Microstructural Evolution and Failure Analysis for 8YSZ/(Y0.5Gd0.5)TaO4 Double-Ceramic-Layer Thermal Barrier Coatings on Copper Substrate
Abstract
:1. Introduction
2. Experimental Details
2.1. Material Preparation
2.2. Characterization
3. Results and Discussion
3.1. Orthogonal Spray Experiment
3.2. Microstructure
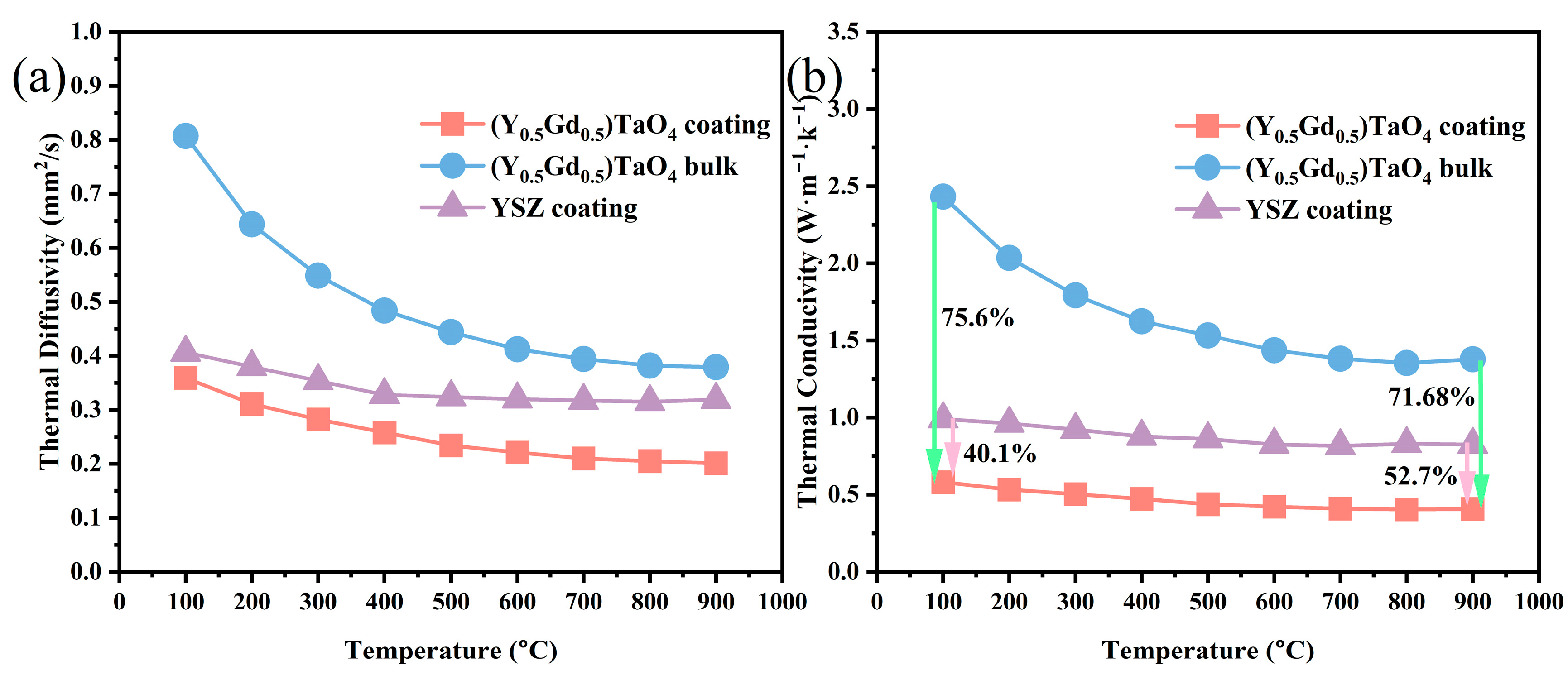
