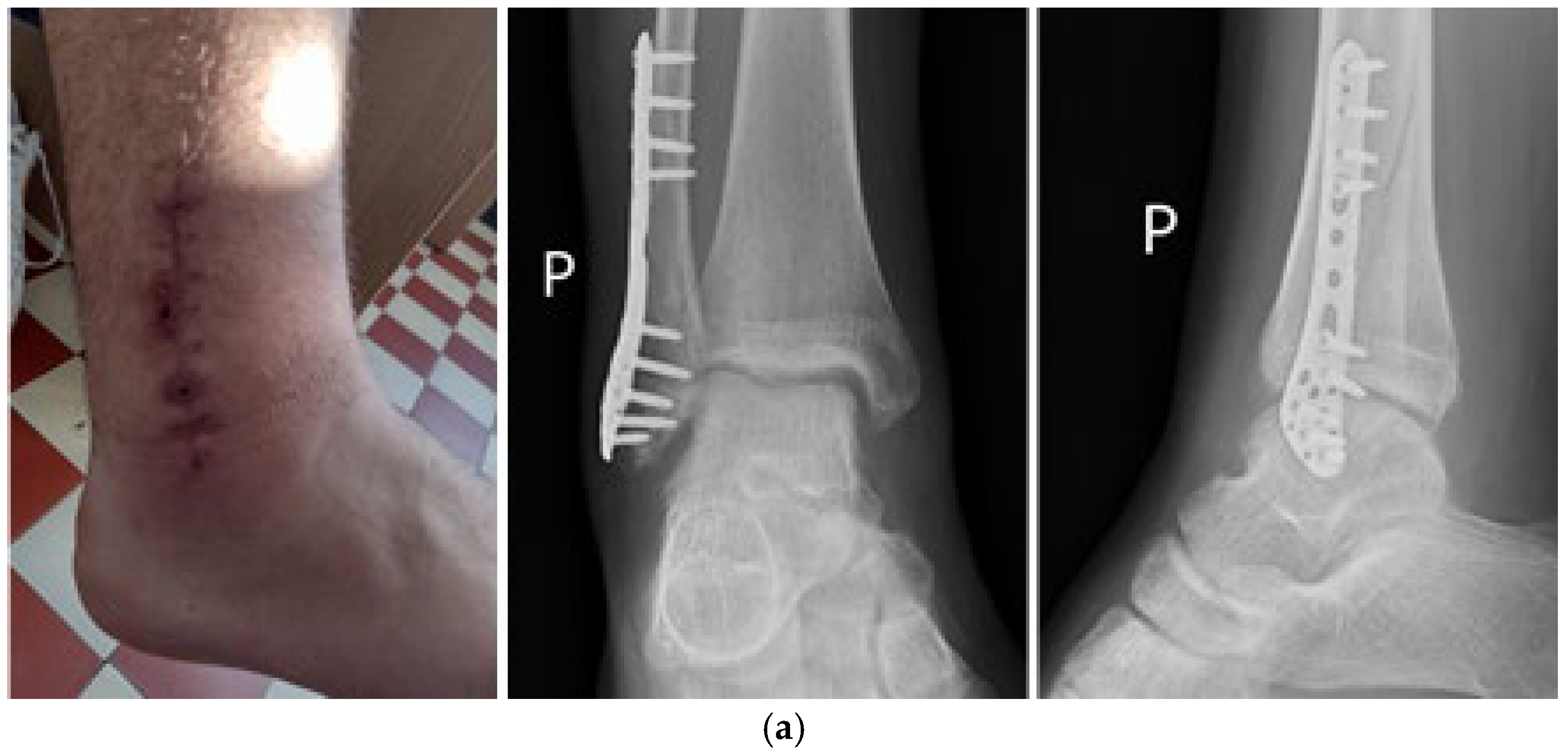
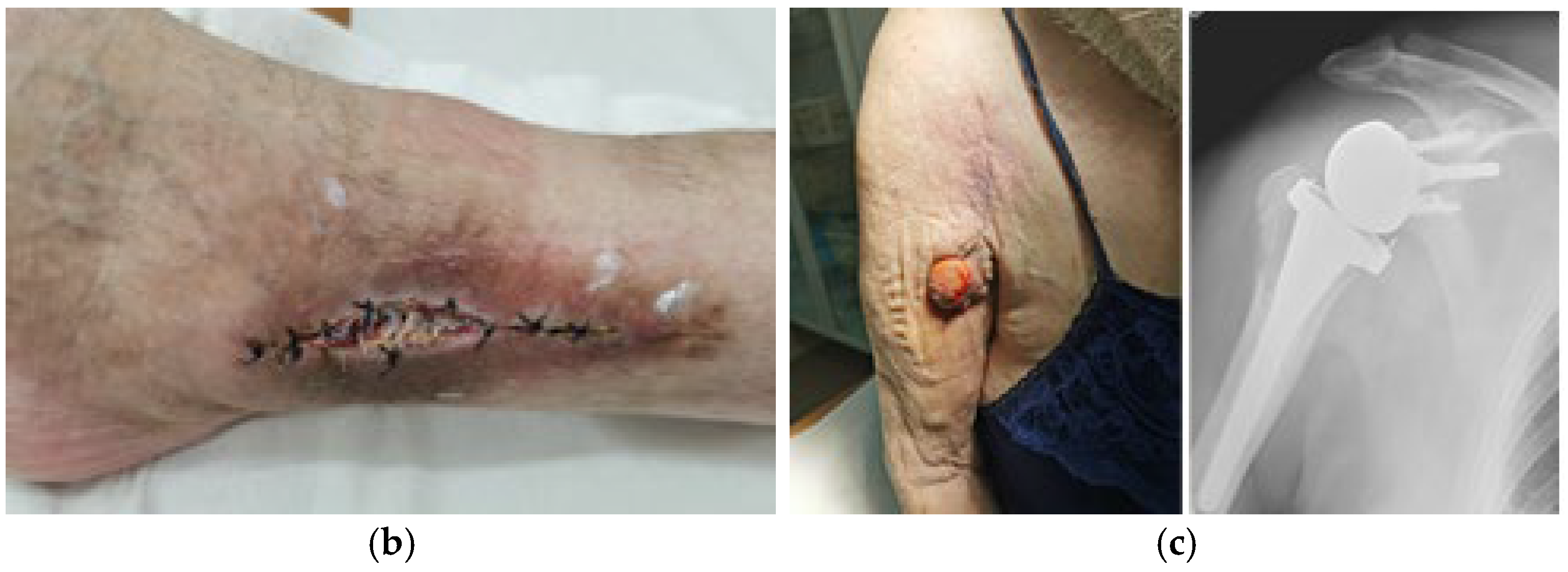
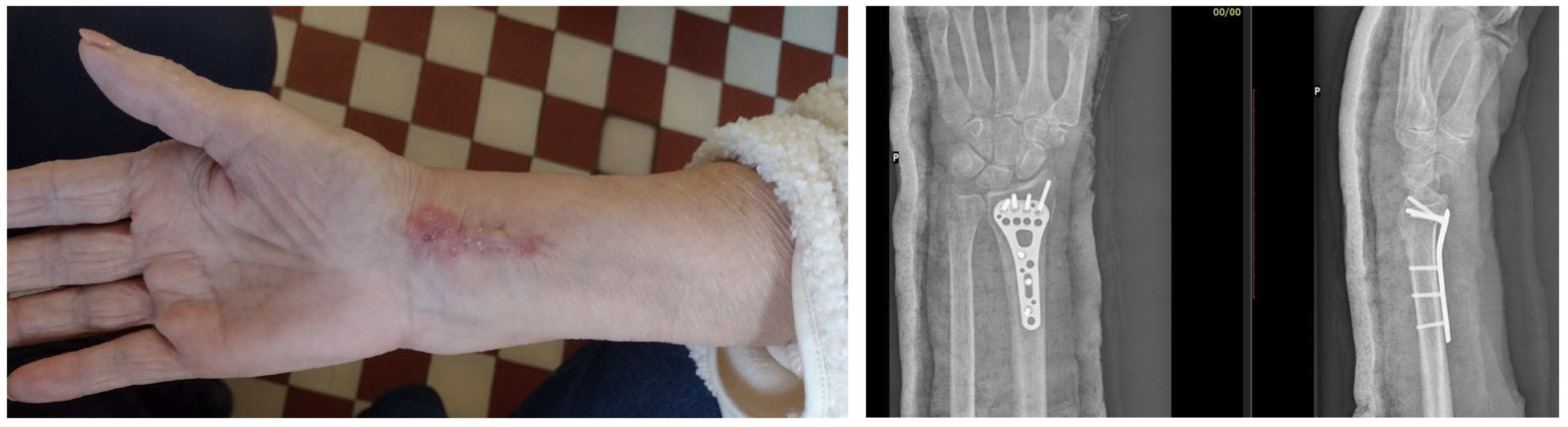
7. Macroscopic Observations of Interacted Surfaces of Removed Implants—Author Observations
8. Discussion and Summary
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bugaj, U.; Nejbert, K.; Ilnicki, S.; Wieciński, P.; Onyszczuk, T.; Garbacz, H.; Włodarczak, P. Copper sulphosalts in early metallurgy (2600–1900 BC)—Chemical-mineralogical investigation of artefacts from southern Poland. Geol. Q. 2019, 63, 302–318. [Google Scholar] [CrossRef]
- Radivojević, M.; Roberts, B.W. Early Balkan Metallurgy: Origins, Evolution and Society, 6200–3700 BC. J. World Prehistory 2021, 34, 195–278. [Google Scholar] [CrossRef]
- Adams, M. Advances in Gold Processing; Elsevier B.V.: Perth, Australia, 2005. [Google Scholar]
- Murr, L.E. A Brief History of Metals. In Handbook of Materials Structures, Properties, Processing and Performance; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Killick, D. From Ores to Metals. In Archaeometallurgy in Global Perspective; Roberts, B., Thornton, C., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Winder, C. The History of Lead, Part 3. 1994. ISSN 1324-6011. Available online: https://lead.org.au/lanv2n3/lanv2n3-22.html (accessed on 10 February 2025).
- Bartoníček, J. Early history of operative treatment of fractures. Arch. Orthop. Trauma Surg. 2010, 130, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S. Identity as Process: An Archaeological and Osteological Study of Early Bronze Age Burials in Northern England. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2013. [Google Scholar]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinha, N.; Hamre, H.J.; Musial, F.; Werner, E.L.; Björkman, L. Health complaints before and at one and five years after removal of dental amalgam restorations—Data from a prospective cohort study in Norway. Acta Odontol. Scand. 2024, 83, 219–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Council of the European Union. Council Signs Off on Measures to Make the EU Mercury-Free. Available online: https://www.consilium.europa.eu/en/press/press-releases/2024/05/30/council-signs-off-on-measures-to-make-the-eu-mercury-free/ (accessed on 10 February 2025).
