Synthesis and Characterization of a Fluorinated Schiff Base from Benzimidazole and Its Metal Complexes for Antimicrobial and UV-Protective Cotton Fabrics
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Chemicals
2.3. Instruments
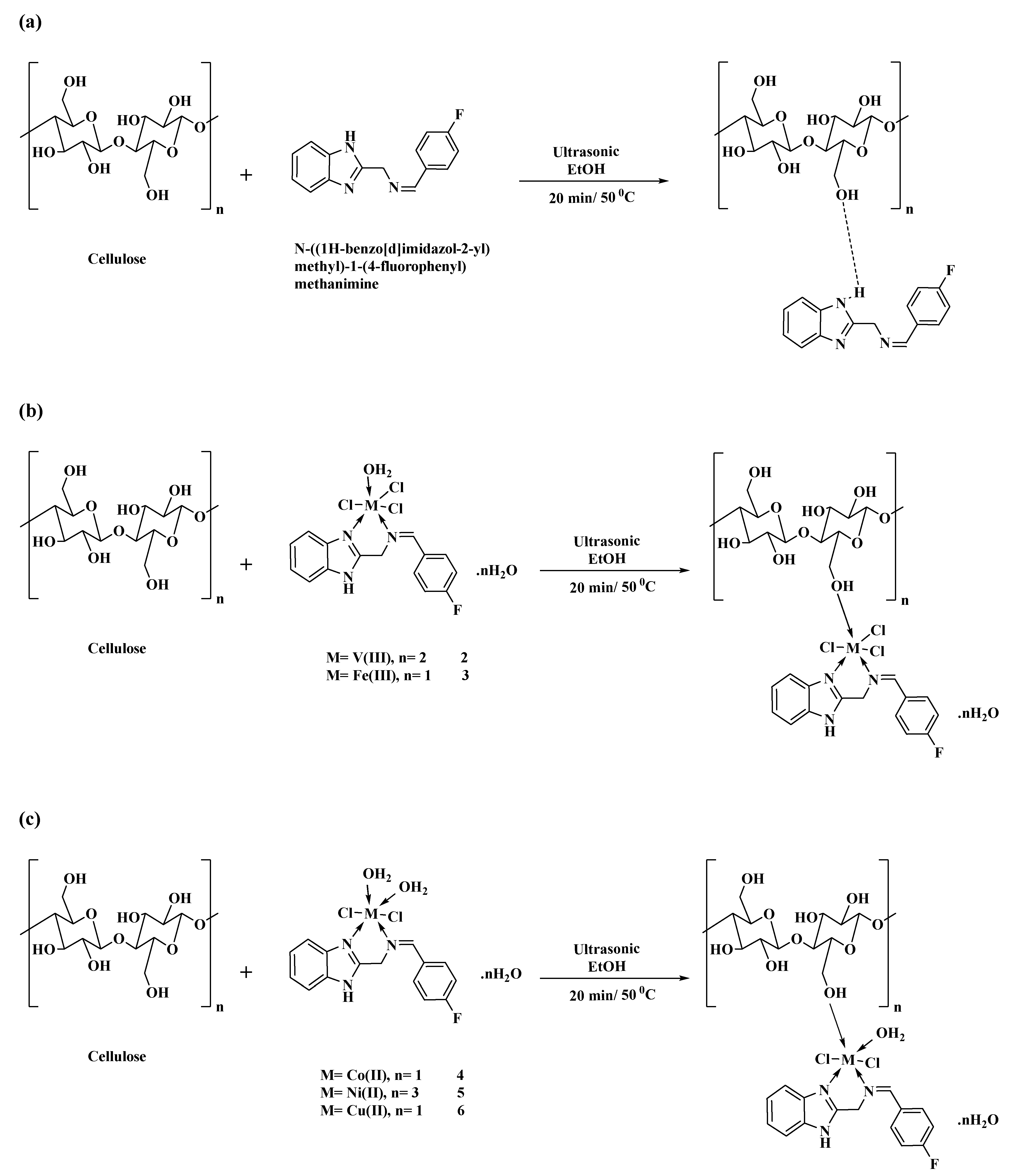
2.4. Preparation of N-((1H-Benzo[d]imidazol-2-yl)methyl)-1-(4-fluorophenyl)methenamine Ligand: (BZI) (1)
2.5. Preparation of Metal Complexes
2.5.1. V(III)-Complex (2)
2.5.2. Fe(III)-Complex (3)
2.5.3. Co(II)-Complex (4)
2.5.4. Ni(II)-Complex (5)
2.5.5. Cu(II)-Complex (6)
2.6. Treatment of Cotton Fabric by Prepared Ligand and Its M-Complexes
2.7. Ultraviolet Protection Factor (UPF)
2.8. In Vitro Antimicrobial
- Gram-positive bacteria “Staphylococcus aureus (S. aureus) (AATCC12600)”
- Gram-negative bacteria “Escherichia coli (E. coli) (ATCC11775)”
- Fungi “Candida albicans (C. albicans) (ATCC 7102)” and Aspergillus flavus (A. flavus) (ATCC 9643)
3. Results and Discussion
3.1. Chemistry
UV–Visible Spectra and Magnetic Susceptibility
3.2. Characterization of the Treated Cotton Fabric
3.2.1. FTIR Spectra
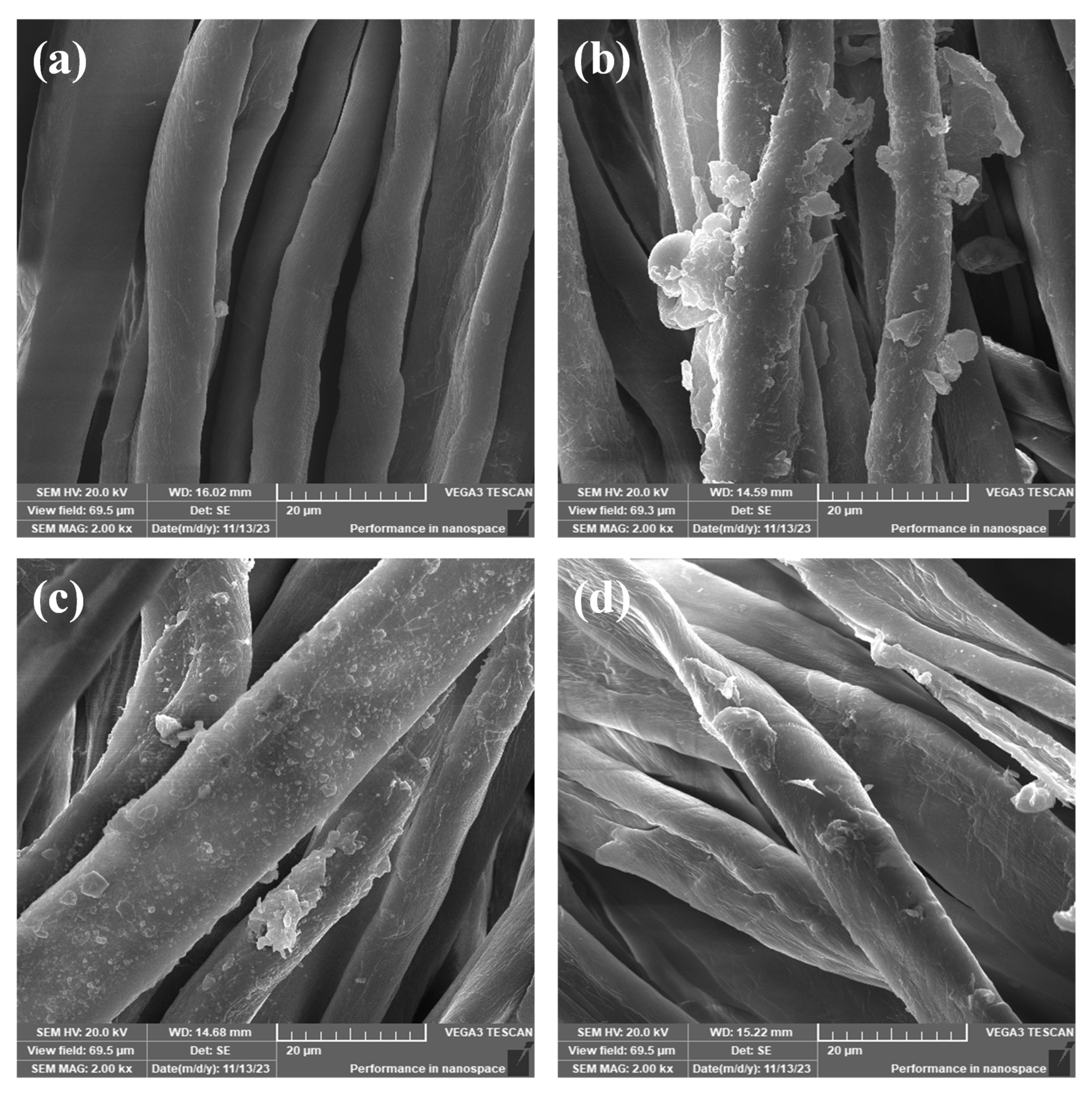
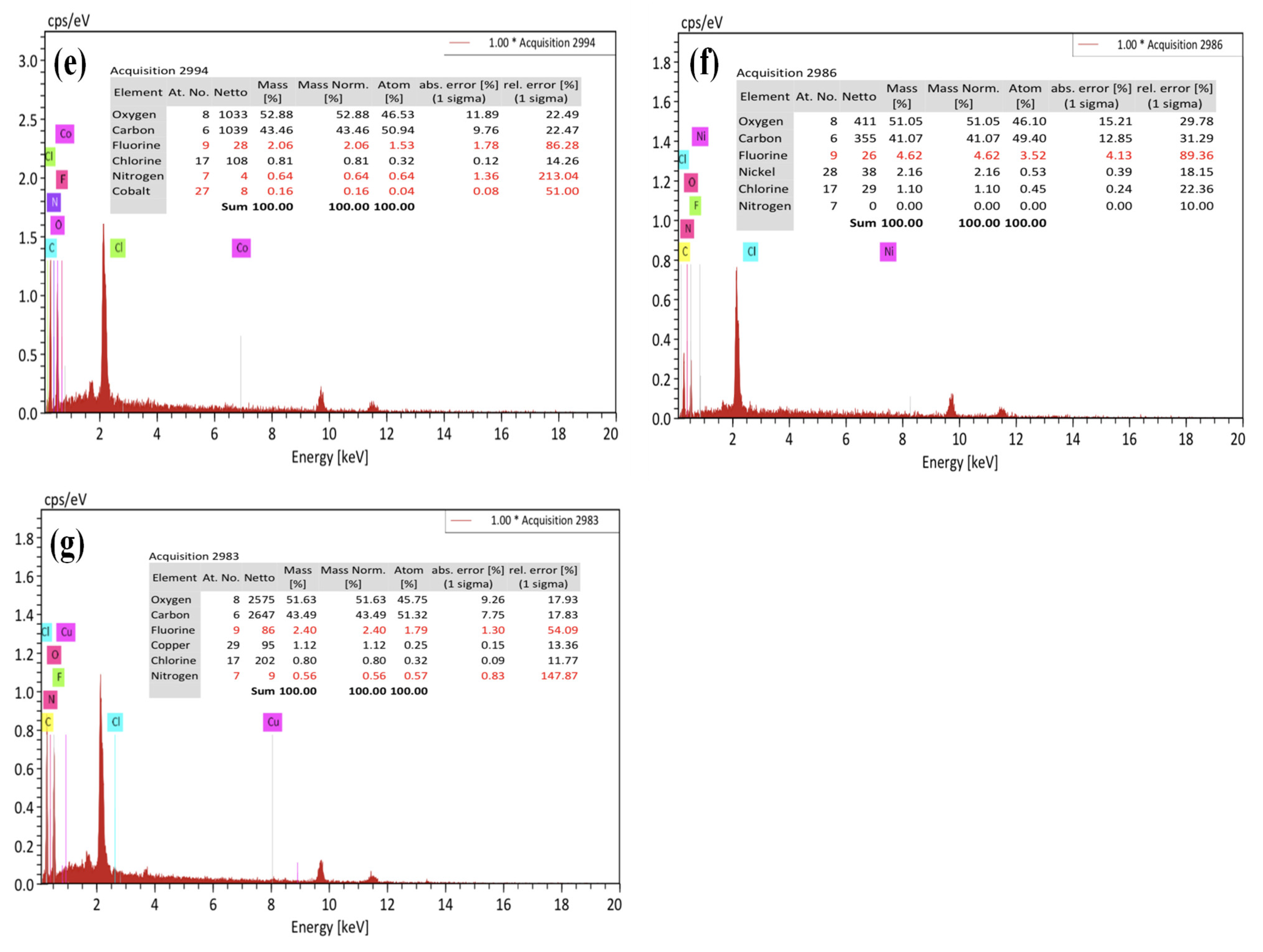
3.2.2. SEM/EDX Analysis
3.2.3. Ultraviolet Protection Properties
3.2.4. Antimicrobial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kale, R.D.; Bansal, P.S.; Gorade, V.G. Extraction of Microcrystalline Cellulose from Cotton Sliver and Its Comparison with Commercial Microcrystalline Cellulose. J. Polym. Environ. 2018, 26, 355–364. [Google Scholar] [CrossRef]
- Wei, D.W.; Wei, H.; Gauthier, A.C.; Song, J.; Jin, Y.; Xiao, H. Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications. J. Bioresour. Bioprod. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Ilić, V.; Šaponjić, Z.; Vodnik, V.; Potkonjak, B.; Jovančić, P.; Nedeljković, J.; Radetić, M. The influence of silver content on antimicrobial activity and color of cotton fabrics functionalized with Ag nanoparticles. Carbohydr. Polym. 2009, 78, 564–569. [Google Scholar] [CrossRef]
- Babu, K.M.; Ravindra, K.B. Bioactive antimicrobial agents for finishing of textiles for health care products. J. Text. Inst. 2015, 106, 706–717. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Abdel-Mohdy, F.A.; Fouda, M.M.G.; El-Sawy, S.M.; Hamdy, I.A.; Al-Deyab, S.S. Antimicrobial activity of monochlorotriazinyl-β-cyclodextrin/ chlorohexidin diacetate finished cotton fabrics. Carbohydr. Polym. 2011, 86, 1389–1394. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, K.; Li, R.; Ren, X.; Huang, T.S. Antibacterial cotton treated with N-halamine and quaternary ammonium salt. Cellulose 2013, 20, 3123–3130. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Jiang, X.; Huang, D.; Yan, Y. Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr. Polym. 2011, 85, 221–227. [Google Scholar] [CrossRef]
