Abstract
Human neural stem cells (hNSCs) are vital for advancing therapies for neurocognitive disorders. However, standard hNSC culture conditions often lack chemically defined and xeno-free substrates, limiting their clinical applicability. Chitosan, known for its biocompatibility, presents a promising alternative for hNSC culture. Hyaluronic acid (HA) and alginate, with their negative charges, enable effective interaction with positively charged chitosan to form films with enhanced mechanical properties. Incorporating chitosan into substrates creates chitosan–alginate (CA) and chitosan–hyaluronic acid (CHA) composites that meet chemically defined, mechanically tunable, and xeno-free standards. Despite their potential, the effects of these composites’ composition and mechanical properties on hNSC behavior, particularly in film form, remain unexplored. To bridge this gap, we fabricated films with varying chitosan-to-alginate and chitosan-to-hyaluronic acid ratios to assess their influence on hNSC pluripotency under xeno-free conditions. Our results reveal that films with higher chitosan content promote hNSC attachment and proliferation. Conversely, increasing alginate generally decreased cell attachment, proliferation, and multipotency, while increasing HA had no impact on attachment or proliferation but decreased multipotency. This investigation provides insights into the impact of substrate composition and mechanical properties on hNSC behavior, guiding the design of analogous materials for three-dimensional cultures and optimizing stem cell-based therapies for clinical applications.
1. Introduction
Over the past two decades, human neural stem cells (hNSCs) have become central to pioneering research aimed at modeling and treating a broad spectrum of diseases, including cancer [1,2,3], Alzheimer’s disease [4,5], Parkinson’s disease [5,6,7,8,9], cerebral palsy [10,11], multiple sclerosis [12,13,14], and many others [15,16]. These diseases can be targeted through hNSC implantation [17,18,19], or by using hNSCs to model disease progression and assess drug efficacy [20]. However, the translation of these experimental approaches into clinical practice is hindered by the stringent requirements of current Good Manufacturing Practice (cGMP) culture conditions. These conditions necessitate the use of chemically defined, xeno-free environments to ensure safety and reproducibility.
Geltrex is the leading substrate for human neural stem cell (hNSC) growth, supporting adhesion, proliferation, and differentiation due to its ECM protein composition. However, derived from murine tumors, it is costly and unsuitable for xeno-free and chemically defined standards critical for clinical use. Human-derived extracellular matrices show promise as alternatives in supporting the proliferation and differentiation of hNSCs [21], but face scalability issues due to high production costs. These limitations highlight the need for affordable, reproducible, and clinically compatible materials, driving interest in innovative, scalable solutions for both research and clinical purposes.
To address these limitations, chitosan-based materials are being explored due to their well-established history as low-cost biomaterials with customizable properties [22,23,24,25,26]. Chitosan (CS) is natural and known for its excellent biocompatibility, non-toxicity, and biofunctional properties such as antiviral and antibacterial effects, making it ideal for various biomedical applications [27,28,29,30,31,32,33]. Its versatility has been demonstrated in the fabrication of thin films and microbeads that support the growth and differentiation of rat hippocampal neurons and human neural progenitor cells (hNPCs) into neural and glial lineages [34]. Additionally, CS-based copolymers have been used to create hydrogels for cultivating and differentiating hNPCs, human neural stem cells (hNSCs), rat NSCs, and human olfactory ectomesenchymal stem cells [35,36,37]. Furthermore, patterned CS thin films have facilitated studies on the differentiation and morphology of embryonic neuronal and glial cells [38]. In one study, chitosan–alginate films were used to culture mouse and human fibroblasts, but the researchers used only one ratio of chitosan to alginate and did not use human neural stem cells [39]. Chitosan–alginate films of different ratios have been characterized but not evaluated for their ability to maintain a multipotent population of human neural stem cells [40]. Chitosan hyaluronic acid films have been cultured with mesenchymal stem cells and corneal epithelial cells, but not with hNSCs nor at different ratios of chitosan and hyaluronic acid [41,42]. Rat-derived neural stem cells have been cultured on chitosan thin films and evaluated for their ability to maintain a multipotent state and differentiate; however, only pure chitosan films and rat neural stem cells were used [43]. Chitosan thin films have also been shown to improve recovery after sciatic nerve injury in rats when treated in conjunction with mesenchymal stem cells [44].
Building on this foundation, the present study investigates the modification of the microenvironment of hyaluronic acid (HA) and alginate (A) by incorporating chitosan. HA and alginate are two non-proteinaceous natural polymers that mimic the structure of glycosaminoglycans, a major component of the extracellular matrix (ECM). HA itself is a major ECM component in the brain [19,20]. Both HA and alginate are negatively charged, which allows them to effectively interact with positively charged chitosan to form films and enhance mechanical properties. Previous research has demonstrated that chitosan complexing with HA and alginate enhances cell attachment and the mechanical properties of biomaterials [45,46,47,48].
In this study, we developed chitosan–alginate (CA) and chitosan–hyaluronic acid (CHA) hybrid materials to meet chemically defined, mechanically tunable, and xeno-free standards, and assess their suitability for supporting hNSC culture. By fabricating thin films from CS, CA, and CHA with varying ratios of CS to alginate and CS to HA (0:1, 1:3, 1:1, 3:1, and 1:0), we aimed to determine how these variations influence hNSC multipotency. These films, characterized by their transparency, uniform topography, and hydrophilicity, provided a conducive environment for studying hNSC adhesion, proliferation, and protein expression. The ability to fine-tune the physiochemical and mechanical properties of chitosan-based materials to optimize neural stem cell culture environments represents a significant advancement in stem cell-based therapies. These findings hold relevance for regenerative medicine, where precise control over cell behavior is essential to achieving consistent and predicable outcomes, especially in the treatment of neurological disorders.