- Björkman, L.; Musial, F.; Alræk, T.; Werner, E.L.; Hamre, H.J. Mercury, silver and selenium in serum before and after removal of amalgam restorations: Results from a prospective cohort study in Norway. Acta Odontol. Scand. 2022, 81, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Fowler, B.A.; Fustinoni, S.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 113–122. ISBN 9780444594532. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier: Philadephia, PA, USA, 2016. [Google Scholar]
- Ottaviano, F.G.; Handy, D.E.; Loscalzo, J. Redox Regulation in the Extracellular Environment. Circ. J. 2008, 72, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef]
- Hashimoto, K.; Asami, K.; Teramoto, K. An X-ray photo-electron spectroscopic study on the role of molybdenum in increasing the corrosion resistance of ferritic stainless steels in HCl. Corros. Sci. 1979, 19, 3–14. [Google Scholar] [CrossRef]
- Gotman, I. Characteristics of Metals Used in Implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Knecht, C.; Polakof, L.; Behrens, J.; Goodman, S.B. Wear debris in metal-on-metal bearings and modular junctions: What have we learned from the last decades? Orthopadie 2023, 52, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, Y.; Su, Y.; Qiao, L. Release of metal ions from nano CoCrMo wear debris generated from tribo-corrosion processes in artificial hip implants. J. Mech. Behav. Biomed. Mater. 2017, 68, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Aro, H.; Eerola, E.; Aho, A.; Niinikoski, J. Tissue oxygen tension in externally stabilized tibial fractures in rabbits during normal healing and infection. J. Surg. Res. 1984, 37, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Laing, P.G. Compatibility of biomaterials. Orthop. Clin. N. Am. 1973, 4, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.B.; Kiridena, S.D.; Wijayaratna, U.N.; Taylor, C.; Anker, J.N.; Tzeng, T.-R.J. pH variation in medical implant biofilms: Causes, measurements, and its implications for antibiotic resistance. Front. Microbiol. 2022, 13, 1028560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lebeaux, D.; Chauhan, A.; Létoffé, S.; Fischer, F.; de Reuse, H.; Beloin, C.; Ghigo, J.-M. pH-Mediated Potentiation of Aminoglycosides Kills Bacterial Persisters and Eradicates In Vivo Biofilms. J. Infect. Dis. 2014, 210, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Hengge-Aronis, R. Signal Transduction and Regulatory Mechanisms Involved in Control of the σS(RpoS) Subunit of RNA Polymerase. Microbiol. Mol. Biol. Rev. 2002, 66, 373–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blair, T.A.; Frelinger, A.L. Platelet surface marker analysis by mass cytometry. Platelets 2020, 31, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vermeulen, N.; Bester, J.; Lipinski, B.; Kell, D.B. A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: The use of scanning electron microscopy. Toxicol. Mech. Methods 2013, 23, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Steven, F.S.; Griffin, M.M.; Brown, B.S.; Hulley, T.P. Aggregation of fibrinogen molecules by metal ion. Int. J. Biol. Macromol. 1982, 4, 367–369. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J.; Gibon, E.F.; Takagi, M. Diagnosis and management of implant debris-associated inflammation. Expert Rev. Med. Devices 2020, 17, 41–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roach, K.; Roberts, J. A comprehensive summary of disease variants implicated in metal allergy. J. Toxicol. Environ. Health B Crit. Rev. 2022, 25, 279–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simkiss, K. Lipid solubility of heavy metals in saline solutions. J. Mar. Biol. Assoc. U. K. 1983, 63, 1–7. [Google Scholar] [CrossRef]
- Rizzollo, F.; More, S.; Vangheluwe, P.; Agostinis, P. The lysosome as a master regulator of iron metabolism. Trends Biochem. Sci. 2021, 46, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.S. Metal-Induced Hepatotoxicity. Semin. Liver Dis. 1996, 16, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Blaby-Haas, C.E.; Merchant, S.S. Lysosome-related Organelles as Mediators of Metal Homeostasis. J. Biol. Chem. 2014, 289, 28129–28136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granchi, D.; Cenni, E.; Ciapetti, G.; Savarino, L.; Stea, S.; Gamberini, S.; Gori, A.; Pizzoferrato, A. Cell death induced by metal ions: Necrosis or apoptosis? J. Mater. Sci. Mater. Med. 1998, 9, 31–37. [Google Scholar] [CrossRef] [PubMed]
- European Commission Taxation and Customs Union. Skin Irritants Health and Safety. Available online: https://ec.europa.eu/taxation_customs/dds2/SAMANCTA/EN/Safety/SkinIrritants_EN.htm (accessed on 10 February 2025).
- Lechuga, M.; Fernández-Serrano, M.; Núñez-Olea, J.; Martínez-Gallegos, J.F.; Ríos, F. Optimization of Toxicity, Biodegradability, and Skin Irritation in Formulations Containing Mixtures of Anionic and Nonionic Surfactants Combined with Silica Nanoparticles. Toxics 2025, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Yatera, K. The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int. J. Environ. Res. Public Health 2022, 19, 2788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tramontana, M.; Hansel, K.; Bianchi, L.; Sensini, C.; Malatesta, N.; Stingeni, L. Advancing the understanding of allergic contact dermatitis: From pathophysiology to novel therapeutic approaches. Front. Med. 2023, 10, 1184289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Filgueira, L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening—Current concepts. J. Biomed. Mater. Res. A 2009, 91, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Aluminum, Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Mercury, Molybdenum, Nickel, Platinum, Thallium, Titanium, Vanadium, and Zinc: Molecular Aspects in Experimental Liver Injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacheco, K.A. Allergy to Surgical Implants. Clin. Rev. Allergy Immunol. 2019, 56, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Afif, W.; Knowles, S.; Lim, G.; Lin, Y.; Mothersill, C.; Nistor, I.; Rehman, F.; Song, C.; Xenodemetropoulos, T. Canadian expert consensus: Management of hypersensitivity reactions to intravenous iron in adults. Vox Sang. 2019, 114, 363–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caimmi, S.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Bianchi, A.; Bottau, P.; Mori, F.; Triggiano, P.; Paglialunga, C.; Saretta, F.; et al. Hypersensitivity to Intravenous Iron Preparations. Children 2022, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szebeni, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Rampton, D.; Weiss, G.; Folkersen, J. Hypersensitivity to intravenous iron: Classification, terminology, mechanisms and management. Br. J. Pharmacol. 2015, 172, 5025–5036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Ohgami, N.; Sakashita, M.; Ogi, K.; Hashimoto, K.; Tazaki, A.; Tong, K.; Aoki, M.; Fujieda, S.; Kato, M. Intranasal levels of lead as an exacerbation factor for allergic rhinitis in humans and mice. J. Allergy Clin. Immunol. 2021, 148, 139–147.e10. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Peana, M.; Dadar, M.; Chirumbolo, S.; Aaseth, J.; Martins, N. Mercury-induced autoimmunity: Drifting from micro to macro concerns on autoimmune disorders. Clin. Immunol. 2020, 213, 108352. [Google Scholar] [CrossRef] [PubMed]