3.3. Thermal Properties
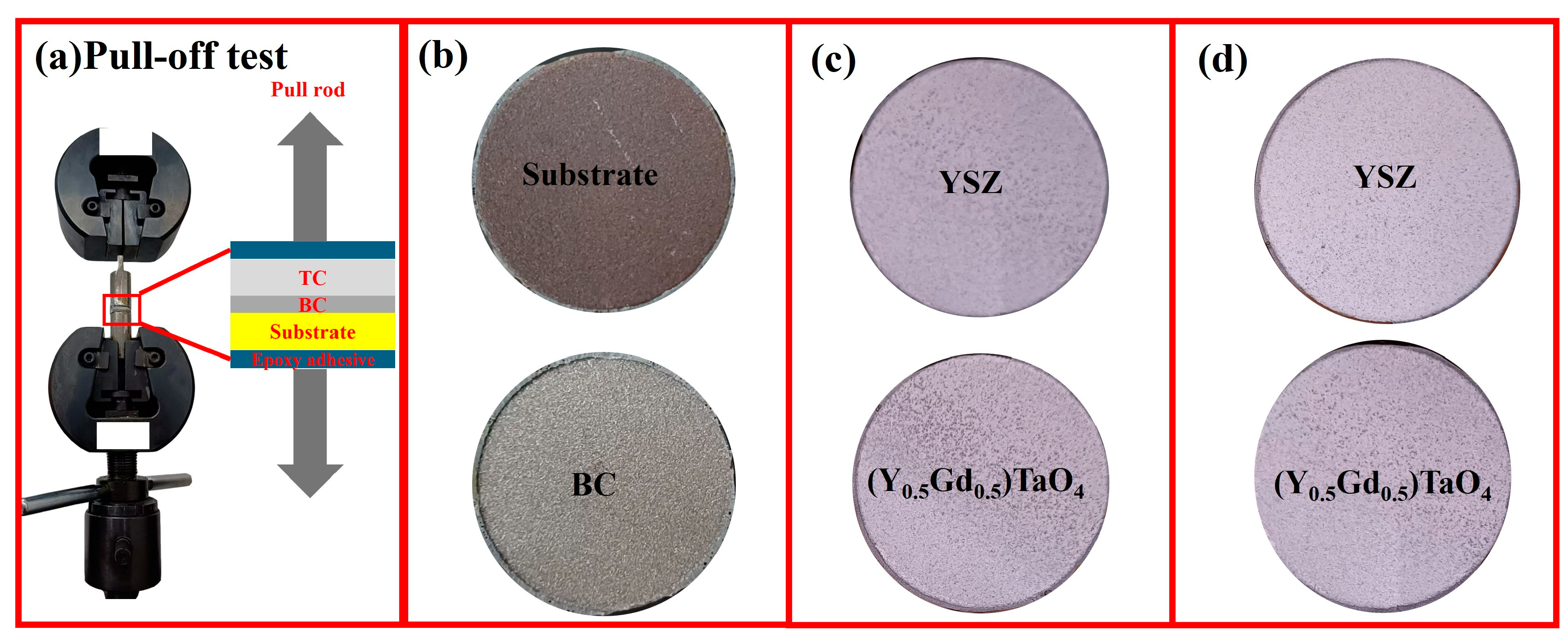
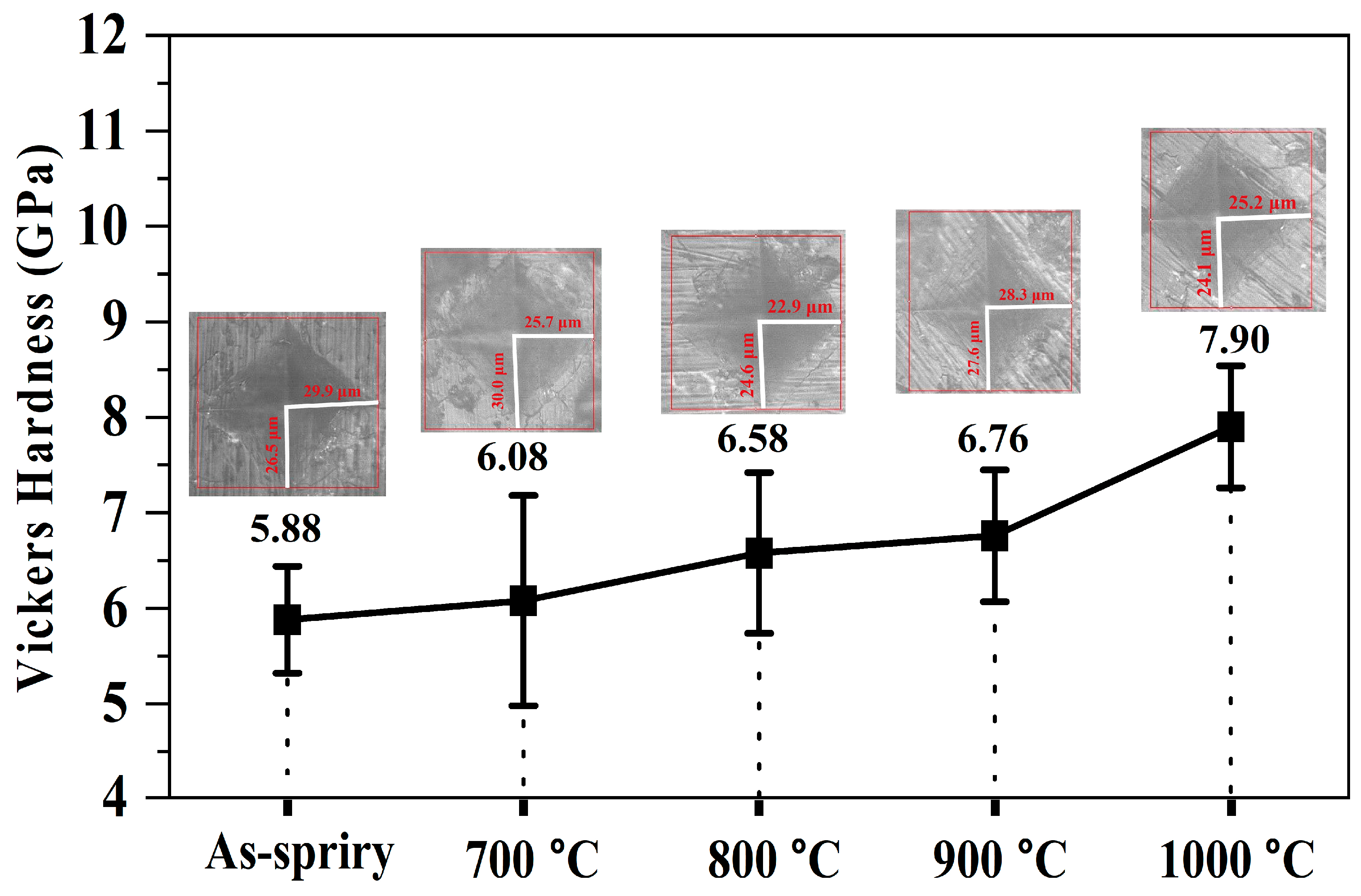
3.4. Mechanical Properties
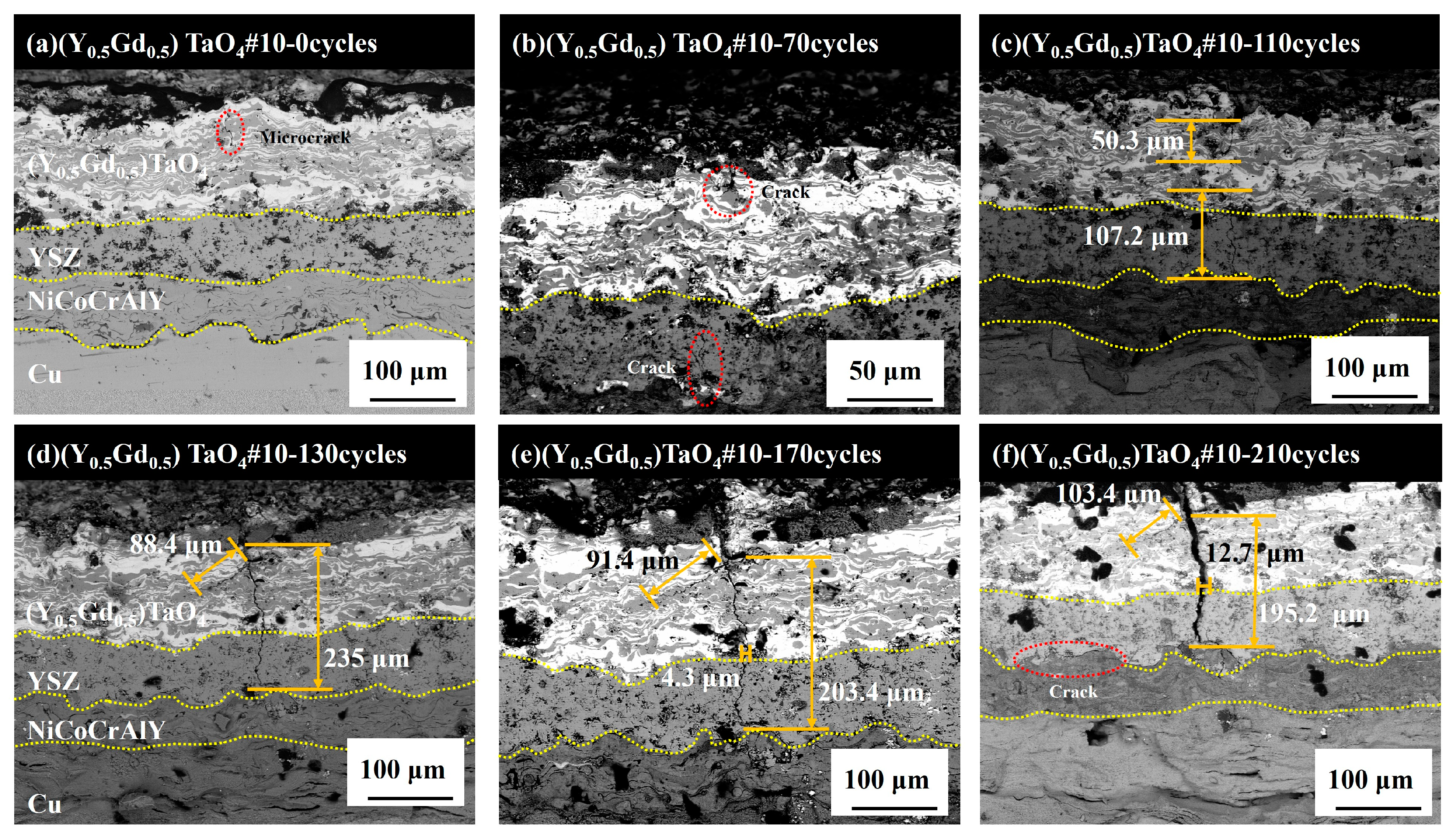
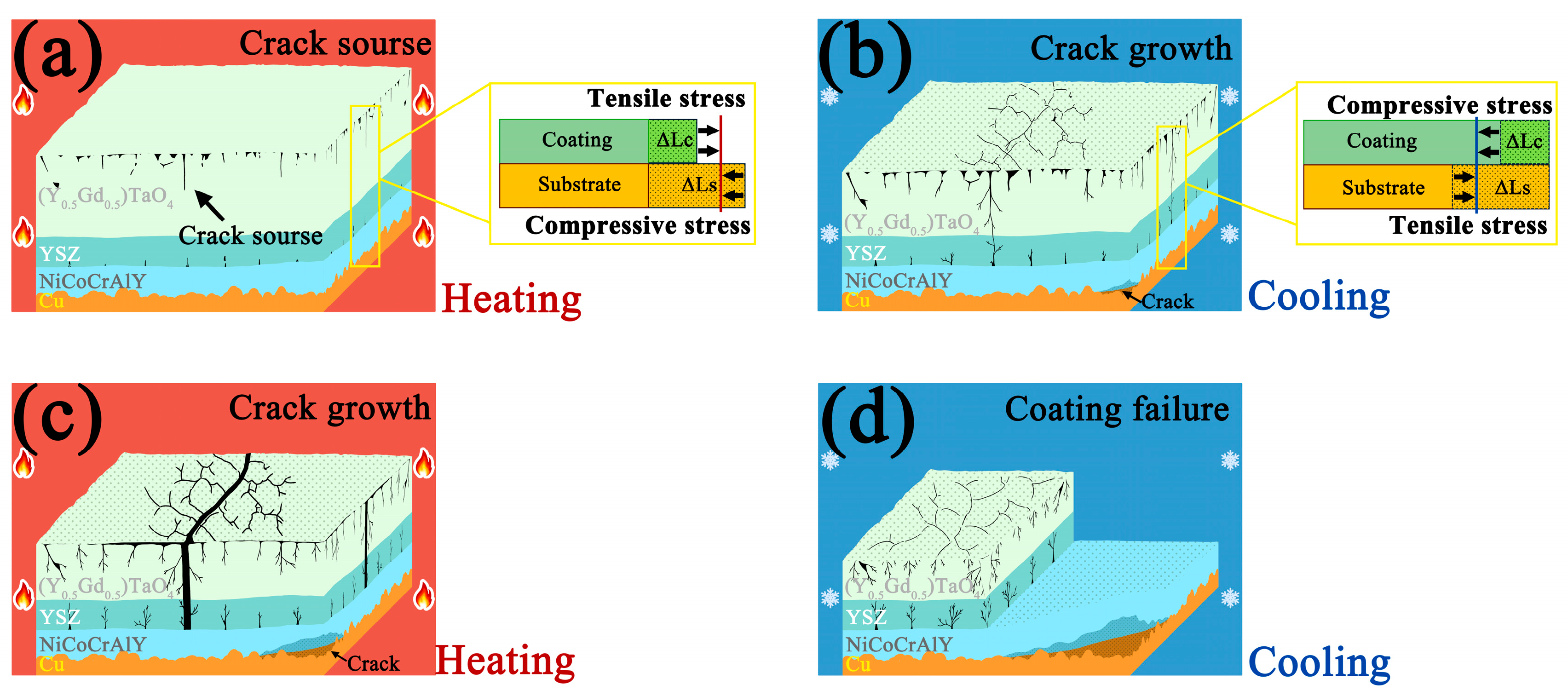
3.5. Failure Mechanism
4. Conclusions
- (1)
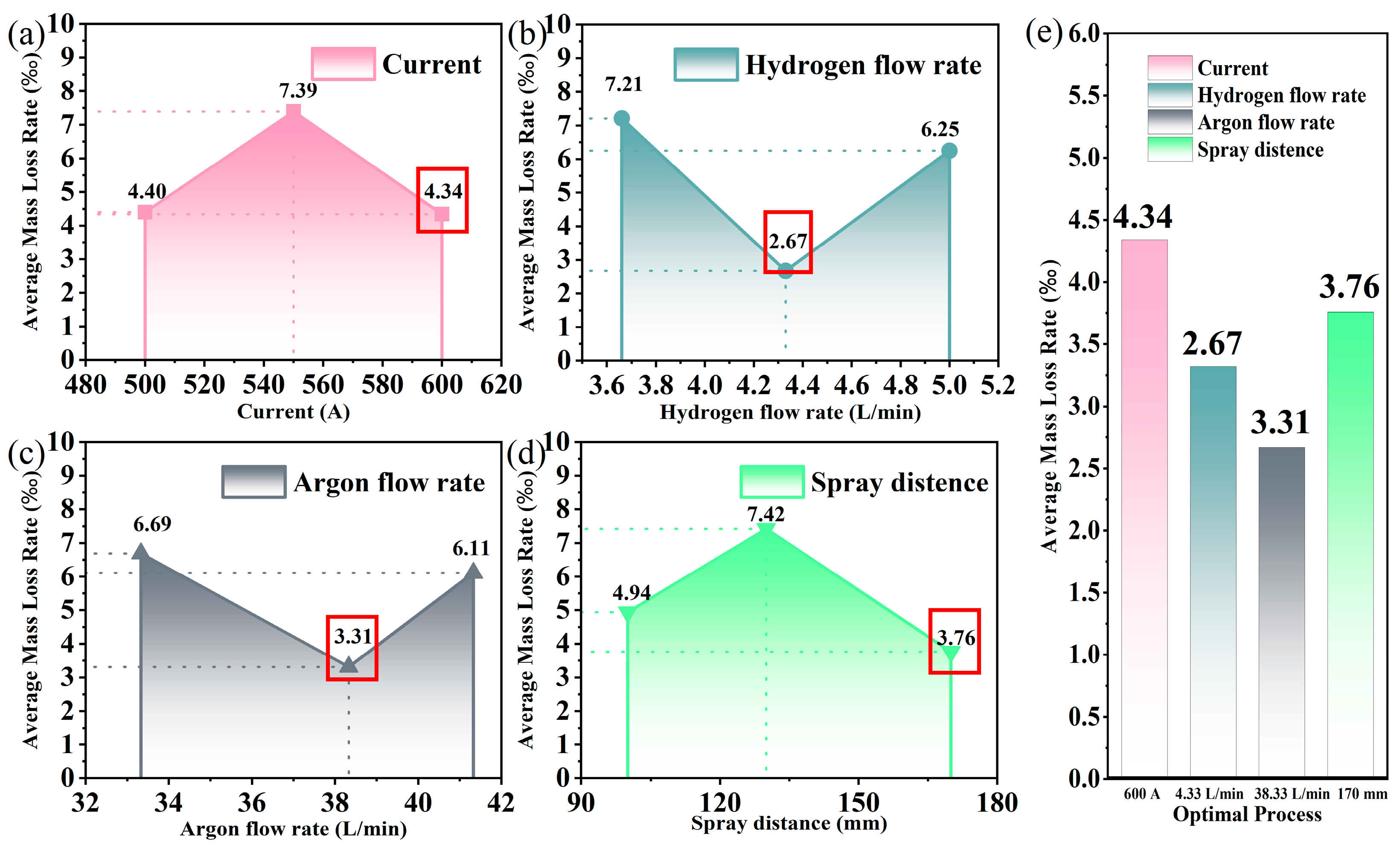
- The range analysis results showed that hydrogen flow rate is the primary influencing factor of the average mass loss rate, followed by the spray distance, argon flow rate, and current. The optimum spraying parameters for (Y0.5Gd0.5)TaO4 coating are a current of 600 A, a hydrogen flow rate of 4.33 L/min, a spray an argon flow rate of 38.33 L/min, and distance of 170 mm.
