Abstract
Alkyd resins are still one of the most important classes of binders for paint systems. They are outstanding in terms of their versatility of formulations and applications, cost-effectiveness, and durability. Traditionally, they are synthesized using phthalic anhydride, polyalcohols with three or four functional groups (pentaerythritol, glycerol, and trimethylolpropane), and fatty acids or oils. In this study, new bio-alkyd resins were synthesized with the objective of increasing the bio-based content by substituting phthalic anhydride, thereby also enhancing the biodegradability of coatings. The newly synthesized alkyd resins, formulated with azelaic acid, were used to develop coatings incorporating additives while avoiding cobalt-based driers. Additional agents such as leveling, wetting, and anti-skinning agents, were also included. Paints were applied to wood substrates and dried at room temperature. The resulting films were characterized by pendulum hardness, transparency, and color by colorimetry, cross-cut test, contact angle, and gloss. Thermal properties were analyzed by Differential Scanning Calorimetry (DSC), and Total Organic Carbon (TOC) content and aerobic biodegradation were also evaluated. The resulting coating films exhibited good mechanical performance, with hardness values ranging from 132 to 148 Persoz oscillations and strong adhesion to wood substrates (smooth cross-cut edges, Class 0). Significant biodegradability (70% in less than 90 days) was demonstrated under composting conditions, which was considerably higher than that of a commercial reference alkyd coating (34.7%) under the same conditions. These findings suggest that the developed bio-alkyd coatings formulated with azelaic acid and DCO-FA without cobalt-based driers represent a promising alternative to conventional phthalic acid-based alkyds. These novel coatings move closer to fully bio-based formulations and offer enhanced biodegradability, making them a more sustainable option for coating applications.
1. Introduction
The paint and coating industry is constantly facing new challenges, such as complying with the stringent rules and regulations regarding the content and release of Volatile Organic Compounds (VOCs) from architectural and industrial coatings. More sustainable coatings are searched for, focusing on the use of bio-based and biodegradable materials. The benefits of using bio-based raw materials in the development of coatings include lesser dependence on fossil resources, lower emissions of fossil-derived CO2, and reduced carbon footprint [1,2,3,4]. Also, increasing the proportion of biodegradable materials in coatings is expected to mitigate the release of microplastic particles into the environment [5].
There is a growing interest in biodegradable resins based on natural feedstocks, such as fully bio-based alkyd resins. Alkyd resins have been one of the most important paint binder classes since they were first introduced in the 1930s. They are widely used due to their affordable price, ready availability of raw materials, partial biodegradability, good durability, flexibility, adhesion, and ease of application [6]. Furthermore, their compatibility with other polymers makes them an interesting alternative for the development of different coating systems [7].
Many different grades of alkyd resins are commercially available. Their chemical structure is defined as polyesters where chain-shaped fatty acid molecules, from natural fatty oils, are connected with a rigid, hard backbone normally containing aromatic diacid groups. They are used as binders, for example, in anticorrosion metal coatings [8,9,10] and wood coatings [11,12], and combined with other raw materials (such as fillers or pigments) for the development of clear coats and/or pigmented paints.
Alkyd resins are branched polyesters synthesized by the reaction between dicarboxylic acids or anhydrides and polyols such as glycerol or pentaerythritol and long-chain unsaturated monocarboxylic fatty acids derived from natural oils [13,14]. A similar definition describes them as products of a polycondensation reaction between polybasic acids and polyhydric alcohols modified by fatty acids [15]. The presence of triglyceride oil and glycerol in alkyd resins suggests better biodegradability and bio content, compared with conventional petroleum-based polymers [16,17]. Anyhow, it is known that terephthalic acid in polyesters, such as PET, reduces biodegradability and hydrolytic degradation in composting conditions [18]. It is therefore of interest to replace the aromatic diacid in the alkyd resins with aliphatic diacids in order to further improve their biodegradability. Alkyd resin synthesis using aliphatic diacids has been reported in the literature, for example, with succinic acid [19], azelaic acid [20], and partially with cardanol derivatives [21]. The degradation mechanisms of polyesters containing aliphatic acids and diacids are especially benign, as the esters bonds can be hydrolyzed in water [22], and the released carboxylic groups are further mineralized by microorganisms through the tricarboxylic acid (TCA) pathway, ultimately converting them into biomass and CO2 [23].
Several types of seed oils such as tall oil, linseed oil, soybean oil, or castor oil are used in alkyd synthesis, depending on the nature of the unsaturated fatty acid present [24]. Alkyd resins are susceptible to oxidation in the presence of oxygen and catalytic systems such as light, heat, enzymes, metals, metalloproteins, and microorganisms. This oxidation process involves the formation of free radicals or reactive oxygen species when molecular oxygen (O2) from the air reacts with carbon–hydrogen bonds adjacent to double bonds within the unsaturated fatty acid, resulting in crosslinking of the resin [25].
Besides the VOC restriction affecting solvent-borne alkyd-based coatings, there are other elements of concern regarding the coating formulations, such as the presence of cobalt-based driers. Cobalt driers have been identified by the European Union as having high toxicological potential, which highlights the need to find cobalt-free alternatives in coating formulations. In this line, modified ferrocenes and oxovanadium (IV) compounds have been explored as alternative driers for alkyds [26,27].