- Baseri, M.; Radmand, F.; Hamedi, R.; Yousefi, M.; Kafil, H.S. Immunological Aspects of Dental Implant Rejection. BioMed Res. Int. 2020, 2020, 7279509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’ambrosi, R.; Nuara, A.; Mariani, I.; Di Feo, F.; Ursino, N.; Hirschmann, M. Titanium Niobium Nitride Mobile-Bearing Unicompartmental Knee Arthroplasty Results in Good to Excellent Clinical and Radiographic Outcomes in Metal Allergy Patients With Medial Knee Osteoarthritis. J. Arthroplast. 2021, 36, 140–147.e2. [Google Scholar] [CrossRef] [PubMed]
- Contuzzi, N.; Casalino, G.; Boccaccio, A.; Ballini, A.; Charitos, I.A.; Bottalico, L.; Santacroce, L. Metals Biotribology and Oral Microbiota Biocorrosion Mechanisms. J. Funct. Biomater. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Liu, T.; Hu, H.; Xiong, Z.; Zhang, K.; He, X.; Liu, W.; Lei, P.; Hu, Y. Differential effect of tantalum nanoparticles versus tantalum micron particles on immune regulation. Mater. Today Bio 2022, 16, 100340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a Better Regeneration through Implant-Mediated Immunomodulation: Harnessing the Immune Responses. Adv. Sci. 2021, 8, e2100446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, N.; Yang, X.; Zhang, Y.; Zhang, J.; Ji, J.; Zhang, Y.; Kong, X.; Xi, Y.; Liu, D.; Ye, L.; et al. A molybdenum oxide-based degradable nanosheet for combined chemo-photothermal therapy to improve tumor immunosuppression and suppress distant tumors and lung metastases. J. Nanobiotechnol. 2021, 19, 428. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Mathur, A.; Murugappan, S.; Sharma, A.; Tanwar, K.; Dua, K.; Singh, S.K.; Mazzola, P.G.; Yadav, D.N.; Rengan, A.K.; et al. Inorganic nanoparticles in dermopharmaceutical and cosmetic products: Properties, formulation development, toxicity, and regulatory issues. Eur. J. Pharm. Biopharm. 2023, 192, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Möller, H. Contact allergy to gold as a model for clinical-experimental research. Contact Dermat. 2010, 62, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zemelka-Wiacek, M. Metal Allergy: State-of-the-Art Mechanisms, Biomarkers, Hypersensitivity to Implants. J. Clin. Med. 2022, 11, 6971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.; Fraser, J. Metal-derivatized Major Histocompatibility Complex: Zeroing in on contact hypersensitivity. J. Exp. Med. 2003, 197, 549–552. [Google Scholar] [CrossRef]
- Büdinger, L.; Hertl, M. Immunologic mechanisms in hypersensitivity reactions to metal ions: An overview. Allergy 2000, 55, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.-J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Environ. Res. Public Health 2021, 18, 10867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, M.; Raghavan, B.; Müller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Thierse, H.-J.; Gamerdinger, K.; Junkes, C.; Guerreiro, N.; Weltzien, H.U. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 2005, 209, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.P.; Deng, T.Z.; Witherden, D.A.; Goldrath, A.W. Origins of CD4+ circulating and tissue-resident memory T-cells. Immunology 2019, 157, 3–12. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. Tissue-resident memory T cells. Immunol. Rev. 2013, 255, 165–181. [Google Scholar] [CrossRef]
- Fransway, A.F.; Zug, K.A.; Belsito, D.V.; DeLeo, V.A.; Fowler, J.F.; Maibach, H.I.; Marks, J.G.; Mathias, C.T.; Pratt, M.D.; Rietschel, R.L.; et al. North American Contact Dermatitis Group Patch Test Results for 2007–2008. Dermatitis 2013, 24, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Degradation of dental implants. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer: New York, NY, USA, 2012; Chapter 3; pp. 57–78. [Google Scholar]
- Simón-Vázquez, R.; Lozano-Fernández, T.; Dávila-Grana, A.; González-Fernández, Á. Metal oxide nanoparticles interact with immune cells and activate different cellular responses. Int. J. Nanomed. 2016, 11, 4657–4668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagay, B.E.; Cordeiro, J.M.; Barao, V.A.R. Insight into Corrosion of Dental Implants: From Biochemical Mechanisms to Designing Corrosion-Resistant Materials. Curr. Oral Health Rep. 2022, 9, 7–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delaunay, C.; Petit, I.; Learmonth, I.; Oger, P.; Vendittoli, P. Metal-on-metal bearings total hip arthroplasty: The cobalt and chromium ions release concern. Orthop. Traumatol. Surg. Res. 2010, 96, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Dikici, B.; Esen, Z.; Duygulu, O.; Gungor, S. Corrosion of Metallic Biomaterials. In Advances in Metallic Biomaterials; Springer Series in Biomaterials Science and Engineering; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 3. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matharu, G.S.; Berryman, F.; Judge, A.; Reito, A.; McConnell, J.; Lainiala, O.; Young, S.; Eskelinen, A.; Pandit, H.G.; Murray, D.W. Blood Metal Ion Thresholds to Identify Patients with Metal-on-Metal Hip Implants at Risk of Adverse Reactions to Metal Debris: An External Multicenter Validation Study of Birmingham Hip Resurfacing and Corail-Pinnacle Implants. J. Bone Jt. Surg. 2017, 99, 1532–1539. [Google Scholar] [CrossRef]
- Přikrylová, J.; Procházková, J.; Podzimek, Š. Side Effects of Dental Metal Implants: Impact on Human Health (Metal as a Risk Factor of Implantologic Treatment). BioMed Res. Int. 2019, 2019, 2519205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortona, E.; Pagano, M.T.; Capossela, L.; Malorni, W. The Role of Sex Differences in Bone Health and Healing. Biology 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller-Heupt, L.K.; Schiegnitz, E.; Kaya, S.; Jacobi-Gresser, E.; Kämmerer, P.W.; Al-Nawas, B. Diagnostic tests for titanium hypersensitivity in implant dentistry: A systematic review of the literature. Int. J. Implant. Dent. 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lechtman, H.; Klein, S. The Production of Copper–Arsenic Alloys (Arsenic Bronze) by Cosmelting: Modern Experiment, Ancient Practice. J. Archaeol. Sci. 1999, 26, 497–526. [Google Scholar] [CrossRef]