- (2)
- The failure of the 8YSZ/(Y0.5Gd0.5)TaO4 double-ceramic-layer thermal barrier coatings is crack growth due to thermal stress mismatch accumulation during thermal cycling.
- (3)
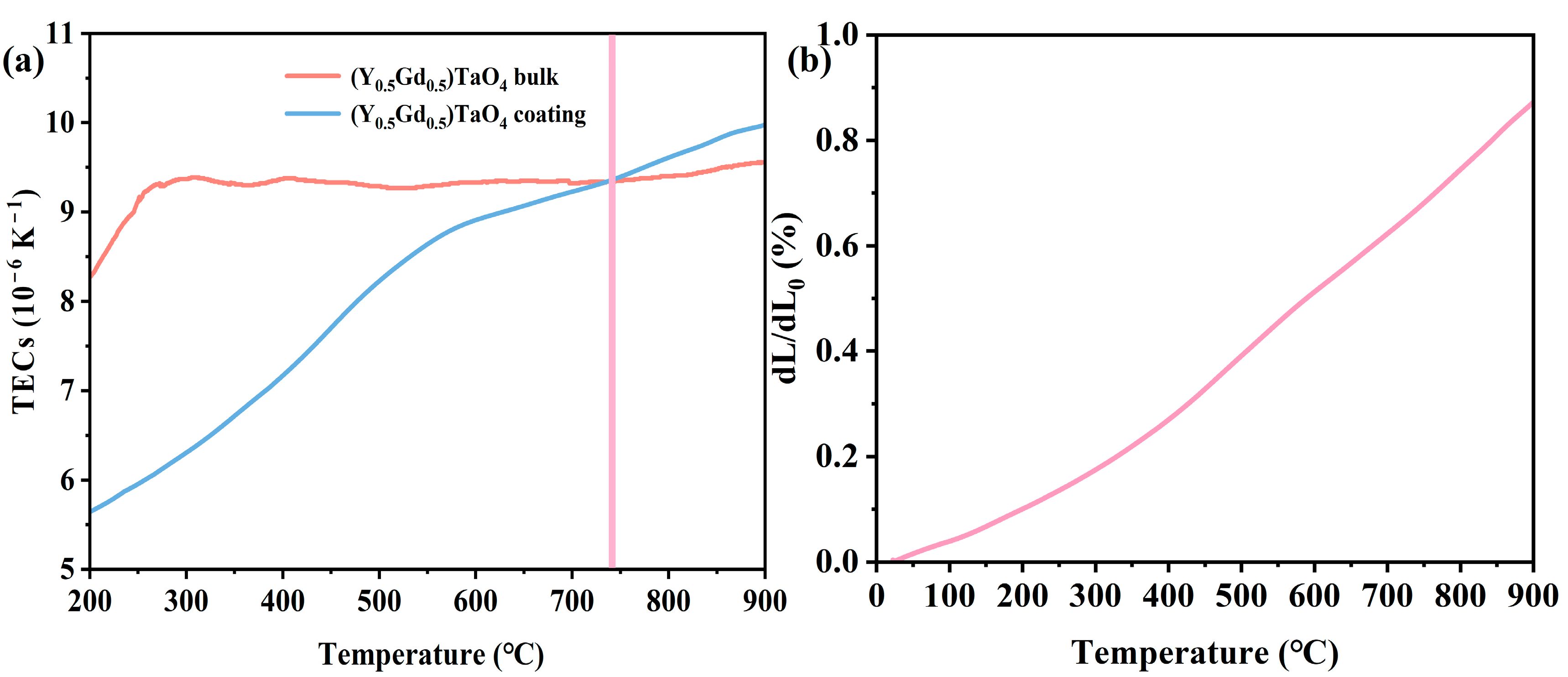
- The TECs of the (Y0.5Gd0.5)TaO4 coatings and dense blocks are close to each other when the temperature exceeds 740 °C, benefiting from the pores and cracks being sintered and compacted under the compressive thermal stress at high temperatures. The thermal conductivity (0.39 W·m−1·K−1 at 900 °C) of (Y0.5Gd0.5)TaO4 coating is 71.68% and 52.7% lower than that of the (Y0.5Gd0.5)TaO4 bulk and 8YSZ.
- (4)
- The phase transition of occurs in (Y0.5Gd0.5)TaO4 when the heat treatment temperature increases to 1000 °C, 1100 °C, 1200 °C, 1300 °C, and 1400 °C for 6 h, respectively; the phase transition of occurs in (Y0.5Gd0.5)TaO4 when the heat treatment temperature to 1400 °C for 12 h.