The aim of this study was to develop a nearly 100% bio-based alkyd coating for wood by replacing the phthalic anhydride monomer, thereby also enhancing biodegradability. Novel bio-alkyd resins were synthesized by substituting the normally used phthalic anhydride with azelaic acid and using dehydrated castor oil fatty acids for their good drying properties. Then, new bio-alkyd-based coatings for wood were developed combining the synthetized resins with non-aromatic solvents and environmentally friendlier additives such as cobalt-free driers. N-butyl acetate was selected to replace the aromatic solvent in the coating formulation, as it effectively dissolved the developed bio-alkyds, can be bio-based, and offers improved labeling and classification under Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and Classification, Labelling and Packaging (CLP) Regulations. Additionally, microcrystalline cellulose was incorporated to evaluate its potential impact on degradation rates and its role in reinforcing the coatings.
2. Materials and Methods
2.1. Materials
Glycerol (1,2,3-propanetriol, 99.5%; Sharlau, Sentmenat, Spain), pentaerythritol (Acros, 98%; Geel, Belgium), potassium hydroxide (KOH ≥ 85%; Sigma-Aldrich, Saint Louis, MO, USA), azelaic acid (Thermo Scientific 98%; Geel, Belgium), dehydrated castor oil fatty acids (DCO-FA, Castor International, Deurne, The Netherlands), cellulose microcrystalline powder (20 µm, Sigma-Aldrich, Saint Louis, MO, USA), Setal® 312 SM-88 alkyd resin, known as Alkyd resin REF (ALLNEX, 88% in D40 solvent, Bergen op Zoom, The Netherlands), Nafta Petrosol D40 solvent (D40, Cepsa, Madrid, Spain), n-butyl acetate solvent (BuAc 98%; Sigma-Aldrich, St. Louis, MO, USA), Calcium catalyst (Ca 10%, Durham NUODEX® Calcium, VENATOR; 45% in hydrocarbon solvent, Birtley, UK), Zirconium catalyst (Zr 12%, NUODEX® Zirconium, VENATOR; 41.1% in hydrocarbon solvent, Birtley, UK), cobalt-free primary co-drier (Mn 1%, ADDITOL dry CF100, ALLNEX, Werndorf, Austria), and anti-skinning (ADDITOL XL 297, 100% ALLNEX, Werndorf, Austria) additives were employed as received.
2.2. Synthesis of Bio-Alkyd Resins
Pentaerythritol (25.23 g, 0.187 mol), azelaic acid (32.63 g, 0.173 mol), DCO-FA (70.66 g, 0.252 mol), glycerol (4.30 g, 0.0467 mol), and a magnetic stirring bar were added to a 250 mL glass reactor. The heating of the reactor was done with a heating mantle connected to a thermostat. Also, a Dean–Stark trap was used to collect the water produced during the esterification reaction. The reactor was slowly heated to 170 °C under nitrogen until all components dissolved and then maintained at this temperature for 5 h (acid number (AN) = 19.4 mg KOH/g). The temperature was then cooled down to room temperature and left overnight. The next day, additional (35.9 g, 0.38 mol) DCO-FA was added, and the temperature was slowly increased to 200 °C and maintained for 3 h (AN after 3 h = 20.2 mg KOH/g) before it was cooled to produce Bio-Alkyd AL1 resin. A second Bio-Alkyd AL2 resin with microcrystalline cellulose was prepared by suspending 4.2 g of the cellulose powder in the initial monomer melt and proceeding to the synthesis in the same manner (final AN = 20.8 mg KOH/g).
2.3. Characterization of Resins
ATR-FTIR on resins was recorded using a Nicolet iS5/ATR iD7 from Thermo Scientific (Madison, WI, USA). Recordings were done with 16 scans and a resolution of 4 cm−1 directly on dried films with the ATR option.
2.4. Coatings Preparation
Formulated coatings (F_REF, F_AL1, and F_AL2) were obtained by mixing the alkyd resin (Alkyd resin REF, Bio-Alkyd AL1, and Bio-Alkyd AL2) with the solvent (D40 or butyl acetate) and other additives: cobalt-free driers (Ca 10%, Zr 12% and Mn 1%) and anti-skinning agent (ADDITOL XL 297), as shown in Table 1. Mechanical stirring was conducted on the mixture with a DISPERMAT® CV3 Plus (VMA-GETZMANN GmbH, Reichshof, Germany) at 103 rpm for 30 min. Additives were gradually added whilst stirring.

Table 1.
Formulation of the alkyd wood coatings.
2.5. Coating Characterization and Performance
Coating characterization, Differential Scanning Calorimetry, Total Organic Carbon, and Gel-Permeation Chromatography analysis were performed using dried biocoating films.
Differential Scanning Calorimetry (DSC) measurements were performed on a DSC2 from Mettler-Toledo. Around 5 mg of the sample was placed inside an aluminum pan, and the following dynamic thermal program was applied: two cycles of a heating ramp of 10 °C/min from −80 °C to 150 °C and a cooling ramp within the same range of temperatures at 20 °C/min. The data were collected from the second cycle to remove any thermal history. The glass transition temperature (Tg) was determined at the inflection point.
Total Organic Carbon content (TOC, % dry weight (%dw)) was measured by the combustion method with IR detection and calculation of Total Inorganic Carbon (TIC), carbonates, and organic matter from measured values according to ISO 10694 [28], UNE EN 13137 [29], and UNE EN 15936 [30]. Dried samples were ground to fine dust at a particle size below 65 µm. HCl was added to remove carbonates and bicarbonates. Afterward, the sample was dried at 70 °C to remove any rest of the HCl. Samples were incinerated in an O2 atmosphere at 900 °C. The CO2 generated was detected by an IR detector and quantified according to the calibration of the instrument. Results are recalculated to the C content.