2. Materials and Methods
2.1. Preparation of CA, CHA, and CS Films
First, 0.3% (w/w) CS (Matexcel NAT-0300, degree of deacetylation: 97.3%), 0.3% (w/w) hyaluronic acid, and 0.3% (w/w) alginate were prepared in separate solutions of 1% acetic acid. Then, 200 μL of the CS solution was added to a 48-well plate. The plate was then air-dried in a chemical fume hood for 18 h to form a base layer of CS. The CS base layer was then submerged in a neutralization solution consisting of 2.7% DI water, 6.4% NH4OH, and 90.9% methanol for 30 min. Then, the films were washed extensively with DI water to remove the base solution and air-dried for another 12 h. Mixtures of CA were then prepared at ratios of CS:A of 0:1, 1:3, 1:1, 3:1, and 1:0 by combining the appropriate amounts of the solutions and using a blender to homogenize them. Mixtures of CHA were prepared at ratios of CS:HA of 0:1, 1:3, 1:1, 3:1, and 1:0. Then, 100 μL of these mixtures was added to the top of the base CS layer in the 48-well plate. The films were then air-dried for 12 h and then submerged in a neutralization solution for 30 min. The films were washed extensively with DI water and sterilized using 70% ethanol. Larger plates were also fabricated by scaling the amounts based on the surface area of the well plate.
2.2. Water Contact Angle
The films’ surface wettability was examined using a goniometer (Ramé-Hart Instrument, Succasunna, NJ, USA) through measuring the water contact angle. Films were cast onto glass slides using the same protocol mentioned in Section 2.1. A 5 μL droplet of DI water was placed onto the coated glass slide, and the measurement was taken immediately at five random locations on the surface.
2.3. SEM
The CA, CHA, and CS films had their surface topographies examined using SEM. Films were prepared as described in Section 2.1, except inside of Petri dishes. Dried films were cut into 0.5 cm × 0.5 cm pieces and mounted onto stubs using carbon adhesive tape (NEM Tape, Nisshin Em. Co. Ltd. Tokyo, Japan). Samples were imaged with a TM3000 tabletop SEM (Hitachi, Tokyo, Japan) at 5 kV.
2.4. AFM
The CA, CHA, and CS films’ mechanical properties were measured using a Nanosurf EasyScan atomic force microscope (Nanosurf AG, Liestal, Switzerland). The mechanical properties were determined under ambient conditions using a ferromagnetic resonance tip with a nominal spring constant of 2.8 N m−1 and a resonance frequency of 60 kHz. The topography of the sample was monitored using scanning probe microscopy (Nanosurf AG, Liestal, Switzerland) in the force modulation mode while simultaneously taking nanomechanical measurements. The tip’s position was controlled using an optical microscope (Nanosurf AG, Liestal, Switzerland) in tandem with the AFM. Films were prepared as described in Section 2.1, except inside Petri dishes. The Petri dishes were then fixed to an aluminum holder using tape and secured onto the AFM stage using magnets. Images were processed using Scanning Probe Image Processor software (SPIP v 10.3; Image Metrology, Lyngby, Denmark).
2.5. FTIR
FTIR analysis of the chitosan films was performed using a Nicolet 5DXB spectrometer (Thermo Scientific, Boston, MA, USA). The films were analyzed after being cast onto well plates. The films were scraped off the well plate using a razor blade, and the shavings were combined with KBr powder. The polymer and the KBr mixture were then pressed to form a thin film. The films were analyzed by averaging 32 scans at a resolution of 2 cm−1 over a range of 500–4000 cm−1. Areas under the curve were calculated using the python (v. 3.12) packages scipy and numpy.
2.6. Cell Culture
Human neural stem cells, namely, hNSC-H14 (WB0195) derived from the NIH-approved hESC-H14 (WA14), were obtained from WiCell Research Institute (Madison, WI, USA). The cells were expanded and cultured following the supplied protocol. Briefly, the cells were cultured on Geltrex- or Matrigel-coated tissue culture dishes prepared according to the WiCell protocol, and the cells were supplied with fresh NSC culture medium every other day. Human recombinant basic fibroblast growth factor (bFGF, Gibco) and epidermal growth factor (EGF, Gibco) were supplied every day at 20 ng mL−1. The NSC culture medium consisted of Neurobasal medium (Gibco) supplemented with B27 (Gibco), N2 (Gibco), heparin (StemCell Technologies Inc., Vancouver, BC, Canada), MEM NEAA (Gibco), and GlutaMAX (Gibco). The cells were passaged when they reached 90%–95% confluency and were dissociated with Accutase. Cells that were seeded onto films had half-medium changes performed every day. BFGF and EGF were added every day at 20 ng mL−1. Cells were maintained in a humidified incubator with 5% CO2 at 37 °C. For characterization experiments, cells were cultured for a maximum of 4 days because this is the typical amount of time it takes for cells to reach confluency in Geltrex- or Matrigel-coated plates.
2.7. Cell Morphology and Proliferation
Cells were monitored using a Nikon Eclipse TE 2000-S (Nikon, Tokyo, Japan) inverted microscope. Live cells were stained with Calcien AM (Thermo Fisher, Washington, DC, USA) and dead cells were stained with propidium iodide according to the manufacturer’s protocol. The cells were then imaged using a Nikon TE 2000-S inverted microscope (Nikon, Tokyo, Japan). Percent area fluorescent measurements were made using ImageJ (v. 1.53t) and were taken over 4 separate images with a total area analyzed of around 15 mm2. Cell proliferation was monitored using an alamarBlue assay (Sigma Aldrich, St. Louis, MO, USA). Briefly, the total amount of medium in each well was measured, and then the alamarBlue reagent (110 μg mL−1 resazurin) was added at 10% of the total volume. The cells were then incubated at 37 °C for 2 h, and the fluorescence intensity of the solution was measured at 590 nm with an excitation of 560 nm using a SpectraMax M2 microplate reader (Molecular Device, Sunnyvale, CA, USA).
2.8. Flow Cytometry
The antibodies Alexa Fluor® 488 anti-Ki67 (350508) and Alexa Fluor® 647 anti-SOX2 (BDB560302) were purchased from BioLegend (San Diego, CA, USA) and BD Bioscience (Franklin Lakes, NJ, USA), respectively, and were used as suggested by the suppliers. Cells were dissociated from their respective coating or film with Accutase, rinsed with PBS, and then fixed in 4% paraformaldehyde for 15 min. Next, the cells were permeabilized in 0.1%v/v Triton-X 100 in PBS and washed with PhosFlow PermWash Buffer (BD Bioscience, San Jose, CA, USA). The cells were resuspended in 100 µL cell PermWash Buffer and incubated with fluorophore-conjugated antibodies for 30 min on ice in the dark. Next, the cells were washed with PermWash Buffer (Thermo Fisher Scientific, Waltham, MA, USA) and resuspended in 0.3 mL Cell Staining Buffer (BioLegend). The cells were analyzed on an LSRII flow cytometer (BD Bioscience) and quantified using FlowJo software (v. 10.6, Tree Star, Ashland, OR, USA).