- (5)
- Heat treatment improves the mechanical properties of the coating. The bond strength increases from 8.86 MPa to 14.03 MPa when the heat treatment time increases to 24 h at 500 °C and the hardness improves from 5.88 ± 0.56 GPa to 7.90 ± 0.64 GPa when the heat treatment temperature increases to 1000 °C for 6 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, R.; Nag, S.; Kundu, S.; Ghosh, U.; Singh, U.; Chandra, S. Impact of Flow Boiling on Blast Furnace Tuyere Life: A Designer’s Perspective. Steel Res. Int. 2022, 93, 2100567. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.D.; Hu, R.; Zhang, C.; Li, G.S.; Zhang, Y.W.; Wu, W.H.; Lu, X.G. Failure mode and mechanism of a blast furnace tuyere. Eng. Fail. Anal. 2022, 137, 106294. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, J.; Zong, Y.; Zhang, L.; Liu, Y.; Yan, L.; Jiao, K. Optimization Study of Annular Wear-Resistant Layer Structure for Blast Furnace Tuyere. Metals 2023, 13, 1109. [Google Scholar] [CrossRef]
- Kumar Das, B. Reduction in heat losses through air tuyeres in blast furnaces at DSP. Mater. Today Proc. 2022, 66, 3944–3948. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, R.; Wu, W.; Zhang, Y.; Wang, D.; Zhang, P.; Chen, Z.; Lu, X. A novel device for investigating the anti-melting loss performance of Ni60A coating on coppery tuyere. Mater. Lett. 2024, 364, 136299. [Google Scholar] [CrossRef]
- Pathak, A.; Sivakumar, G.; Prusty, D.; Shalini, J.; Dutta, M.; Joshi, S.V. Thermal Spray Coatings for Blast Furnace Tuyere Application. J. Therm. Spray Technol. 2015, 24, 1429–1440. [Google Scholar] [CrossRef]
- Goloshchapov, K.V.; Kobelev, O.A.; Titlyanov, A.E.; Borisov, P.V.; Makarov, P.S.; Chicheneva, O.N. Improvement of the Function of Blast-Furnace Air Tuyeres. Metallurgist 2022, 66, 215–220. [Google Scholar] [CrossRef]
- Konrad, W.; Adam, J.; Konietzko, S.; Neinhuis, C. When Lotus Leaves Prevent Metal from Melting—Biomimetic Surfaces for High Temperature Applications. J. Bionic Eng. 2019, 16, 281–290. [Google Scholar] [CrossRef]
- Baby Raga Malikasri, N.; Kishore, K.; Singh Arora, K.; Shankar Mahobia, G. Failure mechanisms of blast furnace tuyeres and mitigation strategies: A comprehensive review. Eng. Fail. Anal. 2025, 167, 108968. [Google Scholar] [CrossRef]
- Pitek, F.M.; Levi, C.G. Opportunities for TBCs in the ZrO2–YO1.5–TaO2.5 system. Surf. Coat. Technol. 2007, 201, 6044–6050. [Google Scholar] [CrossRef]
- Limarga, A.M.; Shian, S.; Leckie, R.M.; Levi, C.G.; Clarke, D.R. Thermal conductivity of single- and multi-phase compositions in the ZrO2-Y2O3-Ta2O5 system. J. Eur. Ceram. Soc. 2014, 34, 3085–3094. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Carvalho, G.B.; Dias, A. Influence of the processing conditions and chemical environment on the crystal structures and phonon modes of lanthanide orthotantalates. Dalton Trans. 2011, 40, 9454–9460. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.K.; Chen, L.; Lv, L.; Wang, Y.C.; Ji, X.L.; Yun, H.T.; Su, C.Q.; Feng, J. Low thermal conductivity and anisotropic thermal expansion of ferroelastic (Gd1-xYx)TaO4 ceramics. J. Adv. Ceram. 2022, 11, 1696–1713. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Fu, H.; Zhi, X.; Guo, X.; Lin, J. Prediction for Dilution Rate of AlCoCrFeNi Coatings by Laser Cladding Based on a BP Neural Network. Coatings 2021, 11, 1402. [Google Scholar] [CrossRef]
- Zalnezhad, E.; Sarhan, A.A.D.; Hamdi, M. Optimizing the PVD TiN thin film coating’s parameters on aerospace AL7075-T6 alloy for higher coating hardness and adhesion with better tribological properties of the coating surface. Int. J. Adv. Manuf. Technol. 2013, 64, 281–290. [Google Scholar] [CrossRef]
- ISO 4624:2002; Paints and Varnishes—Pull-Off Test for Adhesion. German Version EN ISO 4624:2003; International Organization for Standardization: Geneva, Switzerland, 2002.
- Abbas, M.; Guo, L.; Guo, H. Evaluation of stress distribution and failure mechanism in lanthanum–titanium–aluminum oxides thermal barrier coatings. Ceram. Int. 2013, 39, 5103–5111. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Sun, X.G.; He, J.Q.; Pan, Z.Y.; Wang, C.H. Thermal shock behavior of 8YSZ and double-ceramic-layer La2Zr2O7/8YSZ thermal barrier coatings fabricated by atmospheric plasma spraying. Ceram. Int. 2012, 38, 3595–3606. [Google Scholar] [CrossRef]