- Saeed, S.E.; Al-harbi, T.M.; Alhakimi, A.N.; El-hady, M.M.A. Aniline Derivative Schiff Base for Antimicrobial Applications and UV Protection of a Modified Cotton Fabric. Coatings 2022, 12, 1181. [Google Scholar] [CrossRef]
- Saeed, S.E.; Aldubayyan, M.; Al-hakimi, A.N.; El-hady, M.M.A. Synthesis and Characterization of Pyridine Acetohydrazide Derivative for Antimicrobial Cotton Fabric. Materials 2023, 16, 4885. [Google Scholar] [CrossRef]
- Saeed, S.E.; Alomari, B.A.; El-hady, M.M.A.; Al-hakimi, A.N. Novel Pyrimidinethione Hydrazide Divalent and Trivalent Metal Complexes for Improved High-Performance Antimicrobial and Durable UV Blocking Cellulosic Fabric. Inorganics 2023, 11, 231. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, B.; Lv, M.; Li, J.; Zhang, Y.; Jiang, H.; Peng, C. Graphene Oxide-Based Antibacterial Cotton Fabrics. Adv. Healthc. Mater. 2013, 2, 1259–1266. [Google Scholar]
- El-Nahhal, I.M.; Zourab, S.M.; Kodeh, F.S.; Elmanama, A.A.; Selmane, M.; Genois, I.; Babonneau, F. Nanostructured zinc oxide-cotton fibers: Synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2013, 24, 3970–3975. [Google Scholar]
- El-Nahhal, I.M.; Zourab, S.M.; Kodeh, F.S.; Selmane, M.; Genois, I.; Babonneau, F. Nanostructured copper oxide-cotton fibers: Synthesis, characterization, and applications. Int. Nano Lett. 2012, 2, 14. [Google Scholar]
- El-Naggar, M.; Shaheen, T.I.; Zaghloul, S.; El-Rafie, M.H.; Hebeish, A.A. Antibacterial activities and UV-protection of the in-situ synthesized titanium oxide nanoparticles on cotton fabrics. Ind. Eng. Chem. Res. 2016, 55, 2661–2668. [Google Scholar]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinology 2012, 4, 109–117. [Google Scholar]
- Watson, R.E.B.; Gibbs, N.K.; Griffiths, C.E.M.; Sherratt, M.J. Damage to skin extracellular matrix induced by UV exposure, Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef]
- Rahman, M.S.; Alom, J.; Nitai, A.S.; Hasan, M.S.; Ahmed, M.B.; Nam, S.; Mondal, M.I.H. Ultraviolet-Blocking Protective Textiles; Woodhead Publishing: Cawston, UK, 2022. [Google Scholar]
- Yu, Y. UV Interactions with Fibres and Fibrous Structures; Deakin University: Geelong, Australia, 2015. [Google Scholar]
- Tarbuk, A.; Grancarić, A.M.; Šitum, M. Skin cancer and UV protection. AUTEX Res. J. 2016, 16, 19–28. [Google Scholar]
- Kocić, A.; Bizjak, M.; Popović, D.; Stanković, G.B.P.S.B.; More, S. UV protection afforded by textile fabrics made of natural and regenerated cellulose fibres. J. Clean. Prod. 2019, 228, 1229–1237. [Google Scholar]
- Kibria, G.; Repon, M.R.; Hossain, M.F.; Islam, T.; Jalil, M.A.; Aljabri, M.D.; Rahman, M.M. UV-blocking cotton fabric design for comfortable summer wears: Factors, durability and nanomaterials. Cellulose 2022, 29, 7555–7585. [Google Scholar] [CrossRef]