2.9. Immunocytochemistry
A Human Neural Stem Cell ICC kit (Cat. A24354, Thermo Fisher Scientific, Waltham, MA, USA) was used to characterize NSC marker protein expression (SOX2, nestin, and PAX6) following the manufacturer’s protocol. The cells were also stained with the cell proliferation marker protein Ki-67 (BioLegend: San Diego, CA, USA). Briefly, the cells were cultured as described above but on coated glass slides. After five days, the cells were rinsed with PBS, fixed with cold 4% paraformaldehyde for ten minutes on ice, permeabilized for ten minutes on ice, blocked for one hour on ice, and stained with primary antibodies at 4 °C overnight. The cells were then washed and incubated with secondary antibodies for one to two hours on ice. Then, the cells were washed once and stained with DAPI before being mounted in Prolong Gold antifade reagent (Invitrogen; Carlsbad, CA, USA). Fluorescence images were acquired using a Nikon Eclipse TE 2000-S (Nikon, Tokyo, Japan) inverted microscope.
2.10. Statistical Analysis
The statistical significance of the data was determined using an unpaired two-tailed Student’s t-test to evaluate the p-value between two data sets. The number of independent measurements or experiments (n) is indicated in the figure captions. p-values that were less than 0.05 were considered statistically significant. Statistical analyses were performed in Microsoft Excel.
3. Results and Discussion
3.1. Hydrophilicity, Topography, and Mechanical Strength
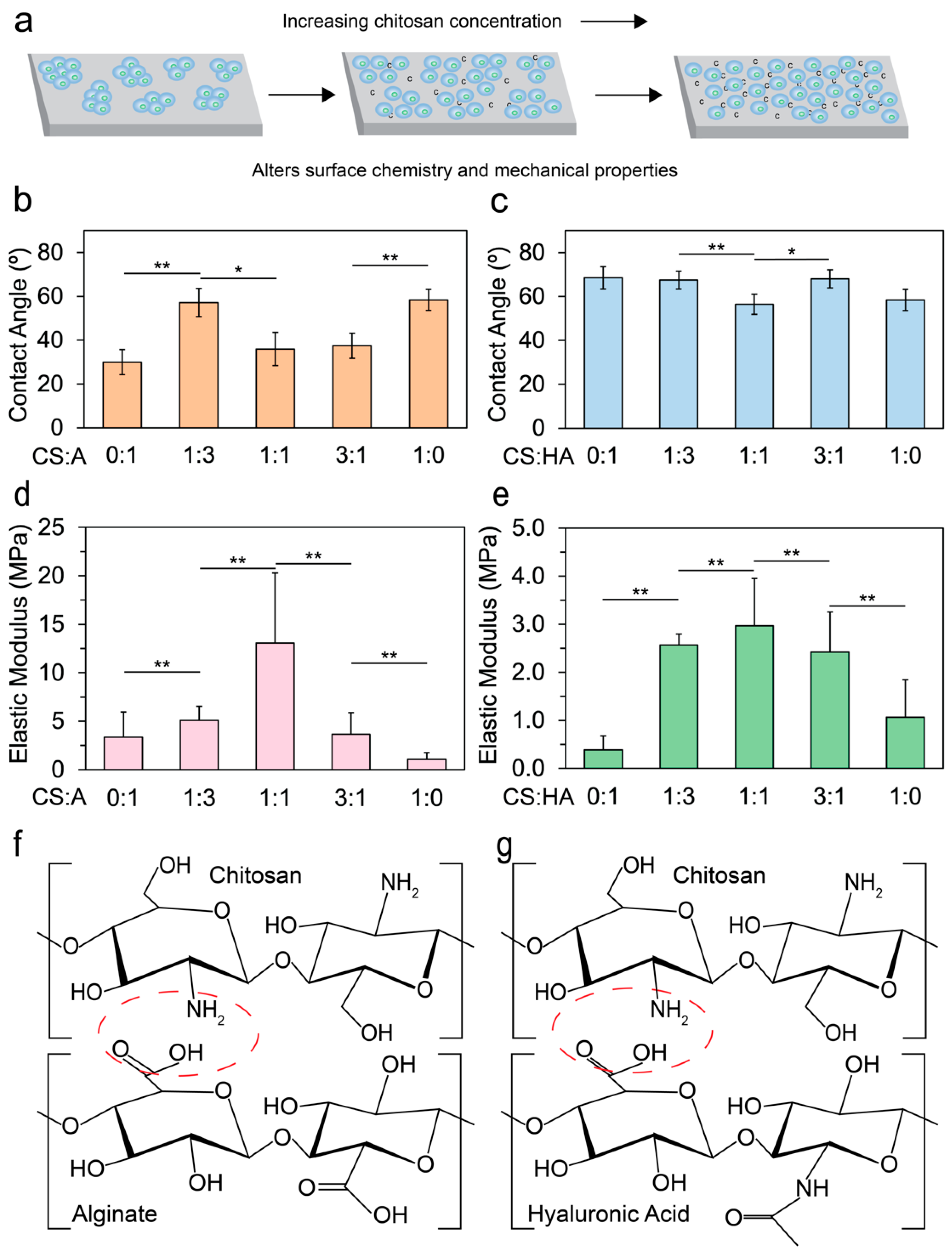
The integration of chitosan into alginate or HA films substantially affects cell adhesion by modifying surface chemistry and mechanical properties, as illustrated in Figure 1a. Hydrophilicity, a critical determinant of cell attachment and proliferation, generally favors more hydrophilic surfaces for optimal cell growth [49,50]. To assess the hydrophilicity of CA and CHA films, we measured the water contact angle immediately (within 60 s) after placing a water droplet on the film using a goniometer. The films analyzed had varying ratios of CS:A and CS:HA of 0:1, 1:3, 1:1, 3:1, and 1:0.