- Spoto, G. Secondary ion mass spectrometry in art and archaeology. Thermochim. Acta 2000, 365, 157–166. [Google Scholar] [CrossRef]
- Ro, S.-H.; Bae, J.; Jang, Y.; Myers, J.F.; Chung, S.; Yu, J.; Natarajan, S.K.; Franco, R.; Song, H.-S. Arsenic Toxicity on Metabolism and Autophagy in Adipose and Muscle Tissues. Antioxidants 2022, 11, 689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarker, A.; Kim, J.-E.; Islam, A.R.M.T.; Bilal, M.; Rakib, R.J.; Nandi, R.; Rahman, M.M.; Islam, T. Heavy metals contamination and associated health risks in food webs—A review focuses on food safety and environmental sustainability in Bangladesh. Environ. Sci. Pollut. Res. 2022, 29, 3230–3245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathec, A.; von Schirnding, Y.; Montgomery, M.; Röllin, H. Lead Poisoning in South African Children: The Hazard is at Horne. Rev. Environ. Health 2021, 19, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, M.; Antonucci, S.; Volpe, A.R.; Carmignani, M.; Di Gioacchino, M. Acute mercury intoxication and use of chelating agents. J. Biol. Regul. Homeost. Agents 2009, 23, 217–223. [Google Scholar] [PubMed]
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Djordjevic, A.B.; Aaseth, J. A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, O.; Le Bot, B.; Verrey, D.; Durand, S.; Harpet, C.; Froment, A.; Jégou, B. High lead level in the Alps in XIXth century, learning from the analysis of 138 historical hair stands. Chemosphere 2022, 286, 131658. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Sun, M.; Ju, J.; Ding, Y.; Zhao, C.; Tian, C. The signaling pathways regulated by KRAB zinc-finger proteins in cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188731. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Wu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Nickel Carcinogenesis Mechanism: DNA Damage. Int. J. Mol. Sci. 2019, 20, 4690. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Górka, I.; Januszewska, L.; Michalak, A.; Prokopowicz, A.; Januszewska, E.; Pawlas, N.; Pawlas, K. Effects of chronic exposure to lead, cadmium, and manganese mixtures on oxidative stress in rat liver and heart. Arch. Ind. Hyg. Toxicol. 2015, 66, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, L.; Zhou, Y.; He, H.; Chen, W.; Lenahan, C.; Li, X.; Deng, Y.; Shao, A.; Huang, J. Crosstalk between Macrophages, T Cells, and Iron Metabolism in Tumor Microenvironment. Oxidative Med. Cell. Longev. 2021, 2021, 8865791. [Google Scholar] [CrossRef]
- Kłys, M. Z rtecia (i…) przez stulecia [Mercury (and…) through the centuries]. Arch. Med. Sadowej Kryminol. 2010, 60, 298–307. (In Polish) [Google Scholar] [PubMed]
- Michaleas, S.N.; Laios, K.; Tsoucalas, G.; Androutsos, G. Theophrastus Bombastus Von Hohenheim (Paracelsus) (1493–1541): The eminent physician and pioneer of toxicology. Toxicol. Rep. 2021, 8, 411–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soler, J.I.; Ellacuria, J.; Triana, R.; Guinea, E.; Osborne, J.W. A history of dental amalgam. J. Hist. Dent. 2002, 50, 109–116. [Google Scholar] [PubMed]
- Motevalian, M.; Shiri, M.; Shiri, S.; Shiri, Z.; Shiri, H. Anti-inflammatory activity of Elaeagnus angustifolia fruit extract on rat paw edema. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, B.; Pactat, I.; Schibille, N. Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production. Minerals 2018, 8, 225. [Google Scholar] [CrossRef]
- Chen, R.J.; Lee, V.R. Cobalt Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Zohoori, F.V.; Duckworth, R.M. Chapter 5: Microelements: Part II: F, Al, Mo and Co. Monogr. Oral Sci. 2020, 28, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Munari, M.; Tinazzi, V. Ricerche sperimentali sull’intossicazione da cobalto e suo trattamento con Ca EDTA Na2 [Experimental research on cobalt poisoning and its treatment with calcium disodium edathamil]. Folia Med. (Napoli) 1956, 39, 260–266. (In Italian) [Google Scholar] [PubMed]
- Moore, B.; Hawkes, J.L. An Investigation of the Toxic Actions of Dilute Solutions of the Salts of certain Heavy Metals (viz.: Copper, Iron, Nickel, Cobalt, Manganese, Zinc, Silver, and Lead) upon the Bacillus Typhosus, with a view to practical application in the Purification of Shell-fish. Biochem. J. 1908, 3, 313–345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, Y.; Wang, Y.; Zhang, C.; Wu, C.; Xu, X.; Xiao, K.; Zhao, Z.; Zhang, H. The Impact of Cobalt Species on the Hazardous Characteristics of Cobalt-Leaching Residue: A Case Study from Guangdong Province, China. Water 2024, 16, 2953. [Google Scholar] [CrossRef]
- Coleman, R.F.; Herrington, J.; Scales, J.T. Concentration of Wear Products in Hair, Blood, and Urine after Total Hip Replacement. BMJ 1973, 1, 527–529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Medicines and Healthcare products Regulatory Agency. Medical Device Alert, Ref: MDA/2012/036; Medicines and Healthcare products Regulatory Agency: London, UK, 2012. Available online: https://assets.publishing.service.gov.uk/media/5485abf6ed915d4c10000273/con155767.pdf (accessed on 10 February 2025).
- Liu, Y.K.; Deng, X.X.; Yang, H. Cytotoxicity and genotoxicity in liver cells induced by cobalt nanoparticles and ions. Bone Jt. Res. 2016, 5, 461–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirk, M.L.; Hille, R. Spectroscopic Studies of Mononuclear Molybdenum Enzyme Centers. Molecules 2022, 27, 4802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colston, K.J.; Basu, P. Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors. Molecules 2022, 27, 3324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Novotny, J.A.; A Peterson, C. Molybdenum. Adv. Nutr. Int. Rev. J. 2018, 9, 272–273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vyskocil, A.; Viau, C. Assessment of molybdenum toxicity in humans. J. Appl. Toxicol. 1999, 19, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (US). Toxicological Profile for Molybdenum; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2020. [Google Scholar] [PubMed]
- Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Gao, J.; Xu, Y.; Wang, J.; Shi, Z.; Cheng, H. Prosthetic Metals: Release, Metabolism and Toxicity. Int. J. Nanomed. 2024, 19, 5245–5267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- 7 Most Abundant Metal Elements in Earth’s Crust. Available online: https://www.refractorymetal.org/7-most-abundant-metal-elements-in-earths-crust/ (accessed on 10 February 2025).