- Alebeid, O.K.; Zhao, T. Review on: Developing UV protection for cotton fabric. J. Text. Inst. 2017, 108, 2027–2039. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Nostro, P.L.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton Fabric Surface Modification for Improved UV Radiation Protection Using Sol–Gel Process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Arunmetha, S.; Manivasakan, P.; Karunakaran, G.; Rajendran, V. Enhanced functional properties of cotton fabrics using TiO2/SiO2 nanocomposites. J. Ind. Text. 2016, 45, 674–692. [Google Scholar] [CrossRef]
- Hou, A.; Zhang, C.; Wang, Y. Preparation and UV-protective properties of functional cellulose fabrics based on reactive azobenzene Schiff base derivative. Carbohydr. Polym. 2012, 87, 284–288. [Google Scholar] [CrossRef]
- Akrman, J.; Přikryl, J. Application of Benzotriazole Reactive UV Absorbers to Cellulose and Determining Sun Protection of Treated Fabric Spectrophotometrically. J. Appl. Polym. Sci. 2008, 108, 334–341. [Google Scholar] [CrossRef]
- Saeed, S.E.S.; Aldubayyan, M.; Al-Hakimi, A.N.; El-Sayed, W.A.; Alnawmasi, J.S.; El-Hady, M.M.A.; Abdel-Mottaleb, M.S.A. UV protective textile: Experimental and DFT computational studies on the function of some metal complexes of hydrazide derivatives on cellulose fabrics. Appl. Organomet. Chem. 2023, 37, e7140. [Google Scholar] [CrossRef]
- Herna, R.; Mederos, A. Acyclic and Macrocyclic Schiff Base Ligands. Compr. Coord. Chem. II 2003, 1, 411–446. [Google Scholar]
- Adaji, M.U.; Iorungwa, M.S.; Salawu, O.W. Mohammed Umar Adaji, Characterization of Schiff Base Ligand and Its Metal Complexes; IntechOpen: London, UK, 2024. [Google Scholar]
- Alreja, P.; Kaur, N. Probing anion and cation with novel salicylidene Schiff base receptor appended with 1, 10-phenanthroline: Mimicking INHIBIT molecular logic gate. Inorganica Chim. Acta 2018, 480, 127–131. [Google Scholar] [CrossRef]
- Al-hakemi, A.N.; Alresheedi, T.M.; Albarrak, R.A.; Shakdofa, M.; Albadri, A.E.; El-hady, M.M.A. Ligational behaviour of tridentate NNO-donor Schiff base and antimicrobial investigation. Biosci. Res. 2024, 21, 35–54. [Google Scholar]
- Wagih, N.; Abdel-Rahman, I.M.; El-Koussi, N.A.; Abuo-Rahma, G.E.-D.A. Anticancer benzimidazole derivatives as inhibitors of epigenetic targets: A review article. RSC Adv. 2025, 15, 966–1010. [Google Scholar] [CrossRef]
- Miller, J.F.; Turner, E.M.; Gudmundsson, K.S.; Jenkinson, S.; Spaltenstein, A.; Thomson, M.; Wheelan, P. Novel N-substituted benzimidazole CXCR4 antagonists as potential anti-HIV agents. Bioorg. Med. Chem. Lett. 2010, 20, 2125–2128. [Google Scholar] [CrossRef]