The molecular weight was determined using gel permeation chromatography (GPC) on an Azura system from Knauer equipped with an IR detector. Tetrahydrofuran (THF) was used as solvent at a flow rate of 1.0 mL/min and a constant temperature of 40 °C. The system was calibrated using polystyrene standards.
Then, formulated coatings were applied to various substrates, including glass, B/N test charts, and wood surfaces for evaluation performance. Multiple tests were carried out to evaluate the coatings’ properties such as (1) water static contact angle, (2) transparency and color, (3) gloss, (4) adhesion, and (5) hardness (Persoz).
(1) The sessile drop method was chosen to measure the CA (water static contact angle). For this purpose, a DataPhysics OCA 15 Plus goniometer (Filderstadt, Germany) was used in conjunction with the image-processing system from Data Physics Instruments GmbH. A 3 μL deionized water drop size was employed, and the value given was the average of eight readings. Samples were applied over wood substrate with a 120 μm Baker applicator and were left to dry for two weeks at room temperature.
(2) The transparency and color of the alkyd coatings were quantified by analyzing the difference in color between the applied films in comparison with the substrates. For this purpose, CIELAB system colorimetric measurements were carried out with a Konica Minolta CM-2600d spectrophotometer (Osaka, Japan). The color determination was performed using the CIE L**b* color system, where Δ* refers to lightness, Δ* to red-green chromatic coordinates, and Δ* to yellow-blue chromatic coordinates. According to this system, the color difference is considered undetectable by the human eye when the ΔE* ab (D65) (Equation (1)) is smaller than 2.
The samples were prepared applying the formulated dispersions over B/N test charts from Neurtek Instruments with a 120 μm thick Baker Applicator from Neurtek Instruments and an ATX Automatic Applicator from Neurtek Instruments at an application rate of 40 mm·min−1 and left to dry for 2 weeks. A total amount of 10 measurements were carried out for each analysis.
(3) A BYK GARDNER micro-TRI-gloss glossmeter (Geretsried, Germany), which combines 20°, 60°, and 85° geometries, was used to determine the gloss according to ISO 2813 [31]. An incident light angle of 60° was first employed. However, at gloss values higher than 70 units the angle was changed to 20°, and at gloss values less than 10 units the angle was changed to 85°. The samples for dark glass were obtained by applying 120 μm of the dispersion with a Baker Applicator from Neurtek Instruments. For wood substrates (sapele and oak), three layers of 80 g·m−2 each were applied. All the applications were left to dry for 2 weeks at room temperature. The results given are the average of 10 measurements.
(4) The adhesion of the alkyd coatings to a wood substrate (pine) was analyzed by cross-cut test according to EN ISO 2409 [32]. For the measurements, a 9 mm wide NT edge cutter, 25 mm wide roll adhesive, and a ZCT 2160.2 cross-cut template (Zehntner GmbH Testing Instruments, Sissach, Switzerland) were employed. For the sample preparation, 3 layers of 80 g·m−2 each were applied over the substrates, then left to dry for 2 weeks at room temperature.
(5) Persoz pendulum hardness measurements were carried out using a TQC Sheen Pendulum Hardness Tester (Capelle aan den IJssel, The Netherlands) according to EN ISO 1522 [33]. The Persoz Hardness determines the number of the pendulum oscillations until it stops, so the higher the number of oscillations, the greater the coating hardness. Samples were prepared by applying the formulated dispersion over dark glasses with a 120 μm thick Baker Applicator from Neurtek Instruments (Eibar, Spain) and leaving to dry for 2 weeks at room temperature. Measures were taken at 48 h and 15 days.
2.6. Coatings Biodegradability
Biodegradability refers to the ability of a material to decompose into base substances (water, carbon dioxide, and other non-toxic compounds), primarily due to microbial action [34]. To determine the aerobic biodegradation potential of newly developed alkyd coatings with increased bio-based content, respirometric tests were conducted according to the ISO 14855-1 standard [35]. This method is based on the measurement of the amount of carbon dioxide evolved during microbial degradation under industrial composting conditions [23]. The test was carried out using a respirometer ECHO (ECHO instruments d.o.o., Slovenske Konjice, Slovenia) with sensors for carbon dioxide, oxygen, methane, temperature, pressure, humidity, and flow rate. A total of 10 g of alkyd coatings were introduced in 3 l cylindrical hermetic vessels containing 133 g compost and 150 g vermiculite, at 50%–55% moisture content. Alkyd samples were previously ground to a particle size less than 1 mm to facilitate homogenization. Compost from a local company (Bolaleku S.A.T, Bizkaia, Spain) with a vs. content of 60% and pH of 8.0, less than three months old, was used in this study. The compost was sieved to remove particles above 2 mm before preparing the inoculum by mixing compost with dried vermiculite in a final ratio of 1:2 (dw) and adjusting the moisture content. The ratio of inoculum (compost and vermiculite mixture) to sample was set at 6 (dw). Reference vessels contained microcrystalline cellulose (ref. 435236, Sigma-Aldrich, St. Louis, MO, USA) instead of sample and blank controls only contained the inoculum. The biodegradability test was kept running for 90 days at 58 °C ± 0.5 °C with continuous CO2, O2, temperature, humidity, and flow rate monitoring. Twice a week the bioreactors were actively aerated by opening and stirring the contents, and water was added to maintain constant moisture level.