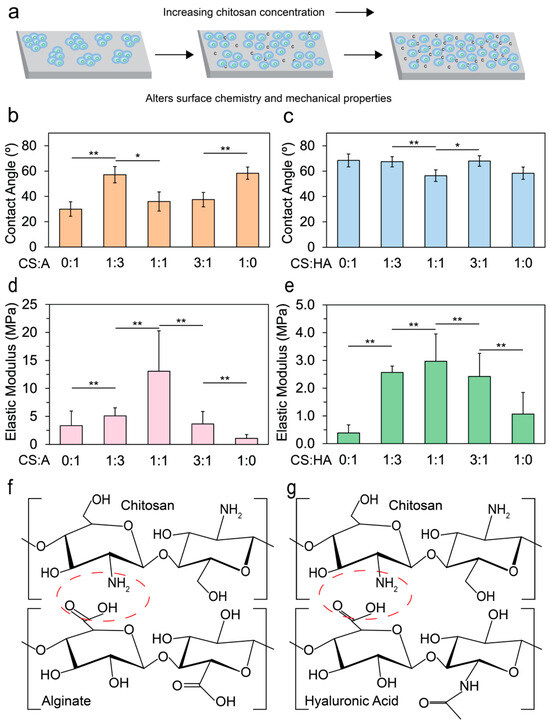
Figure 1.
Outline and physical characterization of the films. (a) An illustration showing how the addition of chitosan aids in cell adhesion. Contact angle measurements taken by a goniometer for (b) CA films (n = 10) and (c) CHA films (n = 10) with varying ratios of 0:1, 1:3, 1:1, 3:1, and 1:0. AFM was used to measure the elastic moduli of (d) CA films (n > 239) and (e) CHA films (n > 239). Statistical significances of p < 0.05 and p < 0.005 are indicated by * and **, respectively. If no marks are present, there is no statistical significance between the samples. Illustrations of (f) chitosan and alginate polyelectrolyte bond formation (red circle) and (g) chitosan and hyaluronic acid polyelectrolyte bond formation (red circle).
Figure 1b shows that the contact angle for CA films decreased initially as the alginate content increased, and reached a minimum at a CS:A ratio of 1:1. There was a large increase in contact angle at 1:3, which may be due to the increase in precipitated alginate or CS. Figure 1c illustrates an increase in the water contact angle upon the introduction of HA to the base chitosan material, and a local minimum in water contact angle was observed at a 1:1 ratio, suggesting that this ratio may represent an optimal balance where CS and HA aggregates are minimized.
When blended, alginate and CS form a polyelectrolyte complex via electrostatic interactions between the positively charged CS and the negatively charged alginate [51,52]. Although mammalian cells typically exhibit poor attachment to alginate [53,54,55,56], CA demonstrates enhanced cell attachment due to its capability to absorb serum proteins [57,58]. As more alginate is added to the base CS material, electrostatic interactions increase until a threshold is reached where there is a higher concentration of alginate than chitosan, resulting in a subsequent decrease in electrostatic interactions and directly affecting the elastic modulus [59,60]. HA, an anionic, non-sulfated GAG, is a major component of some extracellular matrices [61,62,63]. When HA is combined with CS, a polyelectrolyte complex is formed [48,64,65]. Despite being a major component of many extracellular matrices, mammalian cells tend to exhibit poor adhesion to HA due to its negative charge [66].
To understand how the elastic moduli of the films affect the cultivation of hNSCs, CA and CHA films were subjected to AFM nanomechanical measurements. Figure 1d shows that as the amount of alginate in CA films increased, a local maximum in the elastic modulus was reached at a CS:A ratio of 1:1. The local maximum is likely due to an optimal amount of CS and alginate being present to maximize crosslinking. The bond strength from crosslinking is diminished when either CS or alginate is removed from the film. A similar trend in CHA films is observed (Figure 1e). The local maximum occurred at a CS-to-HA ratio of 1:1, indicating the optimal ratio that maximized