- Rathore, A.; Sudhakar, R.; Ahsan, M.J.; Ali, A.; Subbarao, N.; Jadav, S.S.; Umar, S.; Yar, M.S. In vivo anti-inflammatory activity and docking study of newly synthesized benzimidazole derivatives bearing oxadiazole and morpholine rings. Bioorg. Chem. 2017, 70, 107–117. [Google Scholar] [CrossRef]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S.; Hugar, M.H. Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010, 45, 1753–1759. [Google Scholar] [PubMed]
- Datar, P.; Limaye, S. Design and Synthesis of Mannich bases as Benzimidazole Derivatives as Analgesic Agents. Antiinflamm. Antiallergy. Agents Med. Chem. 2015, 14, 35–46. [Google Scholar] [PubMed]
- Dziwornu, G.A.; Coertzen, D.; Leshabane, M.; Korkor, C.M.; Cloete, C.K.; Njoroge, M.; Gibhard, L.; Lawrence, N.; Reader, J.; Der Watt, V.; et al. Antimalarial Benzimidazole Derivatives Incorporating Phenolic Mannich Base Side Chains Inhibit Microtubule and Hemozoin Formation: Structure − Activity Relationship and In Vivo Oral Efficacy Studies. J. Med. Chem. 2021, 64, 5198–5215. [Google Scholar]
- Tonelli, M.; Novelli, F.; Tasso, B.; Vazzana, I.; Sparatore, A.; Boido, V.; Sparatore, F.; La Colla, P.; Sanna, G.; Giliberti, G.; et al. Antiviral activity of benzimidazole derivatives. III. Novel anti-CVB-5, anti-RSV and anti-Sb-1 agents. Bioorg. Med. Chem. 2014, 22, 4893–4909. [Google Scholar]
- Aroua, L.M.; Almuhaylan, H.R.; Alminderej, F.M.; Messaoudi, S.; Chigurupati, S.; Al-mahmoud, S.; Mohammed, H.A. A facile approach synthesis of benzoylaryl benzimidazole as potential α -amylase and α -glucosidase inhibitor with antioxidant activity. Bioorg. Chem. 2021, 114, 105073. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar]
- Sharma, P.; LaRosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Imidazoles as Potential Anticancer Agents: An Update on Recent Studies. Molecules 2021, 26, 4213. [Google Scholar] [CrossRef]
- Elgemeie, G.H.; Azzam, R.A.; Zaghary, W.A.; Aly, A.A.; Metwally, N.H.; Sarhan, M.O.; Abdelhafez, E.M.; Elsayed, R.E. Patents and applications of N-sulfonated N-heterocycles. In N-Sulfonated-N-Heterocycles; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef] [PubMed]
- Al-Wasidi, A.A.E.A.S.; Refat, M.S.; Naglah, A.M. Different Potential Biological Activities of Benzimidazole Derivatives. Egypt. J. Chem. 2021, 64, 2631–2646. [Google Scholar] [CrossRef]
- Abdel-rahman, L.H.; Abu-dief, A.M.; Moustafa, H. Ni (II) and Cu (II) complexes with ONNO asymmetric tetradentate Schiff base ligand: Synthesis, spectroscopic characterization, theoretical calculations, DNA interaction and antimicrobial studies. Appl. Organomet. Chem. 2017, 31, e3555. [Google Scholar]
- Chinnaraj, K.; Kalanithi, M.; Sankarganesh, M.; Jeyakumar, T.C. Novel azo-based benzimidazole Schiff base ligand and its Co (II), Ni (II) Cu (II) complexes: Computational and biological studies. J. Coord. Chem. 2025, 78, 521–537. [Google Scholar]