The percentage of biodegradation () of the test and reference samples was calculated according to the following equation (Equation (2)):
where (CO2)t is the cumulative amount of carbon dioxide evolved in each composting vessel containing the test material, (CO2)B is the mean cumulative amount of carbon dioxide evolved in the blank vessels, and (ThCO2) is the theoretical amount of carbon dioxide that can be produced by the test material (all in grams per vessel). The theoretical amount of carbon dioxide can be determined via the following equation (Equation (3)):
where is the total dry solid content at the start of the test, is the proportion of total organic carbon in the dry solids in the test material, and the ratio 44/12 refers to the molecular masses of carbon dioxide and atomic mass of carbon, respectively.
Statistical Analysis: the contact angle (CA) results were analyzed using a one-way analysis of variance (ANOVA) and Tukey’s post hoc test (p < 0.05).
3. Results and Discussion
3.1. Synthesis and Chemical Characterization of the Bio-Alkyd Resin
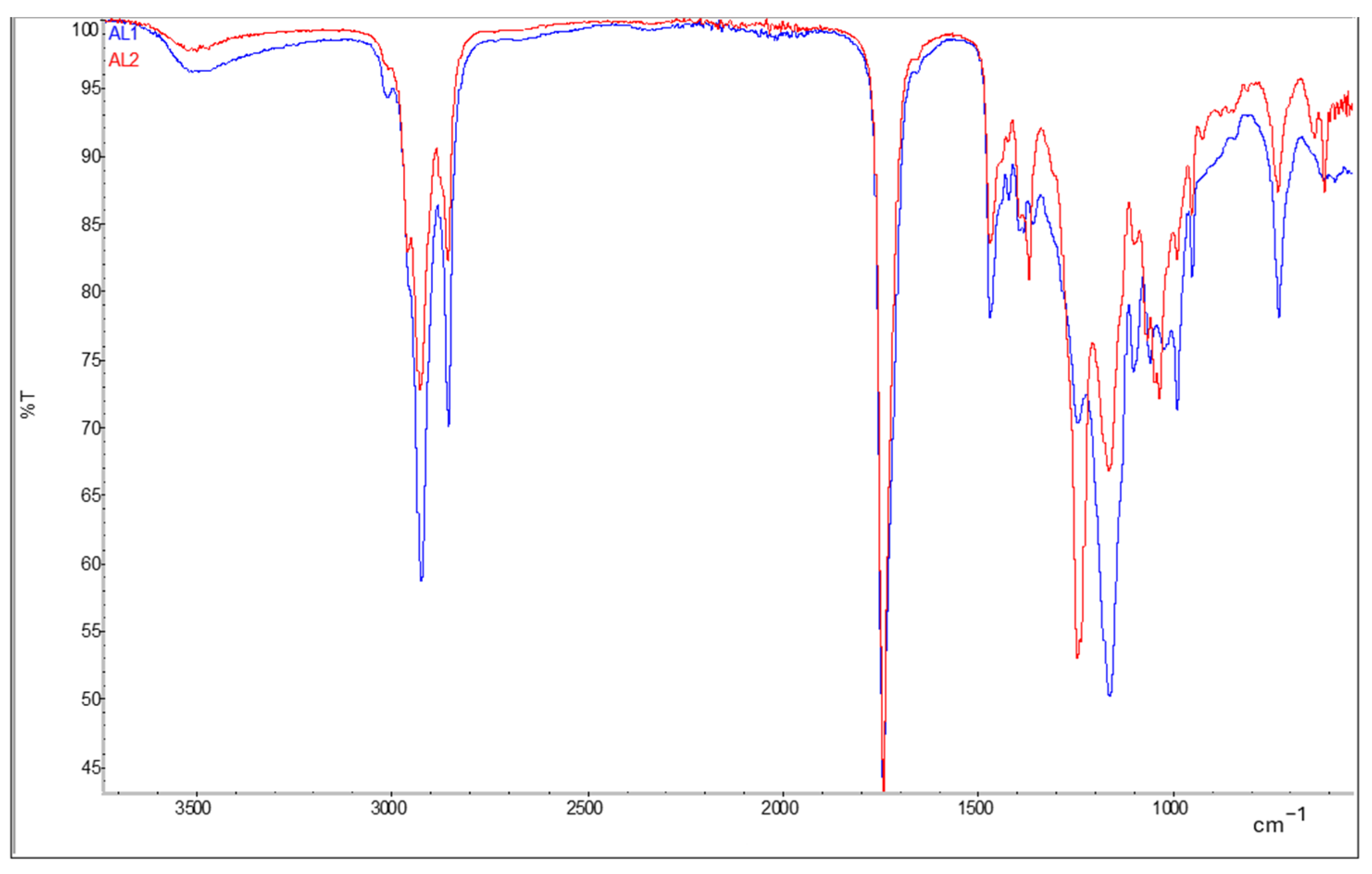
The synthesis of bio-alkyd resins was performed in two steps using an excess of hydroxyls and adding more DCO-FA fatty acids at the second step to avoid gelling in the reactor. Azelaic acid performed better compared to other diacids, avoiding gelification even at 100% substitution of phthalic acid. The reaction of Bio-Alkyd AL1 resulted in a transparent light-yellow resin, and by the addition of 2.5% micro-crystalline cellulose a slightly more viscous and turbid resin was produced (Bio-Alkyd AL2). Both FTIR spectra for the resins AL1 and AL2 show a broad peak at 726 cm−1 and a small peak at 3004 cm−1. The latter corresponds to the C-H bonds of the vinyl carbons present in the fatty acid chains, while the first one might be due to cis C=C double bonds out of plane. There is also a very small peak close to the carbonyl peak, at approximately 1600 cm−1, which might be due to C=C double bonds. In the AL2 sample with added cellulose, a peak appears at 1239 cm−1 that corresponds to the C-O of cellulose esters. Figure 1 shows ATR-FTIR spectra of synthetized bio-alkyd resins.