- Powell, J.J.; Greenfield, S.M.; Thompson, R.P.H.; Cargnello, J.A.; Kendall, M.D.; Landsberg, J.P.; Watt, F.; Delves, H.T.; House, I. Assessment of toxic metal exposure following the Camelford water pollution incident: Evidence of acute mobilization of lead into drinking water. Analyst 1995, 120, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, J.W.C.; Martinez-Lavin, M.; Jara, L.J.; Halpert, G.; Watad, A.; Amital, H.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun. Rev. 2023, 22, 103287. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.; Li, S.; Crean, S.J.; Barrak, F.N. Is titanium alloy Ti-6Al-4 V cytotoxic to gingival fibroblasts—A systematic review. Clin. Exp. Dent. Res. 2021, 7, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bryliński, Ł.; Kostelecka, K.; Woliński, F.; Duda, P.; Góra, J.; Granat, M.; Flieger, J.; Teresiński, G.; Buszewicz, G.; Sitarz, R.; et al. Aluminium in the Human Brain: Routes of Penetration, Toxicity, and Resulting Complications. Int. J. Mol. Sci. 2023, 24, 7228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed]
- De Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials. In Tribology—Fundamentals and Advancements; Gegner, J., Ed.; IntechOpen: London, UK, 2013; Volume 55, pp. 561–565. [Google Scholar]
- Singh, R.; Lehl, G.; Bin Hussain, A.; Abhang, T.N.; Kulkarni, M.M.; Elagib, M.F.A.; Tiwari, R.V.C. Prevalence of Titanium Hypersensitivity in Patients with Titanium Implants: A Systematic Review and Meta-analysis. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S2), S1345–S1349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goutam, M.; Giriyapura, C.; Mishra, S.; Gupta, S. Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, M.; Liu, L.; Ichikawa, T. Are Allergy-Induced Implant Failures Actually Hypersensitivity Reactions to Titanium? A Literature Review. Dent. J. 2023, 11, 263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wawrzynski, J.; Gil, A.; Goodman, A.D.; Waryasz, G.R. Hypersensitivity to Orthopedic Implants: A Review of the Literature. Rheumatol. Ther. 2017, 4, 45–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanski, W.; Kosiorowska, J.; Szymanska-Chabowska, A. Osteoporosis—Risk factors, pharmaceutical and non-pharmaceutical treatment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Dali, S.S.M.; Wong, S.K.; Chin, K.-Y.; Ahmad, F. The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence. Int. J. Mol. Sci. 2023, 24, 7161. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safavi, M.S.; Walsh, F.C.; Visai, L.; Khalil-Allafi, J. Progress in Niobium Oxide-Containing Coatings for Biomedical Applications: A Critical Review. ACS Omega 2022, 7, 9088–9107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chézeau, L.; Tchinda, A.; Pierson, G.; Bravetti, P.; Ferrari, L.; Joubert, O.; Zaiou, M.; Rihn, B.H. In Vitro Molecular Study of Titanium-Niobium Alloy Biocompatibility. Biomedicines 2022, 10, 1898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Santana, F.R.; Dalboni, L.C.; Nascimento, K.F.; Konno, F.T.; Alvares-Saraiva, A.M.; Correia, M.S.; Bomfim, M.D.C.; Casarin, R.C.; Perez, E.C.; Lallo, M.A.; et al. High dilutions of antimony modulate cytokines production and macrophage—Leishmania (L.) amazonensis interaction in vitro. Cytokine 2017, 92, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Lerman, Y.; Kapel, A.; Pardo, A.; Schwarz, Y.; Newman, L.; Maier, L.; Fireman, E. Biological Exposure Metrics of Beryllium-Exposed Dental Technicians. Arch. Environ. Occup. Health 2013, 69, 89–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stearney, E.R.; Jakubowski, J.A.; Regina, A.C. Beryllium Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Moulis, J.-M.; Bulat, Z.; Djordjevic, A.B. Threshold in the toxicology of metals: Challenges and pitfalls of the concept. Curr. Opin. Toxicol. 2020, 19, 28–33. [Google Scholar] [CrossRef]
- Mine, Y.; Makihira, S.; Nikawa, H.; Murata, H.; Hosokawa, R.; Hiyama, A.; Mimura, S. Impact of titanium ions on osteoblast-, osteoclast- and gingival epithelial-like cells. J. Prosthodont. Res. 2010, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robles, D.; Brizuela, A.; Fernández-Domínguez, M.; Gil, J. Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants. Materials 2023, 16, 3650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Fan, H.; Li, H.; Hua, L.; Du, J.; He, Y.; Jin, Y. Recent Advancements in the Surface Modification of Additively Manufactured Metallic Bone Implants. Addit. Manuf. Front. 2025, 4, 200195. [Google Scholar] [CrossRef]
- Szczęsny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A Review on Biomaterials for Orthopaedic Surgery and Traumatology: From Past to Present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef]
- Arevalo, S.; Arthurs, C.; Molina, M.I.E.; Pruitt, L.; Roy, A. An overview of the tribological and mechanical properties of PEEK and CFR-PEEK for use in total joint replacements. J. Mech. Behav. Biomed. Mater. 2023, 145, 105974. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Paltanea, V.M.; Antoniac, A.; Paltanea, G. Magnesium-based alloys with adapted interfaces for bone implants and tissue engineering. Regen. Biomater. 2023, 10, rbad095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viswanathan, V.K.; Jain, V.K.; Sangani, C.; Botchu, R.; Iyengar, K.P.; Vaishya, R. SMART (self-monitoring analysis and reporting technology) and sensor based technology applications in trauma and orthopaedic surgery. J. Orthop. 