- Djuidje, E.N.; Durini, E.; Sciabica, S.; Serra, E.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. Skin damages—Structure activity relationship of benzimidazole derivatives bearing a 5-membered ring system. Molecules 2020, 25, 4324. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ali, S.W. A greener approach to impart multiple functionalities on polyester fabric using Schiff base of vanillin and benzyl amine. Sustain. Chem. Pharm. 2022, 27, 100645. [Google Scholar]
- Song, J.; Long, F.; Shi, Y.; Cao, L. Lyocell fiber modified with Schiff base-Cu(II) Reaction and its excellent antimicrobial properties. Cellulose 2022, 29, 5325–5338. [Google Scholar] [CrossRef]
- Shukla, S.N.; Gaur, P.; Raidas, M.L.; Chaurasia, B.; Bagri, S.S. Novel NNO pincer type Schiff base ligand and its complexes of Fe(IIl), Co(II) and Ni(II): Synthesis, spectroscopic characterization, DFT, antibacterial and anticorrosion study. J. Mol. Struct. 2021, 1240, 130582. [Google Scholar] [CrossRef]
- El-Tabl, A.S.; Shakdofa, M.M.E.; Whaba, M.A. Synthesis, characterization and fungicidal activity of binary and ternary metal(II) complexes derived from 4,4′-((4-nitro-1,2-phenylene) bis(azanylylidene))bis(3-(hydroxyimino)pentan-2-one), Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2015, 136, 1941–1949. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ghosh, S.; Nandi, A.K. Synthesis, structure determination and reactivity of a highly stable vanadium(III) complex. Polyhedron 1997, 16, 2917–2923. [Google Scholar] [CrossRef]
- Alhakimi, A.N.; Shakdofa, M.M.E.; Saeed, S.E.S.; Shakdofa, A.M.E.; Al-Fakeh, M.S.; Abdu, A.M.; Alhagri, I.A. Transition metal complexes derived from 2-hydroxy-4-(p-tolyldiazenyl)benzylidene)-2-(p-tolylamino)acetohydrazide synthesis, structural characterization, and biological activities. J. Korean Chem. Soc. 2021, 65, 93–105. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mohamed, G.G.; El-Dessouky, M.M.I. Synthesis, characterization and in vitro biological activity of mixed transition metal complexes of lornoxicam with 1,10-phenanthroline. Int. J. Electrochem. Sci. 2014, 9, 1415–1438. [Google Scholar] [CrossRef]
- Shakdofa, M.M.E.; Mousa, H.A.; Labib, A.A.; El-All, A.S.A.; Bassyouni, F.A. In vitro antimicrobial activity evaluation of newly synthesized furochromone schiff base complexes. Egypt. J. Chem. 2018, 61, 295–304. [Google Scholar] [CrossRef]
- Wassila, D.; Elkanzi, N.A.A.; Ali, A.M.; Abdou, A. Three Co (II), Ni (II) and Cu (II) Schiff base complexes incorporating 2-[(4-{[(4-methylphenyl) sulfonothioyl] oxy} phenyl) methylene] amino} benzoic acid: Synthesis, structural, dft, biological and molecular docking investigation. Bull. Chem. Soc. Ethiop. 2024, 38, 325–346. [Google Scholar]
- Li, H.C.; Zheng, Y.F.; Wang, M.Z.; Geng, C.C.; Hou, M.; Liu, C.Q.; Li, W.G. Synthesis, crystal structures and anticancer studies of Ni (Ⅱ) and Co (III) complexes based on 2-(2-aminophenyl)-1H-benzimidazole Schiff base derivatives. Inorganica Chim. Acta 2025, 577, 122506. [Google Scholar]