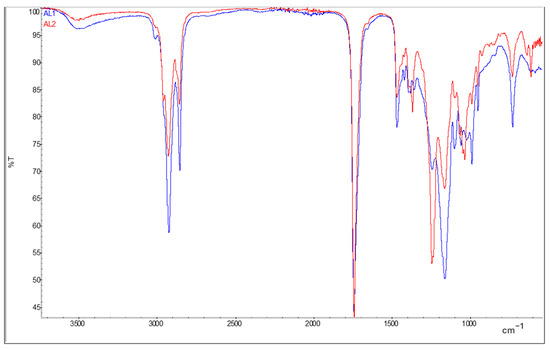
Figure 1.
ATR-FTIR spectra of resins Bio-Alkyd AL1 (in red) and AL2 (in blue).
3.2. Characterization and Performance of the Alkyd Coatings
The DSC measurements on the cured coatings showed low Tg values for both synthesized bio-alkyds and reference commercial wood alkyd (Table 2). Bio-Alkyd F_AL1 and F_AL2 were respectively −25 °C and −23 °C lower than the reference at 2 °C. This implies more flexibility that could be beneficial for wood coatings. Another study, where azelaic acid was also used, showed lower Tg values around −30 °C [20]. This may be due to the use of tall oil fatty acids, resulting in poorer oxidative cross-linking as compared to the use of conjugated DCO-FA in this study.

Table 2.
DSC and contact angle (CA) results of cured coatings.
Regarding the contact angle results, also outlined in Table 2, bio-alkyd coatings showed slightly lower hydrophobicity (p > 0.5). Based on the measured water contact angle values, the bio-alkyd coatings can be considered practically hydrophobic. According to the classical Thomas Young equation, surface wettability behavior is classified based on the contact angle formed between a liquid droplet and a solid surface [36]: hydrophilic (CA < 90°), hydrophobic (CA > 90°), and superhydrophobic (CA > 150°). The higher hydrophobicity observed in the alkyd reference coating (p < 0.001) can be attributed to the influence of surface roughness and surface energies on the contact angle, as proposed by Wenzel. According to this model, surface smoothness affects the hydrophilic behavior, as a smoother surface exhibits a lower contact angle [37].
The TOC values of all dried biocoating films were measured in order to perform the biodegradation tests. Similar TOC values were observed between the biocoatings and the reference alkyd coating (Table 3). These data were then used to calculate the theoretical CO2 production during the test and to evaluate the biodegradability.

Table 3.
Data obtained from TOC results.
GPC analysis was performed after over a year of laboratory storage. Results indicate that the F_AL resins underwent polymerization during this period, yielding Mw values above 60,000 Da, far exceeding the typical 2000–10,000 Da range for alkyd resins.
The transparency and color of the films were measured using a colorimetric test (Table 4). For this purpose, the difference in color between the white and black substrates was analyzed before and after film application.

Table 4.
Colorimeter test results.
The results showed no noticeable differences on the black substrate, indicating that the films are transparent (ΔE*ab (D65) < 1). On the white substrate, a yellowish hue was observed, as evidenced by an increase in the b* value compared to the uncoated substrate. Hence, the ΔE*ab (D65) values obtained were also higher (>7), although both new bio-alkyd coatings exhibited less yellowing than the conventional alkyd-based coating (F_REF). Alkyd resins are prone to yellowing [38,39], primarily due to the use of unsaturated oils [40]. It is worth mentioning that in all the cases sightly yellow transparent films were obtained.
The gloss results for the formulations applied to different surfaces are presented in Table 5. All coatings exhibited a high gloss on dark glass, which is typical for alkyd resins. Furthermore, the incorporation of cellulose (F_AL2) did not affect light reflection. However, when the formulations were applied to wood substrates, the gloss was reduced in all cases. This reduction is attributed to the porous nature of the substrate, which decreases surface smoothness.

Table 5.
Gloss results on different substrates.
Figure 2 shows the appearance of alkyd coatings applied to pine wood substrates. The coatings exhibit good leveling and appearance, confirming the previously noted characteristics: slight yellow coloration, high gloss, and transparency. Moreover, these bio-alkyd coatings effectively preserve the natural look of the wood surface.

Figure 2.
Coatings application to pine wood substrate.
Adhesion of the films to pine wood substrates was evaluated using the cross-cut-adhesion test and the results are presented in Table 6. Very good adhesion was observed, as evidenced by completely smooth cut edges (Class 0, according to EN ISO 2409 [32]).

Table 6.
Adhesion results on wood substrates.
Hardness measurements were conducted using the Persoz pendulum test, with results presented in Table 7. The number of oscillations was low for F_REF, whereas the bio-alkyd coatings exhibited a significant increase with similar values observed for both samples. The higher hardness observed in bio-alkyd samples could be attributed to effective cross-linking, likely resulting from the use of highly reactive conjugated DCO-FA, which in turn accelerates the drying process [40].

Table 7.