crosslinking. As more HA was added to the base CS material, the elastic modulus increased up to a saturation point where the maximum electrostatic interaction occurred. Beyond this threshold, the electrostatic interaction weakened, leading to a decrease in the elastic modulus [67]. An illustration of the polyelectrolyte bond that forms between CS and alginate and between CS and hyaluronic acid is presented in Figure 1f and g, respectively. The electrostatic interaction occurs between the positively charged amine group on chitosan and the carboxylate group on alginate or hyaluronic acid.
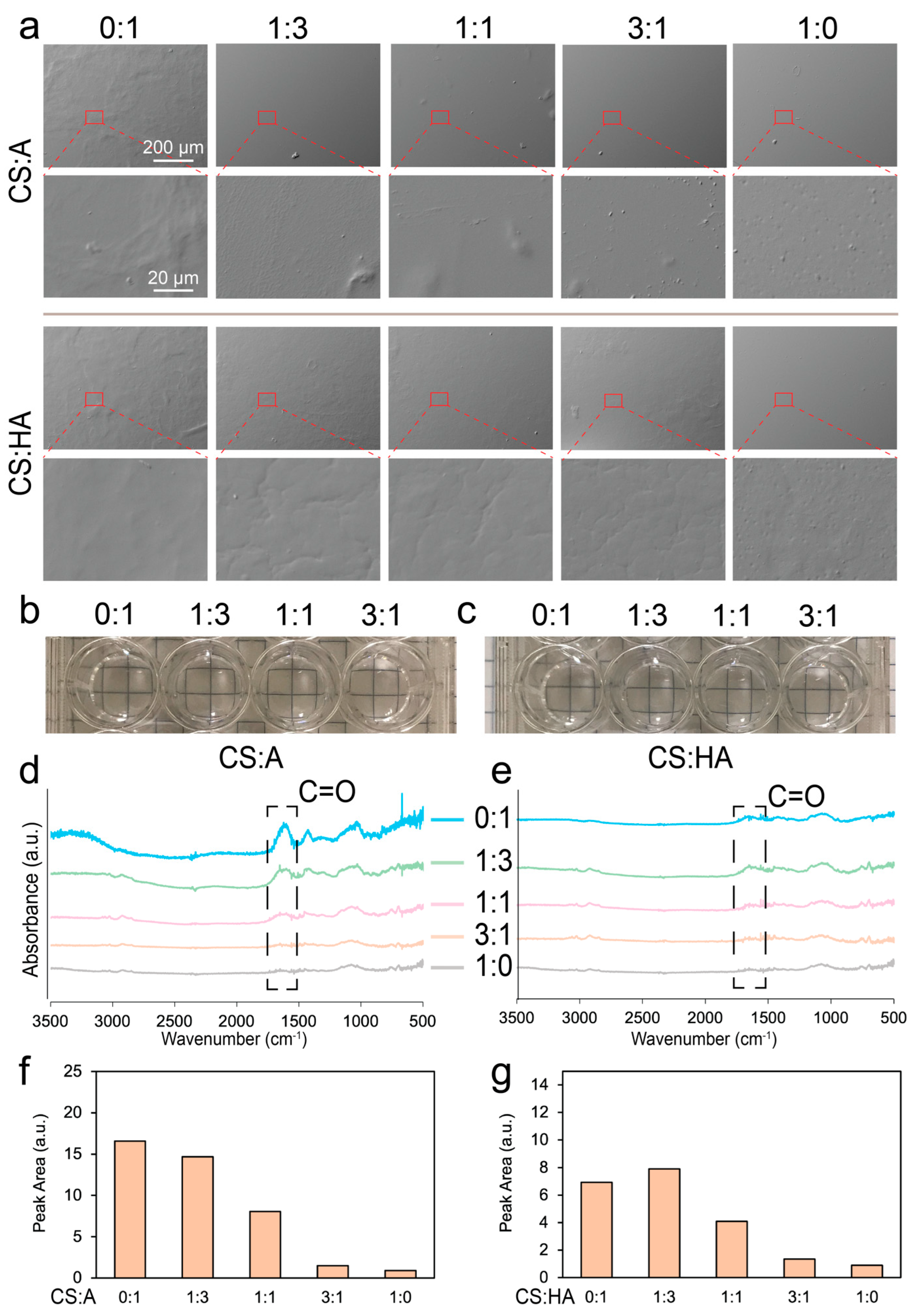
Surface topography plays a key role in cell growth, as sharp features, rough surfaces, and uneven textures can affect cell adhesion and proliferation [68,69]. CA and CHA films with ratios of CS to alginate or CS to HA of 0:1, 1:3, 1:1, 3:1, and 1:0 were cut into 0.5 cm-by-0.5 cm squares for SEM imaging. The pure chitosan and CA films exhibited predominantly flat and smooth surfaces, while pure alginate films exhibited uneven textures (Figure 2a). This difference is likely due to the superior bonding between chitosan and alginate, which facilitates the formation of uniform films and minimizes the presence of aggregates. In contrast, the rougher surface of pure alginate can be attributed to the formation of alginate aggregates. Similarly, CHA films exhibited some uneven or rough surface features, likely attributable to the aggregation of CS or HA within the film matrix. Aggregation could be caused by insoluble chitosan, alginate, or hyaluronic acid, nonhomogeneous mixing, or uneven drying.
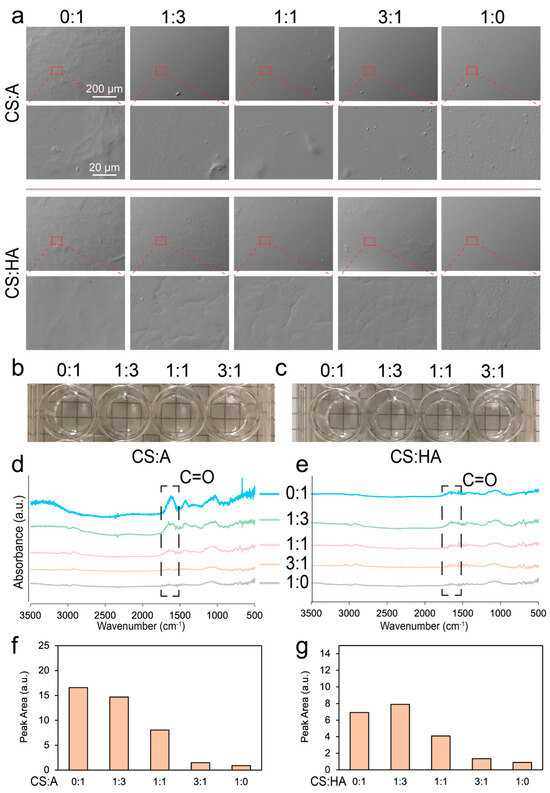
Figure 2.
Surface morphology and composition characterization of the films. (a) SEM images of the CA and CHA films with ratios of CS to alginate and CS to HA of 0:1, 1:3, 1:1, 3:1, and 1:0. Photographs of (b) CS:A and (c) CS:HA films immersed in PBS taken after they were prepared for cell culture. FTIR spectra of (d) CA and (e) CHA films. Area under the highlighted peak in the FTIR spectra for (f) CA and (g) CHA films.
Figure 2b,c show images of the various film blends after immersion in PBS following sterilization. All films appeared transparent, with no visible ridges or aggregates. Figure 2d,e show the FTIR spectra of the CS:A and CS:HA films, respectively. In both cases, as more chitosan was added to the film, the number of carbons double-bonded to oxygen was reduced, and the characteristic peak for this double bond was observed at around 1620 cm−1. Figure 2f,g show the area under the curve of this peak for the CA and CHA films, respectively. This area generally decreased as more chitosan was added into the film, indicating a decrease in carbon–oxygen double bonds. Since chitosan has no carbon–oxygen double bond, it is expected to have no peak here, but the degree of deacetylation of this chitosan is around 97%, indicating the presence of some chitin, which does have a carbon–oxygen double bond.