- Saeed, S.E.-S.; Alomari, B.A.; Al-Hakimi, A.N.; El-Hady, M.M.A.; Alnawmasi, J.S.; Elganzory, H.H.; El-Sayed, W.A. Pyrimidine hydrazide ligand and its metal complexes: Synthesis, characterization, and antimicrobial activities. Egypt. J. Chem. 2023, 66, 315–329. [Google Scholar]
- Gomaa, A.I.; Gomaa, E.A.; Zaky, R.R.; El-Hady, M.N.A. Design and synthesis of pyridine bis-hydrazone metal complexes of Co (II), Cu (II), and Hg (II): Spectral, Gaussian, electrochemical, biological, drug-likeness and molecular docking investigations. Inorg. Chem. Commun. 2024, 162, 112188. [Google Scholar]
- Saeed, S.E.-S.; Al-Harbi, T.M.; Abdel-Mottaleb, M.S.A.; Al-Hakimi, A.N.; Albadria, A.E.A.E.; El-Hady, M.M.A. Novel Schiff base transition metal complexes for imparting UV protecting and antibacterial cellulose fabric: Experimental and computational investigations. Appl. Organomet. Chem. 2022, 36, e6889. [Google Scholar]
- El-Hady, M.M.A.; Sharaf, S.; Farouk, A. Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 2020, 27, 1099–1110. [Google Scholar] [CrossRef]
- Vijayan, T.; Kim, J.; Azam, M.; Al-Resayes, S.I.; Stalin, A.; Kannan, B.S.; Jayamani, A.; Ayyakannu, A.; Nallathambi, S. Influence of co-ligand on the biological properties of Schiff base metal complexes: Synthesis, characterization, cytotoxicity, and antimicrobial studies. Appl. Organomet. Chem. 2022, 36, e6542. [Google Scholar] [CrossRef]
- Ali-Mayada, S.; El-Saied, F.A.; Shakdofa, M.M.E.; Karnik, S.; Jaragh-Alhadad, L.A. Synthesis and characterization of thiosemicarbazone metal complexes: Crystal structure, and antiproliferation activity against breast (MCF7) and lung (A549) cancers. J. Mol. Struct. 2023, 1274, 134485. [Google Scholar]
- Alorini, T.A.; Al-Hakimi, A.N.; Saeed, S.E.-S.; Alhamzi, E.H.L.; Albadri, A.E.A.E. Synthesis, characterization, and anticancer activity of some metal complexes with a new Schiff base ligand. Arab. J. Chem. 2022, 15, 103559. [Google Scholar] [CrossRef]
- Standards Association of Australia. Sun Protective Clothing: Evaluation and Classification; Standards Australia: Sydney, Australia, 1996. [Google Scholar]
- Heydari, N.; Karimi, A.R.; Momeni, H.R.; Azadikhah, F.; Etemadi, T. Chitosan Schiff-Base Hydrogel Sunscreen: A Multifunctional Hybrid Network with Antioxidant, Ultraviolet-Shielding, and Self-Healing Properties. ACS Omega 2025, 10, 8250–8261. [Google Scholar]
- Benos, D.J.; Deamer, D.W.; Kleinzeller, A.; Fambrough, D.M. Membrane Permeability: 100 Years Since Ernest Overton; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Tweedy, B.G. Plant extracts with metal ions as potential antimicrobial agents. Phytopathology 1964, 55, 910–914. [Google Scholar]