Persoz Hardness results for alkyd coatings in time.
3.3. Biodegradability of the Alkyd Coatings
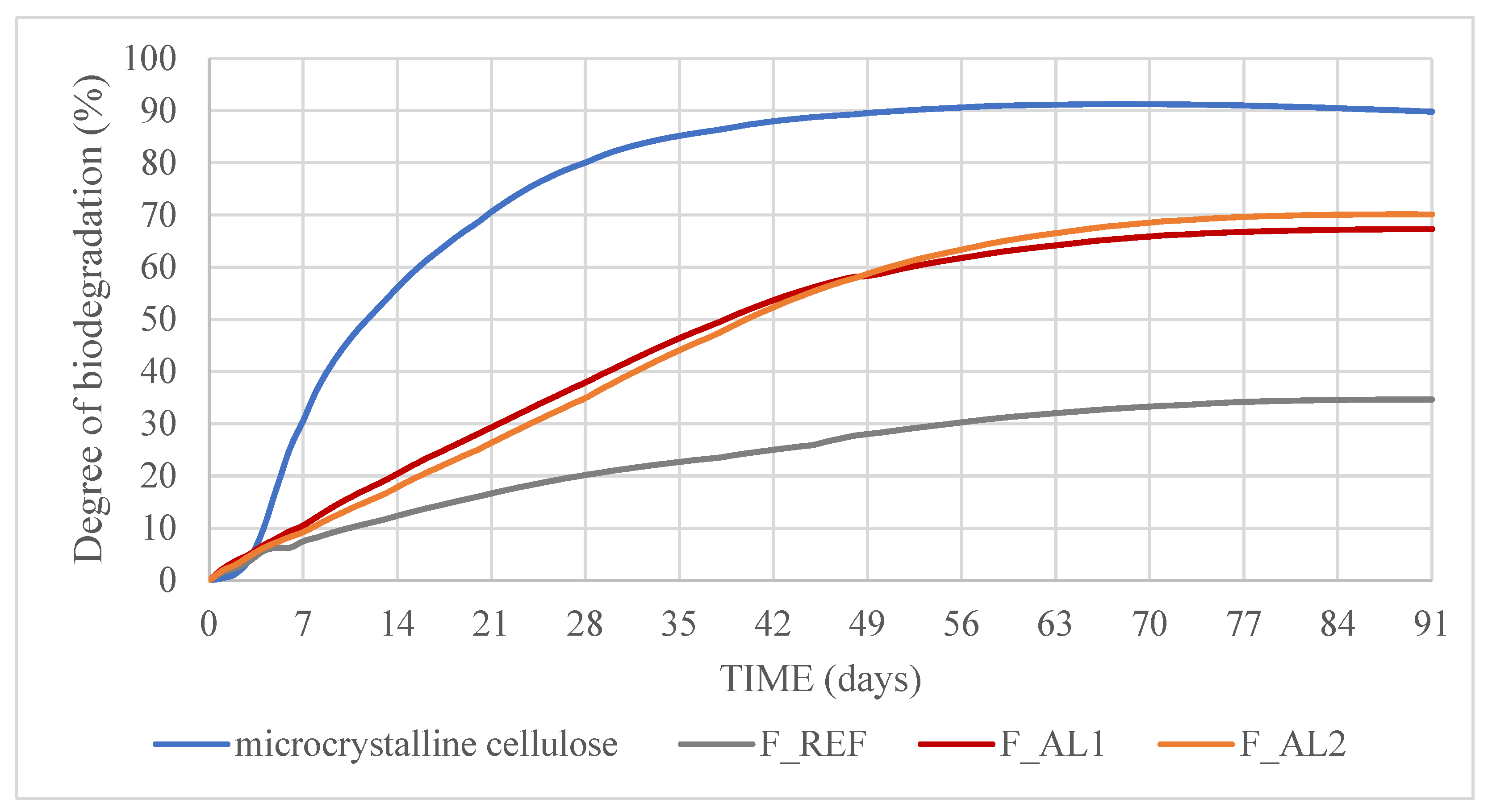
Figure 3 shows the cumulative biodegradation rate of the reference material (micro-crystalline cellulose), the alkyd coating F_REF, and the newly developed bio-alkyd coatings F_AL1 and F_AL2. The validity of the test was confirmed by meeting the conditions established in the standard ISO 14855-1 [35], i.e., (i) biodegradation of the reference material (cellulose) exceeded 70% after 45 days, (ii) the difference in biodegradation percentage of the reference material at the end of the test was less than 20%, and (iii) the inoculum produced more than 50 but less than 150 mg of carbon dioxide per gram of volatile solids (mean value) within the first 10 days of incubation.
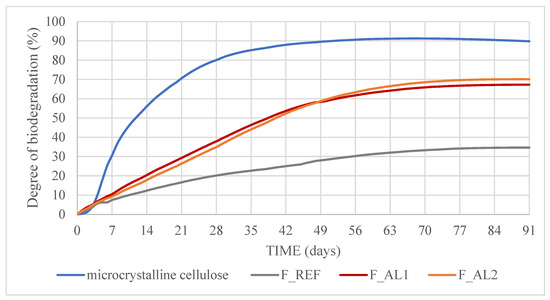
Figure 3.
Biodegradation of test samples: REF, Bio-Alkyd F_AL1 and Bio-Alkyd F_AL2.