2023, 44, 113–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasai, H.; Bergamo, E.; Balderrama, Í.; Imamura, K.; Witek, L.; Jalkh, E.; Bonfante, E.; Inoue, K.; Coelho, P.; Yamano, S. The effect of nano hydroxyapatite coating implant surfaces on gene expression and osseointegration. Med. Oral Patol. Oral Y Cirugia Bucal 2024, 29, e326–e333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, Z.; Zhang, L.; Lan, W.; Du, J.; Hu, Y.; Wei, Y.; Hang, R.; Chen, W.; Huang, D. In situ titanium phosphate formation on a titanium implant as ultrahigh bonding with nano-hydroxyapatite coating for rapid osseointegration. Biomater. Sci. 2023, 11, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Takahashi, H.; Matsugaki, A.; Uemukai, T.; Kogai, Y.; Imagama, T.; Yukata, K.; Nakano, T.; Sakai, T. Novel nano-hydroxyapatite coating of additively manufactured three-dimensional porous implants improves bone ingrowth and initial fixation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 453–462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gamna, F.; Spriano, S. Vitamin E: A Review of Its Application and Methods of Detection When Combined with Implant Biomaterials. Materials 2021, 14, 3691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, F.; Yin, Z.; Ren, X.; Geng, Z.; Su, J. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics 2022, 14, 1069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. Medcomm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.-L.; Lee, Y.-H.; Chou, C.-L.; Chang, Y.-S.; Liu, W.-C.; Chiu, H.-W. Oxidative stress and potential effects of metal nanoparticles: A review of biocompatibility and toxicity concerns. Environ. Pollut. 2024, 346, 123617. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability In vivo in Mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skjöldebrand, C.; Tipper, J.L.; Hatto, P.; Bryant, M.; Hall, R.M.; Persson, C. Current status and future potential of wear-resistant coatings and articulating surfaces for hip and knee implants. Mater. Today Bio 2022, 15, 100270. [Google Scholar] [CrossRef]
- Erkes, D.A.; Selvan, S.R. Hapten-Induced Contact Hypersensitivity, Autoimmune Reactions, and Tumor Regression: Plausibility of Mediating Antitumor Immunity. J. Immunol. Res. 2014, 2014, 175265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumann, C.A.; Crist, B.D. Nickel allergy to orthopaedic implants: A review and case series. J. Clin. Orthop. Trauma 2020, 11, S596–S603. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.K.; Yari, A.; Nikparto, N.; Mehraban, S.H.; Cheperli, S.; Asadi, A.; Darehdor, A.A.; Nezaminia, S.; Dortaj, D.; Nazari, Y.; et al. The current applications of nano and biomaterials in drug delivery of dental implant. BMC Oral Health 2024, 24, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drynda, A.; Drynda, S.; Kekow, J.; Lohmann, C.H.; Bertrand, J. Differential Effect of Cobalt and Chromium Ions as Well as CoCr Particles on the Expression of Osteogenic Markers and Osteoblast Function. Int. J. Mol. Sci. 2018, 19, 3034. [Google Scholar] [CrossRef] [PubMed]


| Element | Effects and Uses | Toxicity | Irritation | Allergy | Mitigation Strategies | Ref. |
|---|---|---|---|---|---|---|
| Gold (Au) | Used in medical and dental implants due to its non-corrosive nature; historically used in jewellery and coins; possesses some antimicrobial properties. | Considered non-toxic; does not corrode; is bioinert in most cases. | Very low; does not commonly cause irritation. | Rare but possible sensitization reactions in some individuals. | Use inert forms when possible. Limit exposure to reactive gold compounds. Monitor for signs of allergic reactions in medical settings. | [13] |
| Silver (Ag) | Used in dental implants, wound dressings, and coatings for medical devices due to its antimicrobial properties. | Low toxicity, but long-term exposure can lead to argyria (blue-grey skin discoloration). | Generally low; can cause irritation, if absorbed in excess. | Rare, but silver allergies have been reported. | Adhere to regulated dosages in medicinal applications. Ensure proper handling in industrial processes to avoid ingestion or prolonged exposure. | [13] |
| Copper (Cu) | Used in alloys such as bronze and brass; has bactericidal properties; is currently not used for dental or orthopaedic applications, but is still in use in gynaecology (contraceptive spirals). | Toxic in high amounts; can cause liver and kidney damage (Wilson’s disease) and neurological symptoms. | High irritation potential; copper salts can cause skin and mucosal irritation. | Can trigger contact dermatitis, particularly in individuals sensitive to metal jewellery. | Monitor dietary and environmental copper levels. Apply chelation therapy in poisoning cases. Control contamination in water supplies and industrial settings. | [13] |
| Iron (Fe) | Essential for red blood cell production; used in stainless steel implants, surgical instruments, and devices. | Generally safe, but excess iron (hemochromatosis) can lead to organ failure and oxidative stress. | Mild; iron supplements may cause gastrointestinal irritation. | Rare, but intravenous iron infusions may trigger allergic reactions. | Use iron chelators (e.g., deferoxamine) and phlebotomy for overload conditions. Regulate iron supplementation and monitor body iron levels. | [38] |
| Nickel (Ni) | Common in stainless steel and orthopaedic implants; is an austenite stabilizer; improves corrosion resistance and the strength of an alloy; is highly allergenic. | Moderate to high toxicity; nickel exposure can lead to systemic toxicity, organ damage, and carcinogenic effects. | Strong irritant; causes skin irritation and can provoke chronic conditions like eczema. | One of the most common metal allergens; causes nickel dermatitis, itching, and rashes. | Limit exposure (especially in sensitized individuals) through substitution in products. Use appropriate personal protective equipment (PPE) in industrial settings. | [13,16] |
| Chromium (Cr) | Found in stainless steel, dental, and orthopaedic implants; improves corrosion resistance. | Hexavalent chromium is highly toxic and carcinogenic; can cause lung and kidney damage. | Strong irritant; chromate salts cause severe skin and respiratory irritation. | Can provoke immune responses and hypersensitivity reactions in some individuals. | Replace or reduce Cr(VI) with Cr(III) when possible. Enforce strict industrial controls and PPE. Implement remediation strategies for contaminated sites. | [16,38] |