- Liu, G.-L.; Zhu, L.; Li, X.-Y.; Sajjad, H.; Song, X.-Y.; Li, G.-R.; Yang, G.-J. Achieving durable double-layered thermal barrier coatings by tailoring multi-scale structures. J. Adv. Ceram. 2024, 13, 2068–2086. [Google Scholar] [CrossRef]
- Sarikaya, O. Effect of some parameters on microstructure and hardness of alumina coatings prepared by the air plasma spraying process. Surf. Coat. Technol. 2005, 190, 388–393. [Google Scholar] [CrossRef]
- Tillmann, W.; Khalil, O.; Baumann, I. Influence of spray gun parameters on inflight particle’s characteristics, the splat-type distribution, and microstructure of plasma-sprayed YSZ coatings. Surf. Coat. Technol. 2021, 406, 126705. [Google Scholar] [CrossRef]
- Friis, M.; Persson, C.; Wigren, J. Influence of particle in-flight characteristics on the microstructure of atmospheric plasma sprayed yttria stabilized ZrO2. Surf. Coat. Technol. 2001, 141, 115–127. [Google Scholar] [CrossRef]
- Sun, G.; He, X.; Jiang, J.; Sun, Y. Parametric study of Al and Al2O3 ceramic coatings deposited by air plasma spray onto polymer substrate. Appl. Surf. Sci. 2011, 257, 7864–7870. [Google Scholar] [CrossRef]
- Limarga, A.M.; Vaßen, R.; Clarke, D.R. Stress Distributions in Plasma-Sprayed Thermal Barrier Coatings Under Thermal Cycling in a Temperature Gradient. J. Appl. Mech. 2010, 78, 011003. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Zhong, X.; Zhao, H.; Wang, L.; Shao, F.; Liu, C.; Tao, S. Thermal shock behaviors of YSZ thick thermal barrier coatings fabricated by suspension and atmospheric plasma spraying. Surf. Coat. Technol. 2014, 249, 48–55. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, M.; Peng, Y.; Wang, J. Advances in Understanding the Evolution Mechanism of Micropore Defects in Metal Materials under External Loads. Metals 2024, 14, 522. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Yang, Y.; Li, C.G.; Wang, L. Investigation of stress field and failure mode of plasma sprayed Al2O3–13%TiO2 coatings under thermal shock. Mater. Sci. Eng. A 2009, 516, 103–110. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Yang, K.L.; Rong, J. Microstructure and performance of YTaO4 coating deposited by atmospheric plasma spraying on TC4 titanium alloy surface. Surf. Coat. Technol. 2022, 431, 128004. [Google Scholar] [CrossRef]
- Moghaddam, K.S.; Usher, J.S. Sensitivity analysis and comparison of algorithms in preventive maintenance and replacement scheduling optimization models. Comput. Ind. Eng. 2011, 61, 64–75. [Google Scholar] [CrossRef]
- Scrivani, A.; Rizzi, G.; Bardi, U.; Giolli, C.; Miranda, M.M.; Ciattini, S.; Fossati, A.; Borgioli, F. Thermal Fatigue Behavior of Thick and Porous Thermal Barrier Coatings Systems. J. Therm. Spray Technol. 2007, 16, 816–821. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Y.; Qu, W.; Guo, W.; Zhang, H.; Pei, Y.; Li, S.; Gong, S. Thickness-related failure behaviors of the thermal barrier coatings under thermal gradient cycling. Surf. Coat. Technol. 2023, 468, 129748. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.; Shi, X.; Huo, C.; He, Q.; Zhang, Y. Effect of spraying power on oxidation resistance and mechanical properties of plasma sprayed La-Mo-Si coating. Surf. Coat. Technol. 2017, 311, 138–150. [Google Scholar] [CrossRef]
- Sudharshan Phani, P.; Srinivasa Rao, D.; Joshi, S.V.; Sundararajan, G. Effect of Process Parameters and Heat Treatments on Properties of Cold Sprayed Copper Coatings. J. Therm. Spray Technol. 2007, 16, 425–434. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q.; Shi, X.L.; Li, D.B.; Yang, Y.; Li, C.; Feng, J. Microstructural evolution and thermal-physical properties of YTaO4 coating after high-temperature exposure. Surf. Coat. Technol. 2023, 456, 129222. [Google Scholar] [CrossRef]
- Shian, S.; Sarin, P.; Gurak, M.; Baram, M.; Kriven, W.M.; Clarke, D.R. The tetragonal-monoclinic, ferroelastic transformation in yttrium tantalate and effect of zirconia alloying. Acta Mater. 2014, 69, 196–202. [Google Scholar] [CrossRef]
- Macauley, C.A.; Fernandez, A.N.; Levi, C.G. Phase equilibria in the ZrO2-YO1.5-TaO2.5 system at 1500 °C. J. Eur. Ceram. Soc. 2017, 37, 4888–4901. [Google Scholar] [CrossRef]
- Flamant, Q.; Gurak, M.; Clarke, D.R. The effect of zirconia substitution on the high-temperature transformation of the monoclinic-prime phase in yttrium tantalate. J. Eur. Ceram. Soc. 2018, 38, 3925–3931. [Google Scholar] [CrossRef]