The mean biodegradation achieved after 90 days of composting was 70.1 ± 3.6% for F_AL1 and 67.3 ± 0.1% for F_AL2 (mean and standard deviation of two replicates). Consequently, neither sample met the established target of 90% biodegradation and cannot be classified as biodegradable. However, both coatings exhibited significantly higher biodegradability compared to the F_REF sample, which achieved only 34.7 ± 0.4% after 90 days. The biodegradation curves for all three alkyd coatings followed a similar trend, progressing at a slower rate than the reference material (cellulose) and reaching the plateau at approximately 70 days. The enhanced biodegradability observed in the newly developed alkyd coatings can be attributed to their bio-based and aliphatic composition, as well as their specific formulation. Ester bonds in vegetable oils are more susceptible to microbial degradation, and the presence of aliphatic chains facilitates enzymatic attack. No significant difference was observed between the two experimental coating formulations (F_AL1 and F_AL2), suggesting that the incorporated cellulose does not influence biodegradation. No comparative data in the literature on alkyd resin biodegradability have been found. However, for biodegradable polyesters, faster mineralization and enhanced degradation are generally observed in polyesters with low aromatic or cyclic content as well as in those containing intermediate aliphatic molecules, which promote higher mobility and lower Tg [23].
4. Conclusions
Despite the growing shift in focus from conventional alkyd coatings to bio-based and renewable formulations using vegetable oils, non-aromatic solvents, and non-toxic additives driven by environmental regulations, there remains a lack of experimental studies on their biodegradability and overall environmental impacts. There is an intrinsic expectation that these bio-based formulas will also be biodegradable, but this assumption needs to be confirmed by performing biodegradability studies. In this work, the aerobic biodegradability of bio-based formulations for alkyd coatings was evaluated using a partially bio-based commercial resin as a reference. The results confirm that the newly developed resin formulations exhibit increased biodegradability compared to a commercially available alkyd resin, without compromising their performance and functionality. These novel biocoatings demonstrated good appearance and natural aspect when applied to wood (slight yellow coloration, high gloss, and transparency), strong adhesion to wood (“Class 0” rating in cross-cut adhesion test), and enhanced hardness (Persoz Hardness exceeding 100 oscillations after 48 h at room temperature) compared to a reference coating containing phthalic acid. While the environmental profile of these sustainable coatings is mostly dependent on the selected vegetable oil source, their improved biodegradability (70% in less than 90 days) suggests that they can contribute to reducing the amount of microplastics released to the environment and the dependence on fossil resources.
Author Contributions
Writing (original draft preparation, conceptualization, review, and editing) I.E., I.S., A.I.D. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The SPRI-ELKARTEK FRONTIERS (KK-2022/00109 & KK-2024/00099) of the Basque Country grant to TECNALIA and GAIKER.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
ALLNEX for providing raw materials (alkyd resin and additives) and recommendations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Juhl, M.; Hauschild, M.; Dam-Johansen, K. An eco-strategy for development of more sustainable coatings. Prog. Org. Coat. 2024, 197, 108781. [Google Scholar]
- Paraskar, P.; Prabhudesai, M.; Hatkar, V.; Kulkarni, R. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar]
- Czachor-Jadacka, D.; Biller, K.; Pilch-Pitera, B. Recent development advances in bio-based powder coatings: A review. J. Coat. Technol. Res. 2024, 21, 435–444. [Google Scholar] [CrossRef]
- Malik, P.; Sarkhel, D. Advancements in bio-based materials and low-VOC formulations: Paving the way for sustainable innovation in the coatings industry. J. Coat. Technol. Innov. 2024, 2, 34–38. [Google Scholar]
- Bertling, J.; Dau, K.; Selig, U.; Werner, S. Releases of Microplastic into the Marine Environment—State of Knowledge and Options for Action. German Roundtable on Marine Litter. 2022. Available online: https://www.muell-im-meer.de/sites/default/files/2022-03/Issuepaper_microplastics_final.pdf (accessed on 5 September 2024).
- Chiplunkar, P.P.; Pratap, A.P. Utilization of sunflower acid oil for synthesis of alkyd resin. Prog. Org. Coat. 2016, 93, 61–67. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Hogerat, R.; Ghosal, K.; Shaheen-Mualim, M.; Farad, S. Renewable polyol-based biodegradable polyesters as greener plastics for industrial applications. Chem. Eng. J. 2023, 459, 141211. [Google Scholar]
- Cadena, C.; Irusta, L.; Fernandez-Berridi, M.J. Performance evaluation of alkyd coatings for corrosion protection in urban and industrial environments. Prog. Org. Coat. 2013, 76, 1273–1278. [Google Scholar]
- Guo, H.; Zhou, K.; Feng, Z.; Li, C.; Xie, J.; Ma, J.; Zhang, X.; Wang, X.; Xu, K.; Li, C.; et al. Corrosion Behavior of alkyd-resin-coated carbon steel under cathodic polarization in both static and flowing seawater. Coatings 2023, 13, 1296. [Google Scholar] [CrossRef]
- Kizilkonca, E.; Erim, F.B. Development of Anti-aging and Anticorrosive nanceria dispersed Alkyd coating for decorative and industrial purposes. Coatings 2019, 9, 610. [Google Scholar] [CrossRef]
- de Meijer, M. Review on the durability of exterior wood coatings with reduced VOC-content. Prog. Org. Coat. 2001, 43, 217–225. [Google Scholar]
- Ersoy, O.; Fidan, S.; Köse, H.; Güler, D.; Özdöver, Ö. Effect of calcium carbonate particle size on the scratch resistance of rapid Alkyd-based wood coatings. Coatings 2021, 11, 340. [Google Scholar] [CrossRef]
- Aigbodion, A.I.; Pillai, C.K. Synthesis and molecular weight characterization of rubber sedd oil-modified alkyd resins. J. Appl. Polym. Sci. 2001, 79, 2434–2438. [Google Scholar] [CrossRef]
- Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 2012, 73, 274–282. [Google Scholar] [CrossRef]
- Otabor, G.O.; Ifijen, I.H.; Mohammed, F.U.; Aigbodion, A.I.; Ikhuoria, E.U. Alkyd resin from rubber seed oil/linseed oil blend: A comparative study of the physiochemical properties. Heliyon 2019, 5, e01621. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Müller, R.J.; Kleeberg, I.; Deckwer, W.D. Biodegradation of polyesters containing aromatic constituents. J. Biotechnol. 2001, 86, 87–95. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Leclair, A.; Romand, A.; Choule, O.; Coggio, W.D.; Florent, N. Development of Low-Color Alkyd Resins with High Content of Biobased Succinic Acid. Paint & Coatings Industry, 1 October 2014. Available online: https://www.pcimag.com/articles/99678-development-of-low-color-alkyd-resins-with-high-content-of-biobased-succinic-acid (accessed on 23 March 2025).