| Cobalt (Co) | Mechanically very hard; used in cobalt–chromium-molybdenum (CoCrMo) alloys to manufacture various medical implants; is also essential in vitamin B12 (cobalamin). | Toxic in high exposure; linked to cardiomyopathy, neurological disorders, and thyroid dysfunction. | Moderate; can cause dermatitis, rashes, and respiratory irritation. | High allergenic potential; can trigger cobalt dermatitis and asthma. | Enforce industrial hygiene practices and limit airborne exposure. Use PPE and continuous monitoring in occupational settings. | [13,16] |
| Molybdenum (Mo) | Strengthens stainless steel and CoCrMo alloys; is an essential trace dietary element. | Low toxicity; rare cases of molybdenum poisoning exist, often occupational. | Mild irritant, particularly in dust or fume form. | Rare allergic reactions, though not commonly recognized as an allergen. | Monitor exposure in occupational environments. Ensure balanced dietary intake to avoid imbalances with copper levels. | [13,16] |
| Aluminium (Al) | Used in alloys, implants, vaccines, antacids, and food packaging; is lightweight and corrosion-resistant. | Generally considered non-toxic, but possible links between aluminium poisoning and Alzheimer’s disease and neurodegeneration are debated. | Mild; can cause skin irritation and granulomas, when implanted. | Possible, but rare aluminium hypersensitivity reactions exist. | Reduce exposure through water treatment and controlled use in consumer products. Use alternative materials in medical applications (e.g., dialysis fluids). | [16,38] |
| Titanium (Ti) | Used extensively in orthopaedic and dental implants; is bioinert and corrosion-resistant. | Low toxicity, but some concerns over long-term accumulation in tissues. | Low irritation; metallosis can occur in rare cases around implants. | Rare cases of titanium hypersensitivity reported, leading to implant rejection. | Control nanoparticle release in industrial settings. Use adequate ventilation and PPE to limit inhalation exposure. | [13] |
| Lead (Pb) | In the past, used in dental amalgams and anti-infectious medicines (i.e., syphilis); also used in paints, plumbing, and batteries but is highly toxic to humans. | Highly toxic; causes neurological damage, developmental disorders, kidney failure, and anaemia. | Strong irritant; can cause severe skin and mucosal inflammation. | Not typically allergenic, but exposure can affect the immune system. | Remove lead sources from environments (e.g., lead abatement programs). Apply chelation therapy when necessary. Enforce strict industrial and public health regulations. | [34] |
| Mercury (Hg) | In the past, used in dental amalgams and thermometers; is highly toxic. | Neurotoxic; affects the central nervous system, kidneys, and immune system. | Strong irritant; can cause burns, ulcers, and respiratory issues. | Rare, but mercury exposure can sometimes provoke immune reactions. | Limit consumption of high-mercury fish and control industrial emissions. Use chelation therapy for mercury poisoning. Monitor and remediate environmental contamination. | [38] |
| Strontium (Sr) | Used in bone-strengthening treatments (strontium ranelate) and some medical alloys. | Low toxicity; large amounts can disrupt calcium metabolism. | Mild irritant in high doses. | Rare, but can theoretically trigger immune responses. | Monitor and regulate industrial and environmental exposures. Remediate radioactive contamination and use safe handling practices. | [128] |
| Vanadium (V) | Found in some orthopaedic alloys; is considered for medical applications. | Moderate toxicity; excessive exposure can cause neurotoxicity and respiratory issues. | High irritation potential, particularly in airborne forms. | Rare, but sensitization reactions have been reported. | Limit exposure through strict industrial standards. Monitor environmental levels and enforce the use of PPE. | [9,79] |
| Niobium (Nb) | Used in orthopaedic implants to enhance biocompatibility. | Low toxicity; well-tolerated by human tissues. | Low irritation; does not commonly provoke adverse effects. | May reduce allergic reactions to other metals in alloys. | Follow standard industrial hygiene protocols. Monitor exposure where applicable and promote further research on long-term effects. | [53] |
| Antimony (Sb) | Historically used in medicine for antiparasitic and emetic treatments. | Toxic in excess; affects the liver, heart, and respiratory system. | Strong irritant; can cause skin inflammation and mucosal damage. | Possible allergic reactions, particularly in occupational exposure. | Employ strict industrial controls and proper PPE. Monitor air quality and ensure safe handling/disposal of antimony compounds. | [13] |
| Beryllium (Be) | Industrially used but highly toxic; in medicine, used as a radiographic dye (BaSO4). | Very toxic; causes lung disease (berylliosis) and is carcinogenic. | Strong irritant; beryllium compounds cause severe skin and respiratory inflammation. | Highly allergenic; can trigger chronic immune disorders (beryllium sensitization). | Implement rigorous industrial controls and respiratory protection. Substitute with less toxic materials when possible. Regular health screening for exposed workers. | [13,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczęsny, G.; Kopec, M.; Kowalewski, Z.L. Toxicity, Irritation, and Allergy of Metal Implants: Historical Perspective and Modern Solutions. Coatings 2025, 15, 361. https://doi.org/10.3390/coatings15030361
Szczęsny G, Kopec M, Kowalewski ZL. Toxicity, Irritation, and Allergy of Metal Implants: Historical Perspective and Modern Solutions. Coatings. 2025; 15(3):361. https://doi.org/10.3390/coatings15030361
Chicago/Turabian StyleSzczęsny, Grzegorz, Mateusz Kopec, and Zbigniew L. Kowalewski. 2025. "Toxicity, Irritation, and Allergy of Metal Implants: Historical Perspective and Modern Solutions" Coatings 15, no. 3: 361. https://doi.org/10.3390/coatings15030361
APA StyleSzczęsny, G., Kopec, M., & Kowalewski, Z. L. (2025). Toxicity, Irritation, and Allergy of Metal Implants: Historical Perspective and Modern Solutions. Coatings, 15(3), 361. https://doi.org/10.3390/coatings15030361