- Feng, J.; Shian, S.; Xiao, B.; Clarke, D.R. First-principles calculations of the high-temperature phase transformation in yttrium tantalate. Phys. Rev. B 2014, 90, 094102. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Q.; Song, J.; Zhang, D.; Xu, B.; Ren, Z.; Wang, M.; Yan, S.; Sun, X.; Liu, C.; et al. Revealing the low thermal conductivity of high-entropy rare-earth tantalates via multi-scale defect analysis. J. Adv. Ceram. 2023, 12, 2087–2100. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Y.; Chong, X.; Zhang, M.; Jin, Q.; Sun, Y.; Tang, X.; Wu, P.; Feng, J. Superior thermal and oxygen barrier properties of high-entropy ferroelastic rare earth tantalate (8RE1/8)TaO4. J. Adv. Ceram. 2024, 13, 2051–2067. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Chen, G.; Zhang, H.; Zou, Y.; Ye, Z.; Ouyang, J.; Jia, D.; Zhou, Y. High-temperature broadband infrared radiation from rare earth monosilicate-based ceramics. J. Eur. Ceram. Soc. 2024, 44, 6510–6517. [Google Scholar] [CrossRef]
- Cernuschi, F.; Ahmaniemi, S.; Vuoristo, P.; Mäntylä, T. Modelling of thermal conductivity of porous materials: Application to thick thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 2657–2667. [Google Scholar] [CrossRef]
- Wang, S.; Ye, Z.; Zhang, H.; Wang, Y.; Zhang, T.; Zou, Y.; Ouyang, J.; Jia, D.; Zhou, Y. High-entropy strategy for high-temperature broadband infrared radiation and low thermal conductivity. Ceram. Int. 2024, 50, 18806–18813. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Wang, Y.; Chen, G.; Zou, Y.; Wang, M.; Zhao, D.; Jin, R.; Ouyang, J.; Jia, D.; et al. Doping engineering for high-temperature broadband high emissivity and low thermal conductivity of ytterbium chromate-based ceramics. Ceram. Int. 2024, 50, 17657–17664. [Google Scholar] [CrossRef]
- Moteb, A.; Chen, K. Modelling and evaluating thermal conductivity of porous thermal barrier coatings at elevated temperatures. Ceram. Int. 2020, 46, 21939–21957. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, T.; Pan, W. Defect chemistry and thermal conductivity of the εSmO1.5·(1−ε)ZrO2 (0.2 ≤ ε ≤ 0.65) ceramics: Effect of the nonstoichiometry. J. Adv. Ceram. 2024, 13, 1823–1834. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, B.; Xu, H.; Jiang, X.; Li, J.-F.; Feng, J. Evolutions of mechanical and thermal properties of TmNbO4/Tm3NbO7 composites as protective coating materials. J. Adv. Ceram. 2024, 13, 1771–1785. [Google Scholar] [CrossRef]
- Godiganur, V.S.; Nayaka, S.; Kumar, G.N. Thermal barrier coating for diesel engine application—A review. Mater. Today Proc. 2021, 45, 133–137. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.-J.; He, G.-Y.; Chiang, F.-K.; Wang, H.; Wu, Y.; Jiang, S.-H.; Zhang, X.-B.; Lu, Z.-P. Novel high-entropy ultra-high temperature ceramics with enhanced ablation resistance. Rare Met. 2024, 43, 6559–6570. [Google Scholar] [CrossRef]
- Dharuman, N.; Arulmozhi, M.; Babu, M.S.; Berchmans, L.J.; Sreedhar, G. Investigations on oxidation, hot corrosion and thermal gradient performance of low k-La2Mo2O9 thermal barrier coating. Bull. Mater. Sci. 2021, 44, 8. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Dikici, H.; Kucuk, Y. Effects of heat treatment on adhesion strength of thermal barrier coating systems. Eng. Fail. Anal. 2013, 32, 16–22. [Google Scholar] [CrossRef]
- Paul, S. Assessing Coating Reliability Through Pore Architecture Evaluation. J. Therm. Spray Technol. 2010, 19, 779–786. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Feng, M.; Yu, C.-T.; Bao, Z.-B.; Zhu, S.-L.; Wang, F.-H. Thermal cycling behavior of nanostructured and conventional yttria-stabilized zirconia thermal barrier coatings via air plasma spray. Rare Met. 2023, 42, 3859–3869. [Google Scholar] [CrossRef]
- Wang, A.; Li, S.-H.; Bao, H. Thermal transport mechanism of electrons and phonons in pristine and defective HfB2. Rare Met. 2023, 42, 3651–3661. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.; Dahm, K.L.; Wang, F. Oxidation behaviour of sputter-depositedNi–Cr–Al micro-crystalline coatings. Acta Mater. 1998, 46, 1691–1700. [Google Scholar] [CrossRef]
- Naumenko, D.; Pillai, R.; Chyrkin, A.; Quadakkers, W.J. Overview on Recent Developments of Bondcoats for Plasma-Sprayed Thermal Barrier Coatings. J. Therm. Spray Technol. 2017, 26, 1743–1757. [Google Scholar] [CrossRef]