- Nguyen, H.D.; Löf, D.; Hvilsted, S.; Daugaard, A.E. Highly branched bio-based Unsaturated Polyesters by Enzymatic Polymerization. Polymers 2016, 8, 363. [Google Scholar] [CrossRef]
- Denis, M.; Totee, C.; Le Borgne, D.; Caillol, S.; Negrell, C. Cardanol-modified alkyd resins: Novel route to make greener alkyd coatings. Prog. Org. Coat. 2022, 172, 107087. [Google Scholar] [CrossRef]
- Rowe, M.D.; Eyiler, E.; Walters, K.B. Hydrolytic degradation of bio-based polyesters: Effect of pH and time. Polym. Test. 2016, 52, 192–199. [Google Scholar] [CrossRef]
- Wang, Y.; van Putten, R.-J.; Tieterna, A.; Parsons, J.R.; Gruter, G.-J.M. Polyester biodegradability: Importance and potential for optimisation. Green Chem. 2024, 26, 3698–3716. [Google Scholar] [PubMed]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar]
- İşeri-Çağlar, D.; Baştürk, E.; Oktay, B.; Kahraman, M.V. Preparation and evaluation of linseed oil based alkyd paints. Prog. Org. Coat. 2014, 77, 81–86. [Google Scholar]
- Honzicek, J.; Fedorova, T.; Vinklárek, J.; Mikysek, T.; Cisarová, I. Modified Ferrocenes as Primary Driers for Formulations of Alkyd Paints. Coatings 2020, 10, 873. [Google Scholar] [CrossRef]
- Charamzová, I.; Vinklárek, J.; Kalenda, P.; Honzicek, J. Application of oxovanadium complex stabilized by N,N,N,N-chelating ligand in air-drying paints. Coatings 2018, 8, 204. [Google Scholar] [CrossRef]
- ISO 10694:1995; Soil Quality—Determination of Organic and Total Carbon After Dry Combustion (Elementary Analysis). International Organization for Standardization—ISO: Geneva, Switzerland, 1995.
- UNE-EN 13137:2002; Characterization of Waste—Determination of Total Organic Carbon (TOC) in Waste, Sludges and Sediments. UNE: Asociación Española de Normalización: Madrid, Spain, 2002.
- UNE-EN 15936:2022; Soil, Waste, Treated Biowaste and Sludge—Determination of Total Organic Carbon (TOC) by Dry Combustion. UNE: Asociación Española de Normalización: Madrid, Spain, 2022.
- ISO 2813:2014; Paints and Varnishes—Determination of Gloss Value at 20°, 60° and 85°. International Organization for Standardization—ISO: Geneva, Switzerland, 2014.
- EN ISO 2409:2020; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization—ISO: Geneva, Switzerland, 2020.
- EN ISO 1522: 2022; Paints and Varnishes—Pendulum Damping Test. International Organization for Standardization—ISO: Geneva, Switzerland, 2022.
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- ISO 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions-Method by Analysis of Evolved Carbon Dioxide-Part 1: General Method. International Organization for Standardization—ISO: Geneva, Switzerland, 2012.
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar]
- Ifijen, I.H.; Maliki, M.; Odiachi, I.J.; Aghedo, O.N.; Ohiocheoya, E.B. Review on Solvents Based Alkyd Resins and Water Borne Alkyd Resins: Impacts of Modification on Their Coating Properties. Chem. Afr. 2022, 5, 211–225. [Google Scholar] [CrossRef]
- Wicks, Z.W. Alkyd resins. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 978-047-004-610-4. [Google Scholar]
- Hood, D.K.; Musa, O.M. Application of Maleic Anhydride-based Materials. In Handbook of Maleic Anhydride-Based Materials; Musa, O.M., Ed.; Springer EBooks; Springer Nature: Cham, Switzerland, 2016; ISBN 978-3-319-29454-4. [Google Scholar]
- Muizebelt, W.J.; Hubert, J.C.; Nielen, M.W.; Klaasen, R.J.; Zabel, K.H. Crosslink mechanisms of high-solids alkyd resins in the presence of reactive diluents. Prog. Org. Coat. 2000, 40, 121–130. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).