3.2. Cell Behavior on CA/CHA/CS Films
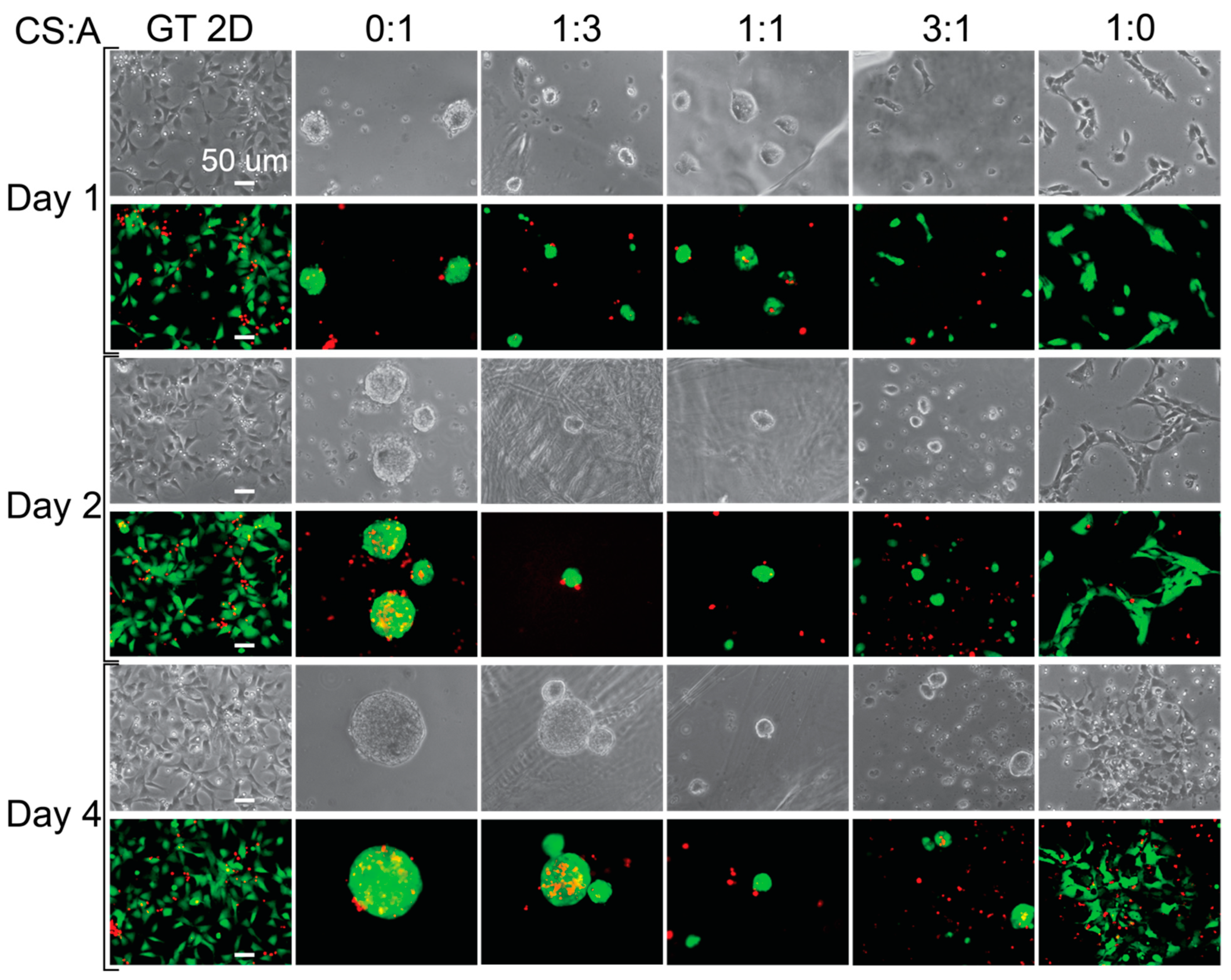
After sterilization, hNSC-H14 cells were plated onto the films at 30k cm−2 cell density and cultured for up to four days. Cell morphology was examined for CS:A films of various chitosan-to-alginate ratios, including 0:1, 1:3, 1:1, 3:1, and 1:0. Figure 3 shows images taken of the cells on days 1, 2, and 4. Cell morphology provides information on how the cells are attached to the substrates. The pure alginate film (CS:A = 0:1) promoted the formation of large cell clusters and had low cell adhesion. Cell adhesion decreased across the 4-day period for all of the conditions, with the exception of the CS:A 1:0 ratio film (pure chitosan) and the Geltrex control (GT 2D), where cell adhesion increased or remained consistent. These decreases in cell adhesion are likely due to an unfavorable interaction between the cells and CA films, caused by either poor electrostatic interactions due to the negative charge of alginate, a lack of cell adhesion motifs, or a combination of the two [70,71]. This may also be caused by the unfavorable mechanical properties of the films. Stem cells are influenced by the mechanical properties of their surroundings through a process known as mechanotransduction [72,73,74,75]. When the cells begin to grow as clusters, it becomes more difficult for nutrients to reach the cells in the core of the cluster, causing the center of the cluster to become necrotic. Pure CS films provide the most ideal surface due to the presence of more protonated amine groups. They support the growth of single cells, while the other film types tend to support the growth of cell clusters. As more alginate is added to the film, the number of protonated amine groups on the film is reduced, while the number of negatively charged carboxylic acid groups increases. On day four, cells grew as clusters in the 0:1 and 1:3 conditions and weakly attached clusters for the rest of films except for the pure CS film and Geltrex control.
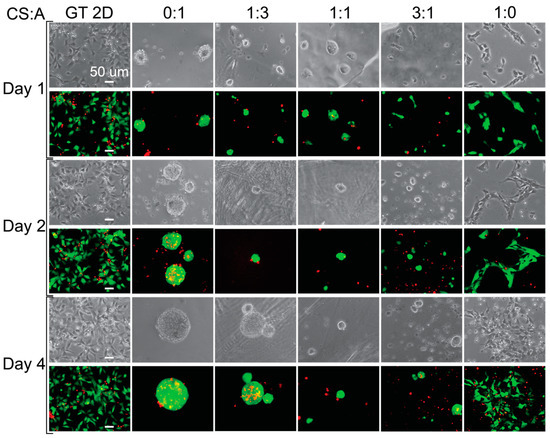
Figure 3.
Cell morphology and live staining of hNSCs grown on CA films. Phase-contrast and live (green) and dead (red) stained images showing the morphology of the cells grown on different films. The scale bar represents 50 μm.