- Sharma, R.; Nagar, M.; Agarwal, M.; Sharma, H. Synthesis, characterization and antimicrobial activities of some mixed ligand complexes of Co(II) with thiosemicarbazones and N-protected amino acids. J. Enzym. Inhib. Med. Chem. 2009, 24, 197–204. [Google Scholar]
- He, B.; Helmann, J.D. Metalation of extracytoplasmic proteins and bacterial cell envelope homeostasis. Annu. Rev. Microbiol. 2024, 78, 83–102. [Google Scholar]
- El-All, A.S.A.; Ragab, F.A.F.; El-Din, A.A.M.; Abdalla, M.M.; El-Hefnawi, M.M.; El-Rashedy, A.A. Design, synthesis and anticancer evaluation of some selected schiff bases derived from benzimidazole derivative. Glob. J. Pharmacol. 2013, 7, 143–152. [Google Scholar]
- Akande, A.A.; Salar, U.; Khan, K.M.; Syed, S.; Aboaba, S.A.; Chigurupati, S.; Wadood, A.; Riaz, M.; Taha, M.; Bhatia, S.; et al. Substituted Benzimidazole Analogues as Potential α-Amylase Inhibitors and Radical Scavengers. ACS Omega 2021, 6, 22726–22739. [Google Scholar]
- Noori, M.; Davoodi, A.; Iraji, A.; Dastyafteh, N.; Khalili, M.; Asadi, M.; Khanaposhtani, M.M.; Mojtabavi, S.; Dianatpour, M.; Faramarzi, M.A.; et al. Design, synthesis, and in silico studies of quinoline-based-benzo[d]imidazole bearing different acetamide derivatives as potent α-glucosidase inhibitors. Sci. Rep. 2022, 12, 14019. [Google Scholar]
- Yarkandi, N.H.; El-Ghamry, H.A.; Gaber, M. Synthesis, spectroscopic and DNA binding ability of CoII, NiII, CuII and ZnII complexes of Schiff base ligand (E)-1-(((1H-benzo[d]imidazol-2-yl)methylimino)methyl)naphthalen-2-ol. X-ray crystal structure determination of cobalt (II) complex. Mater. Sci. Eng. C 2017, 75, 1059–1067. [Google Scholar]
- Erdem, E.; Sari, E.Y.; Kilinçarslan, R.; Kabay, N. Synthesis and characterization of azo-linked Schiff bases and their nickel(II), copper(II), and zinc(II) complexes. Transit. Met. Chem. 2009, 34, 167–174. [Google Scholar]
- Şahin, N. Dege, Synthesis, characterization, X-ray, HOMO-LUMO, MEP, FT-IR, NLO, Hirshfeld surface, ADMET, boiled-egg model properties and molecular docking studies with human cyclophilin D (CypD) of a Schiff base compound: (E)-1-(5-nitro-2-(piperidin-1-yl)phenyl)-N-(3-ni. Polyhedron 2021, 205, 115320. [Google Scholar]
- El-Sonbati, A.Z.; Diab, M.A.; El-Bindary, A.A.; Mohamed, G.G.; Morgan, S.M.; Abou-Dobara, M.I.; Nozha, S.G. Geometrical structures, thermal stability and antimicrobial activity of Schiff base supramolecular and its metal complexes. J. Mol. Liq. 2016, 215, 423–442. [Google Scholar]
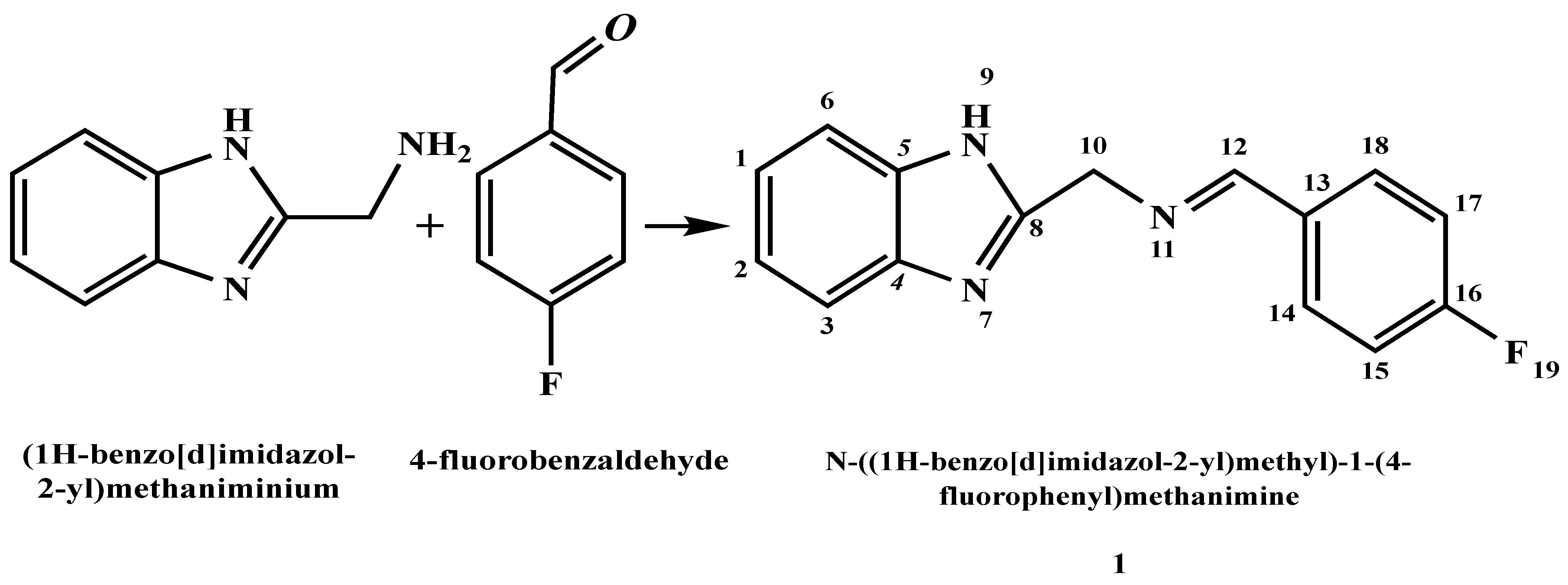
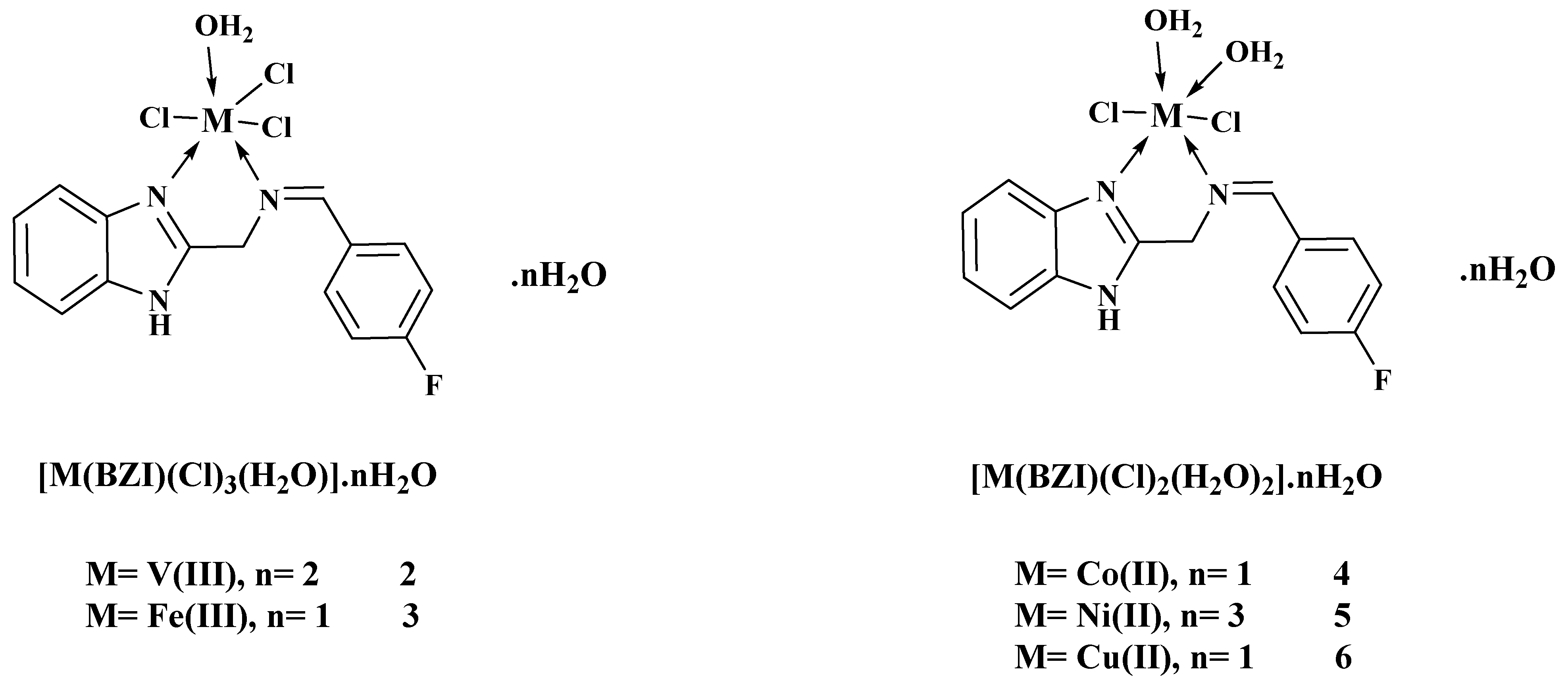
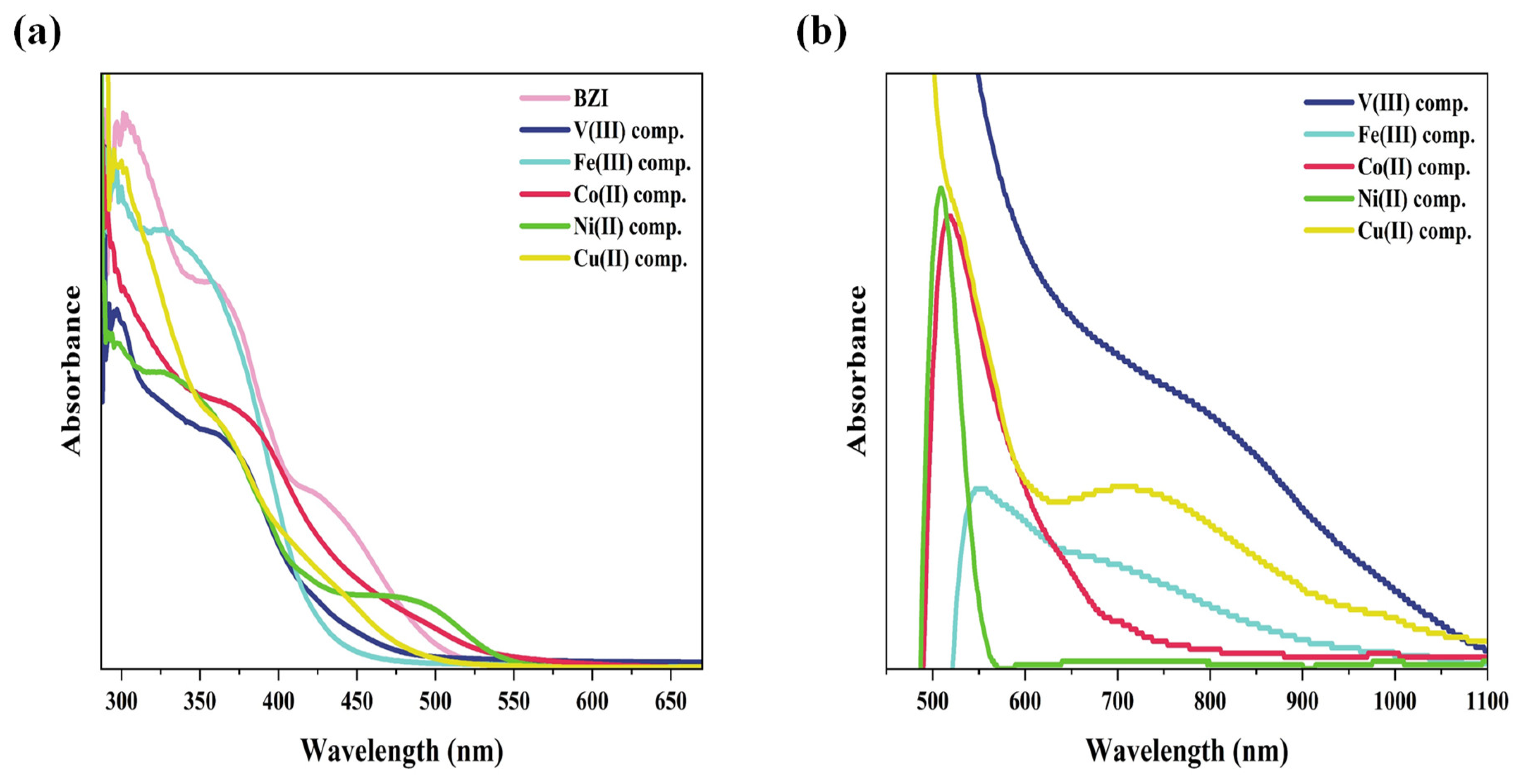
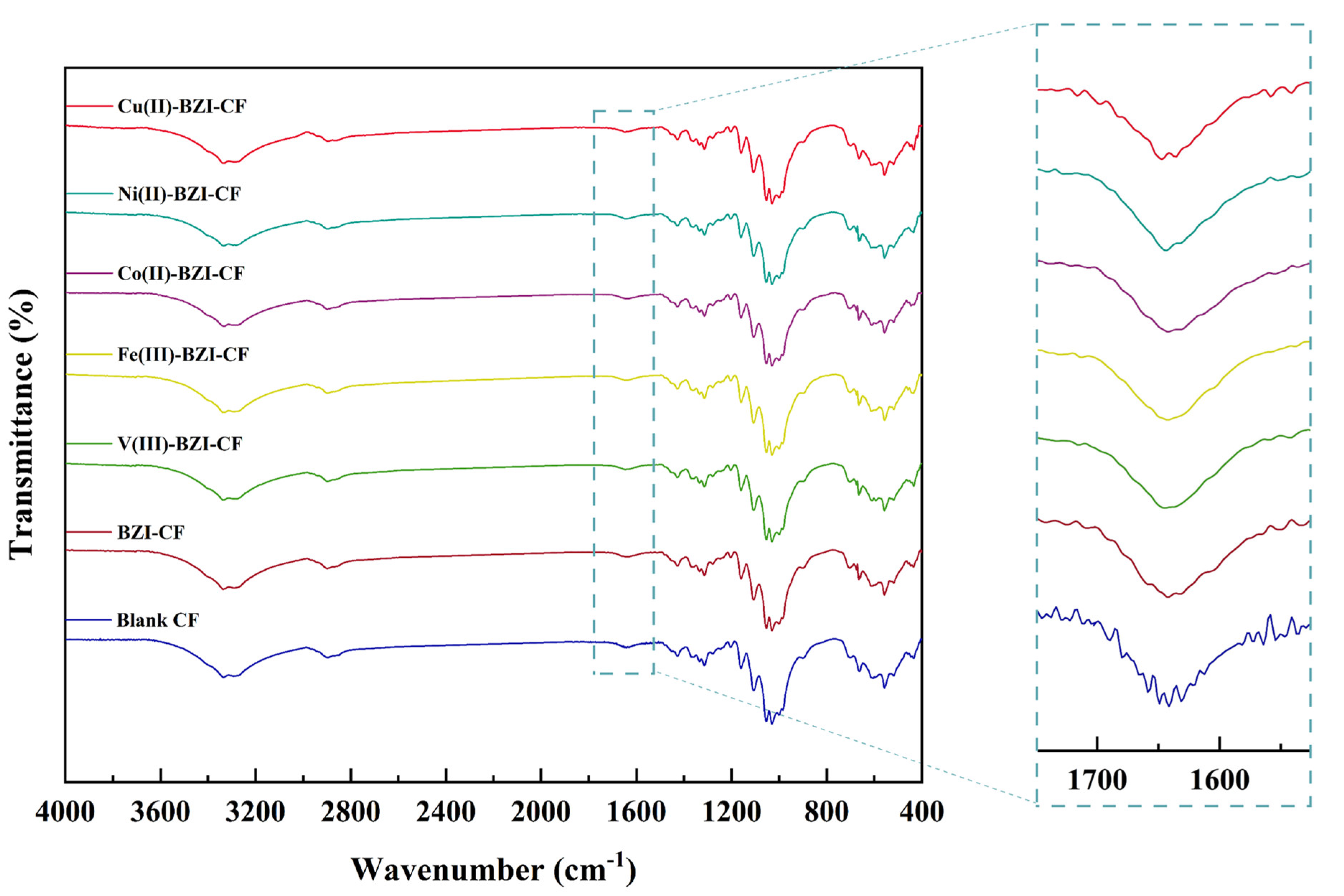


| Comp. No. | Compounds | λmax (nm) | ν (cm−1) | Electronic Transitions |
|---|---|---|---|---|
| 1 | BZI | 421 358 301 | 24,272 27,933 33,223 | n → π* π → π* π → π* |