- Li, M.H.; Sun, X.F.; Li, J.G.; Zhang, Z.Y.; Jin, T.; Guan, H.R.; Hu, Z.Q. Oxidation Behavior of a Single-Crystal Ni-Base Superalloy in Air. I: At 800 and 900 °C. Oxid. Met. 2003, 59, 591–605. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behaviour of NiAl-I. Phase transformations in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Toscano, J.; Gil, A.; Hüttel, T.; Wessel, E.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Temperature dependence of phase relationships in different types of MCrAlY-coatings. Surf. Coat. Technol. 2007, 202, 603–607. [Google Scholar] [CrossRef]
- Li, G.; Huang, P.; Cheng, P.; Wu, W.; Zhang, Y.; Pang, Z.; Xu, Q.; Zhu, K.; Zou, X.; Li, R.; et al. Analysis of the failure mechanism of a blast furnace tuyere sleeve with protective coating. Eng. Fail. Anal. 2023, 153, 107537. [Google Scholar] [CrossRef]
- Curiotto, S.; Battezzati, L.; Johnson, E.; Pryds, N. Thermodynamics and mechanism of demixing in undercooled Cu–Co–Ni alloys. Acta Mater. 2007, 55, 6642–6650. [Google Scholar] [CrossRef]
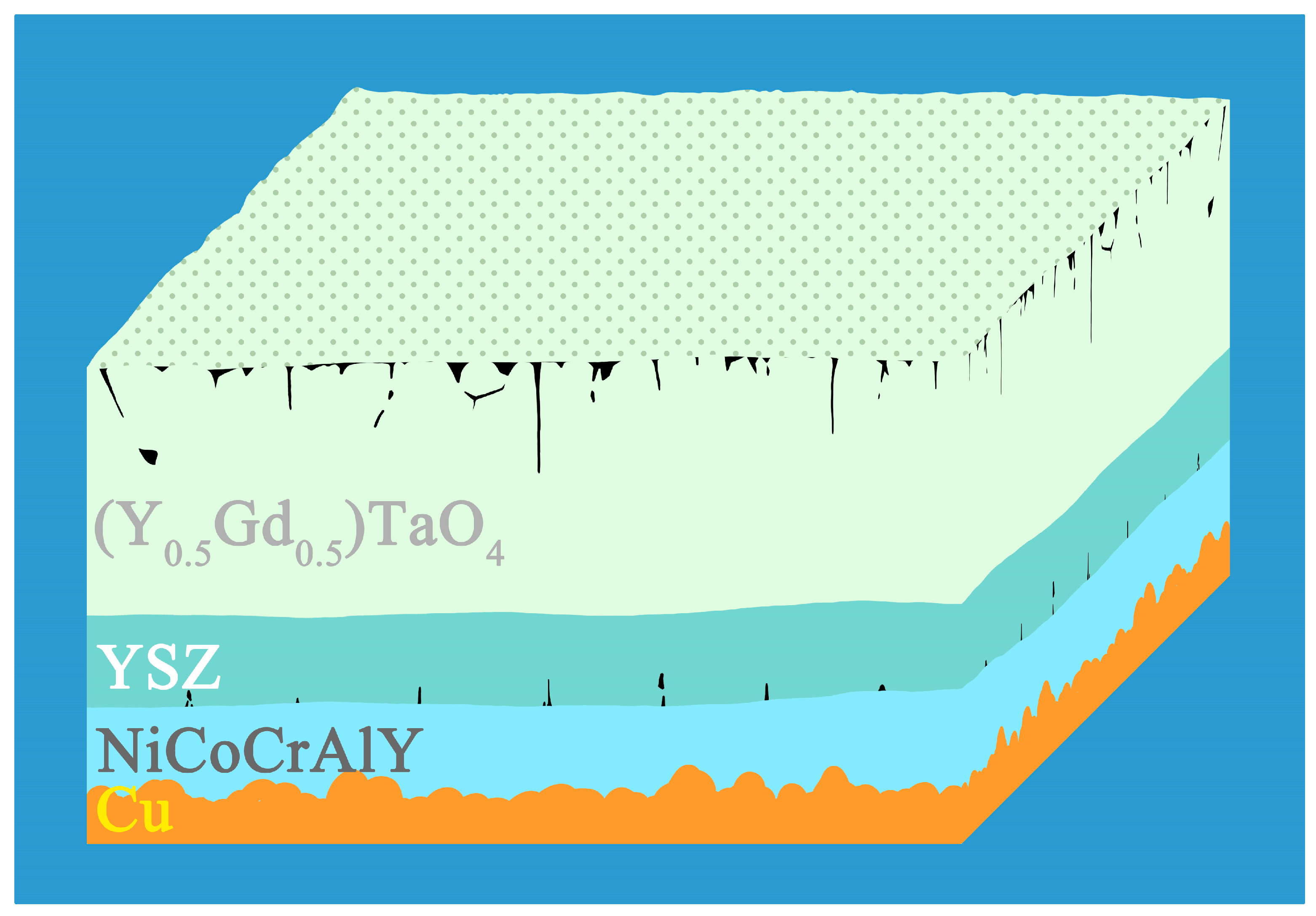




| Element | Ni | Cr | Co | Al | Y |
|---|---|---|---|---|---|
| W | 46.37 | 25.95 | 21.54 | 5.63 | 0.51 |
| Coating Name | I (A) | U (V) | PHydrogen (L/min) | PArgon (L/min) | Spring Distance (mm) |
|---|---|---|---|---|---|
| NiCoCrAlY | 420 | 64 | 0.83 | 33.33 | 130 |
| 8 wt.% YSZ | 500 | 64 | 4.33 | 41.33 | 130 |
| Level (i) | I (A) | U (V) | PHydrogen (L/min) | PArgon (L/min) | Spring Distance (mm) |
|---|---|---|---|---|---|
| 1 | 500 | 64 | 3.66 | 33.33 | 100 |
| 2 | 550 | 64 | 4.33 | 38.33 | 130 |
| 3 | 600 | 64 | 5 | 41.33 | 170 |
| Samples | I (A) | U (V) | PHydrogen (L/min) | PArgon (L/min) | Spring Distance (mm) |
| (Y0.5Gd0.5)TaO4#1 | 500 | 64 | 3.66 | 33.33 | 100 |
| (Y0.5Gd0.5)TaO4#2 | 500 | 64 | 4.33 | 38.33 | 130 |
| (Y0.5Gd0.5)TaO4#3 | 500 | 64 | 5 | 41.33 | 170 |
| (Y0.5Gd0.5)TaO4#4 | 550 | 64 | 4.33 | 33.33 | 170 |
| (Y0.5Gd0.5)TaO4#5 | 550 | 64 | 5 | 38.33 | 100 |
| (Y0.5Gd0.5)TaO4#6 | 550 | 64 | 3.66 | 41.33 | 130 |
| (Y0.5Gd0.5)TaO4#7 | 600 | 64 | 5 | 33.33 | 130 |
| (Y0.5Gd0.5)TaO4#8 | 600 | 64 | 3.66 | 38.33 | 170 |
| (Y0.5Gd0.5)TaO4#9 | 600 | 64 | 4.33 | 41.33 | 100 |
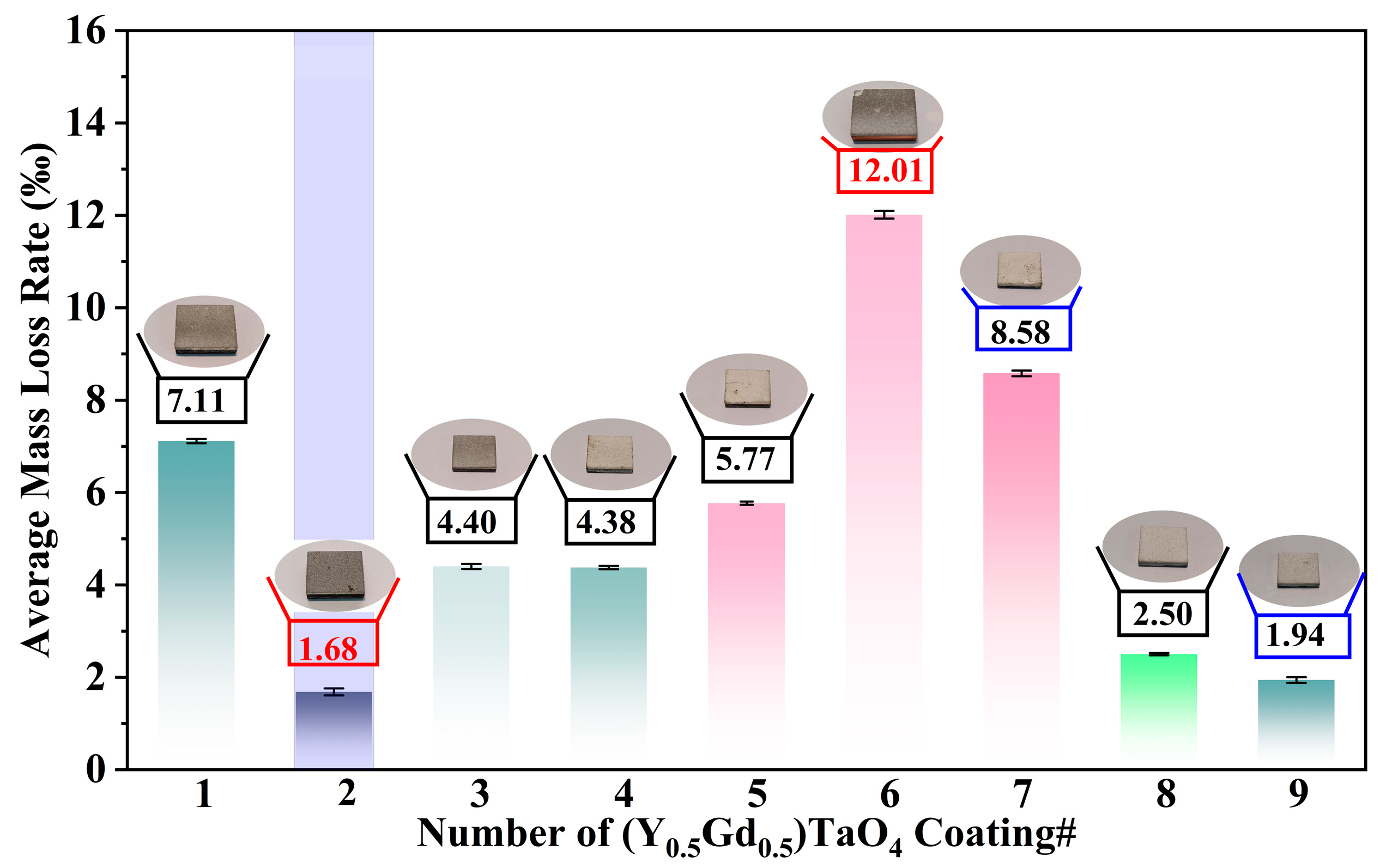
| Samples | Current (A) | Hydrogen Flow Rate (L/min) | Argon Flow Rate (L/min) | Spray Distance (mm) | The Average Mass Loss Rate (‰) |
|---|---|---|---|---|---|
| (Y0.5Gd0.5)TaO4#1 | 500 | 3.66 | 33.33 | 100 | 7.11 |
| (Y0.5Gd0.5)TaO4#2 | 500 | 4.33 | 38.33 | 130 | 1.68 |
| (Y0.5Gd0.5)TaO4#3 | 500 | 5 | 41.33 | 170 | 4.40 |
| (Y0.5Gd0.5)TaO4#4 | 550 | 4.33 | 33.33 | 170 | 4.38 |
| (Y0.5Gd0.5)TaO4#5 | 550 | 5 | 38.33 | 100 | 5.77 |
| (Y0.5Gd0.5)TaO4#6 | 550 | 3.66 | 41.33 | 130 | 12.01 |
| (Y0.5Gd0.5)TaO4#7 | 600 | 5 | 33.33 | 130 | 8.58 |
| (Y0.5Gd0.5)TaO4#8 | 600 | 3.66 | 38.33 | 170 | 2.50 |
| (Y0.5Gd0.5)TaO4#9 | 600 | 4.33 | 41.33 | 100 | 1.94 |
| k1 | 4.40 | 7.02 | 6.69 | 4.94 | --- |
| k2 | 7.39 | 2.86 | 3.31 | 7.42 | --- |
| k3 | 4.34 | 6.25 | 6.11 | 3.76 | --- |
| R | 9.14 | 13.62 | 10.12 | 10.99 | --- |
| Factor | Df | Sum Sq | Mean Sq | F Value | p Value | Optimal Parameter |
|---|---|---|---|---|---|---|
| Current | 2 | 54.68 | 27.34 | 2.92 | 0.07331 | 600 A |
| Hydrogen flow rate | 2 | 103.1 | 51.55 | 7.02 | 0.00399 | 4.33 L/min |
| Argon flow rate | 2 | 58.64 | 29.32 | 3.19 | 0.0592 | 38.33 L/min |
| Spray distance | 2 | 62.94 | 9.02 | 3.49 | 0.04677 | 170 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, J.; Lin, H.; Jiang, Q.; Wang, J.; Feng, J. Microstructural Evolution and Failure Analysis for 8YSZ/(Y0.5Gd0.5)TaO4 Double-Ceramic-Layer Thermal Barrier Coatings on Copper Substrate. Coatings 2025, 15, 451. https://doi.org/10.3390/coatings15040451
Zhang X, Ma J, Lin H, Jiang Q, Wang J, Feng J. Microstructural Evolution and Failure Analysis for 8YSZ/(Y0.5Gd0.5)TaO4 Double-Ceramic-Layer Thermal Barrier Coatings on Copper Substrate. Coatings. 2025; 15(4):451. https://doi.org/10.3390/coatings15040451
Chicago/Turabian StyleZhang, Xiao, Jing Ma, Huizhi Lin, Qingwei Jiang, Jun Wang, and Jing Feng. 2025. "Microstructural Evolution and Failure Analysis for 8YSZ/(Y0.5Gd0.5)TaO4 Double-Ceramic-Layer Thermal Barrier Coatings on Copper Substrate" Coatings 15, no. 4: 451. https://doi.org/10.3390/coatings15040451
APA StyleZhang, X., Ma, J., Lin, H., Jiang, Q., Wang, J., & Feng, J. (2025). Microstructural Evolution and Failure Analysis for 8YSZ/(Y0.5Gd0.5)TaO4 Double-Ceramic-Layer Thermal Barrier Coatings on Copper Substrate. Coatings, 15(4), 451. https://doi.org/10.3390/coatings15040451