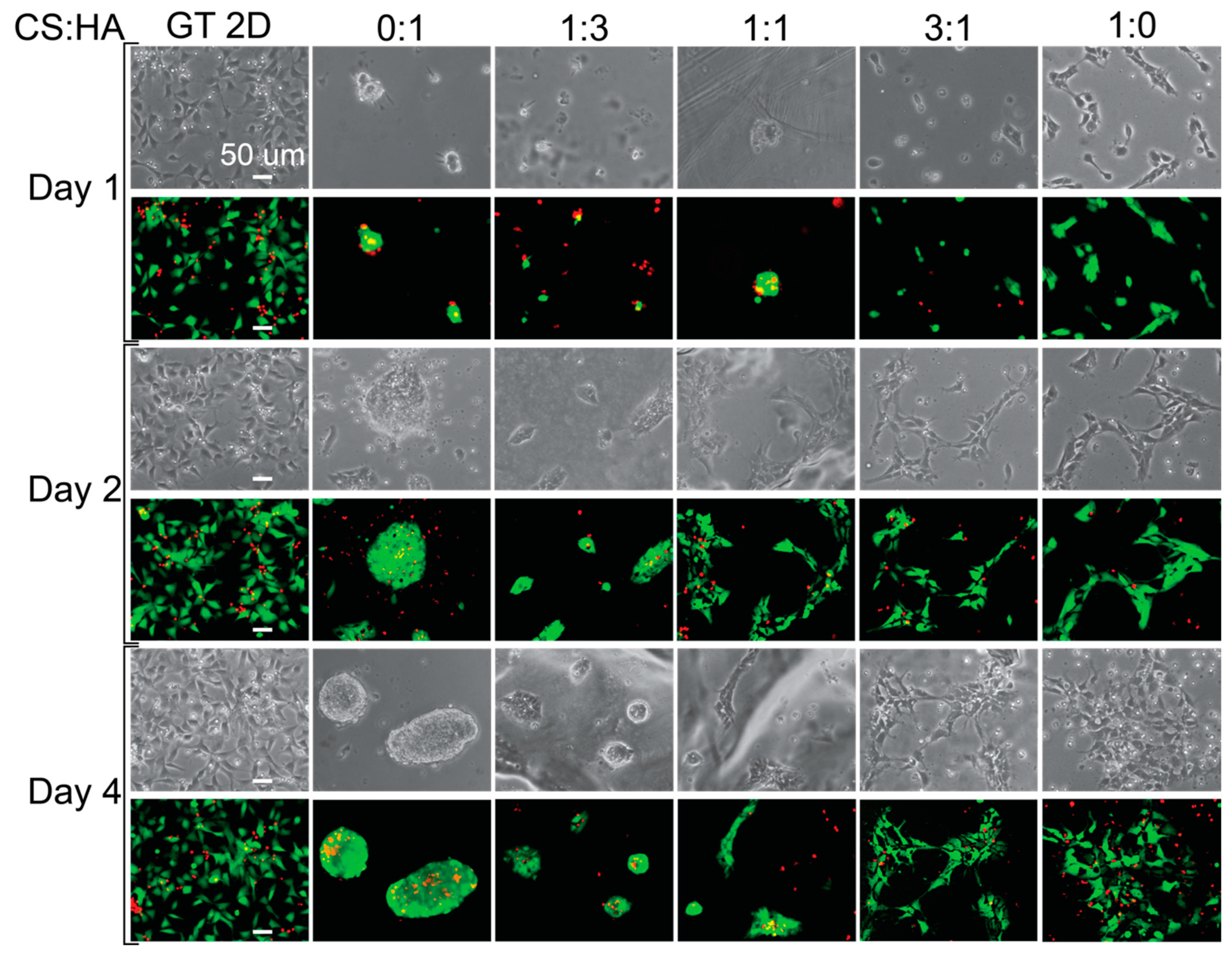
Next, we examined cell morphology on the various CHA films. Figure 4 shows images of cells grown on CHA films taken on days 1, 2, and 4. The cells do not appear to adhere to the pure HA film, likely because of the negative surface charge of the film due to the hydroxide groups. The 1:3 ratio films do not appear adherent on day 1 but appear to be weakly adherent on days 2 and 4, while the 1:1 ratio film does not appear adherent on day 1 but appears to be more adherent on days 2 and 4 than the 1:3 ratio film. This is likely due to the addition of chitosan, which increases the surface charge. The 3:1 and 1:0 films appear more adherent than the films under other conditions, and the pure chitosan film appears more adherent than the 3:1 film on day 1. This is likely due to a favorable interaction between the cells and CS:HA films, with an increasing surface charge as chitosan is added, and increasing interactions between relevant surface receptors and the films, such as CD44 and RHAMM [76]. Even though we added negatively charged hydroxides with HA, we also added various surface receptors, which have been shown to enhance cellular activities [76,77]. These surface receptors enhance cell adhesion, making CS:HA films better substrates than CS:A films for cell adhesion.
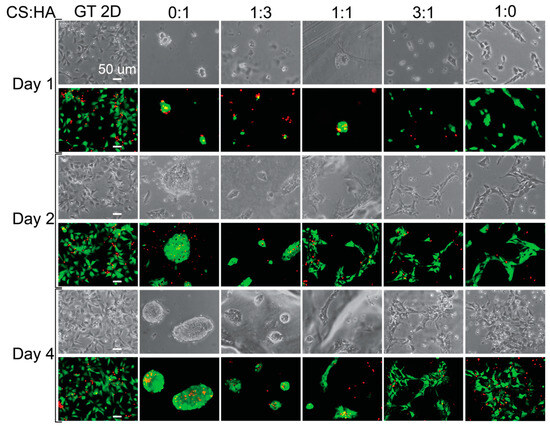
Figure 4.
Cell morphology and live staining of hNSCs grown on CHA films. Phase-contrast and live (green) and dead (red) stained images showing the morphology of cells grown on different films. The scale bar represents 50 μm.
To assess metabolic activity, an alamarBlue assay was conducted on days 1, 2, and 4. Cell viability is directly proportional to metabolic activity [78]. This study investigated the effect of varying CS:A ratios on hNSCs. The data shown in Figure 5a indicate a strong trend in which as chitosan is added to the film, metabolic activity increases. A consistent increase in cell proliferation was observed across all film conditions over the four-day period, with the pure CS film demonstrating the most significant overall proliferation.