| 2 | V(III) comp. | 719 424 351 297 | 13,908 24,096 28,490 33,670 | 3T1g (F) → 3T2g (F) n→ π* π → π* π → π* |
| 3 | Fe(III) comp. | 655 554 437 327 294 | 15,267 18,050 22,883 30,581 34,014 | 6A1g → 4T2g (G) 6A1g → 4T1g (G) n → π* π → π* π → π* |
| 4 | Co(II) comp. | 705 622 519 449 349 292 | 14,184 16,077 19,268 22,272 28,653 34,247 | 4T1g (F) → 4T2g (F) 4T1g (F) → 4A2g (F) 4T1g (F) → 4T1g (P) n → π* π → π* π → π* |
| 5 | Ni(II) comp. | 500 466 327 289 | 20,000 21,459 30,581 34,602 | 3A2g (F) → 3T1g (P) n → π* π → π* π → π* |
| 6 | Cu(II) comp. | 989 707 513 440 352 300 | 10,111 14,144 19,493 22,727 28,409 33,333 | 2B1g → 2A1g 2B1g → 2B2g 2B1g → 2Eg n → π* π → π* π → π* |
| Sample | Value of UPF | UV Protection Category |
|---|---|---|
| Blank CF | 3.7 | Non rating |
| BZI-CF | 18.9 | Good |
| V(III)-BZI-CF | 30.3 | Very good |
| Fe(III)-BZI-CF | 57.7 | Excellent |
| Co(II)-BZI-CF | 34.4 | Very good |
| Ni(II)-BZI-CF | 28.7 | Very good |
| Cu(II)-BZI-CF | 51.8 | Excellent |
| Sample | Inhibition Zone Diameter (mm/cm Sample) | |||
|---|---|---|---|---|
| Bacterial Species | Fungal Species | |||
| S. aureus (G+) | E. coli (G−) | C. albicans | A. flavus | |
| Blank CF | 0 | 0 | 0 | 0 |
| BZI-CF | 13 | 12 | 0 | 0 |
| V(III)-BZI-CF | 0 | 0 | 0 | 0 |
| Fe(III)-BZI-CF | 0 | 0 | 0 | 0 |
| Co(II)-BZI-CF | 17 | 15 | 12 | 0 |
| Ni(II)-BZI-CF | 15 | 13 | 0 | 0 |
| Cu(II)-BZI-CF | 14 | 13 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hakimi, A.N.; Alresheedi, T.M.; Albarrak, R.A.; Albadri, A.E.A.E.; Abd El-Hady, M.M.; Saeed, S.E.-S. Synthesis and Characterization of a Fluorinated Schiff Base from Benzimidazole and Its Metal Complexes for Antimicrobial and UV-Protective Cotton Fabrics. Coatings 2025, 15, 380. https://doi.org/10.3390/coatings15040380
Al-Hakimi AN, Alresheedi TM, Albarrak RA, Albadri AEAE, Abd El-Hady MM, Saeed SE-S. Synthesis and Characterization of a Fluorinated Schiff Base from Benzimidazole and Its Metal Complexes for Antimicrobial and UV-Protective Cotton Fabrics. Coatings. 2025; 15(4):380. https://doi.org/10.3390/coatings15040380
Chicago/Turabian StyleAl-Hakimi, Ahmed N., Tahani M. Alresheedi, Reema A. Albarrak, Abuzar E. A. E. Albadri, Marwa M. Abd El-Hady, and Saeed El-Sayed Saeed. 2025. "Synthesis and Characterization of a Fluorinated Schiff Base from Benzimidazole and Its Metal Complexes for Antimicrobial and UV-Protective Cotton Fabrics" Coatings 15, no. 4: 380. https://doi.org/10.3390/coatings15040380
APA StyleAl-Hakimi, A. N., Alresheedi, T. M., Albarrak, R. A., Albadri, A. E. A. E., Abd El-Hady, M. M., & Saeed, S. E.-S. (2025). Synthesis and Characterization of a Fluorinated Schiff Base from Benzimidazole and Its Metal Complexes for Antimicrobial and UV-Protective Cotton Fabrics. Coatings, 15(4), 380. https://doi.org/10.3390/coatings15040380