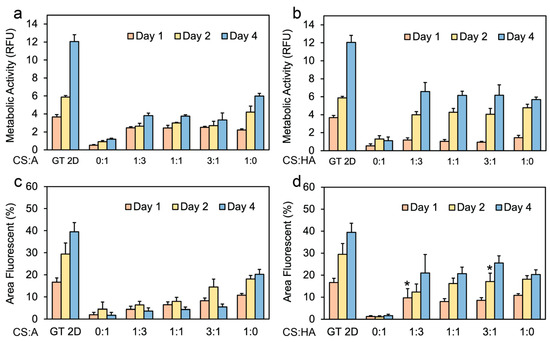
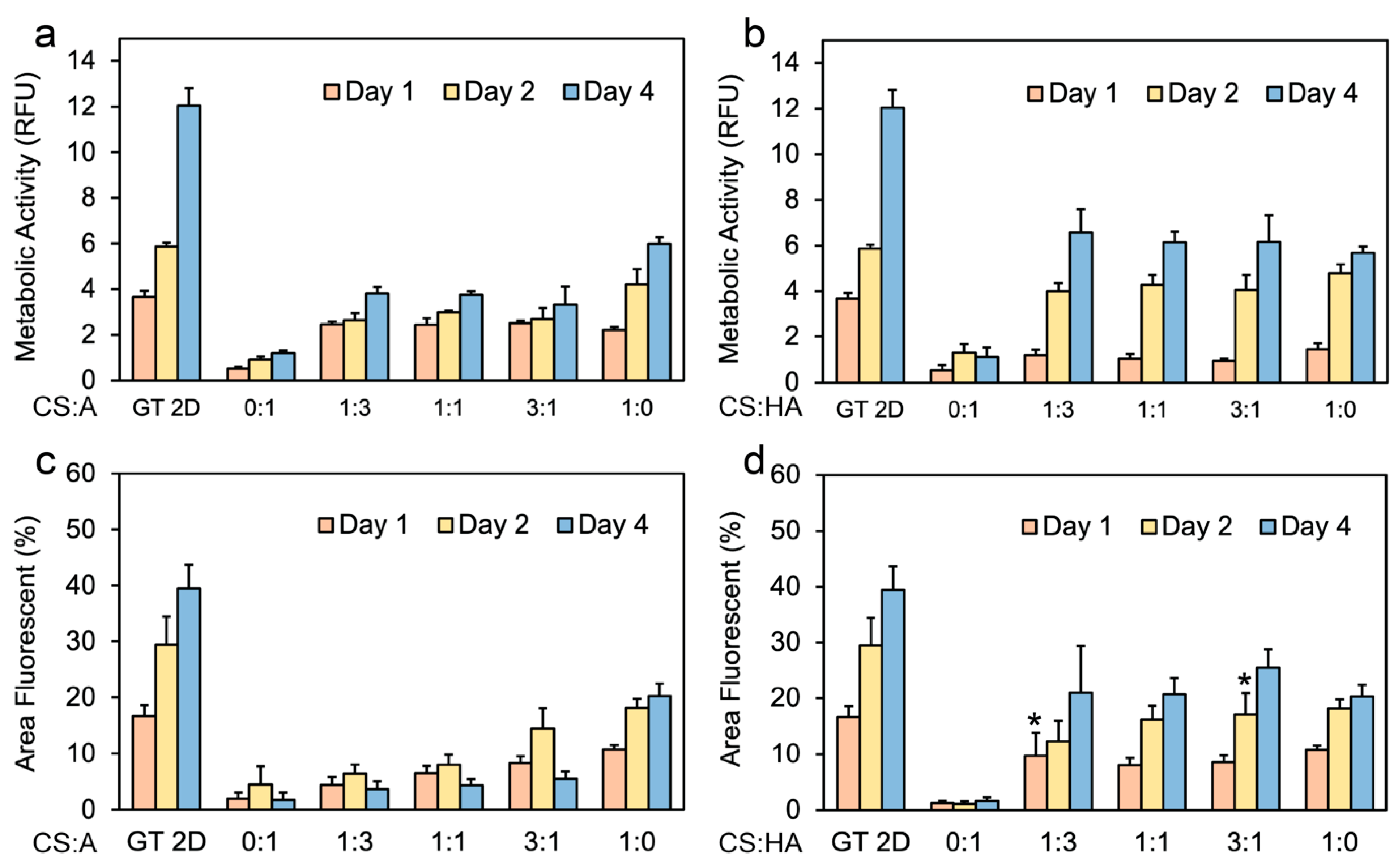
Figure 5.
Cell metabolic activity on CS:A and CS:HA films. Graphs illustrating metabolic activity of hNSCs on (a) CS:A films and (b) CS:HA films with n = 6. Percentage of live stained area on (c) CS:A films and (d) CS:HA films with n = 4. A p-value of less than 0.05 was observed for all samples when compared to the 2D control at the same time point except for samples labeled with *.
Figure 5b shows the metabolic activity data for CHA films. All CHA films exhibited an increase in metabolic activity over the four-day period, with the exception of the 0:1 condition, which exhibited a decrease in cell proliferation between days 2 and 4. This decrease is likely attributable to necrosis within the formed spheroids, or cell loss during routine medium changes or characterization procedures. It should be noted that in the conditions where the cells grew as spheroids, metabolic activity may not be accurate because not all of the cells were directly exposed to the medium, and the cores of the spheroids may have started to become necrotic. The spheroids were not dissociated into single cells before measurement because this may also affect metabolic activity.
Figure 5c shows the percentage of the area of CA films exhibiting Calcien AM staining, indicative of viable cells. The results largely corroborate previous observations in Figure 3 and Figure 4, despite a notable exception: a decline in viable cell area is observed between days 2 and 4 for all CA films, save for the pure CS film, which demonstrates an increase during this period. Figure 5d illustrates the relationship between the percentage of fluorescent area and time for live stained CHA films. Here, a consistent increase across all time points is observed for all films, aligning with our previous observations of enhanced cell adhesion as seen in Figure 3 and Figure 4. As anticipated, the Geltrex control consistently outperformed all other culture methods in terms of cell proliferation and overall fluorescent area.
While the pure CS films demonstrated the highest overall proliferation compared to CA films, no discernible difference in proliferation was observed between the CHA films with ratios of 1:3, 1:1, and 3:1 when compared to the pure CS film. This favorable interaction with the CHA films can be attributed to either the incorporation of surface receptors or the unique mechanical properties of these films. These results agree with the observed trends in cell adhesion in Figure 3 and Figure 4. In a previous study, rat Schwann cells were grown on chitosan films for 7 days, showing that depending on the assay used (metabolic activity or proliferation), the control samples had higher metabolic activity than the chitosan films [79].
Following a four-day culture period on their respective films, cells were collected and stained for flow cytometry analysis. The observed trends in SOX2 (a pluripotency marker responsible for gene regulation [80]) and Ki67 (a proliferation marker), as seen in Figure 6a, corroborate findings from both live/dead imaging and viability assays as seen in Figure 3, Figure 4 and Figure 5.
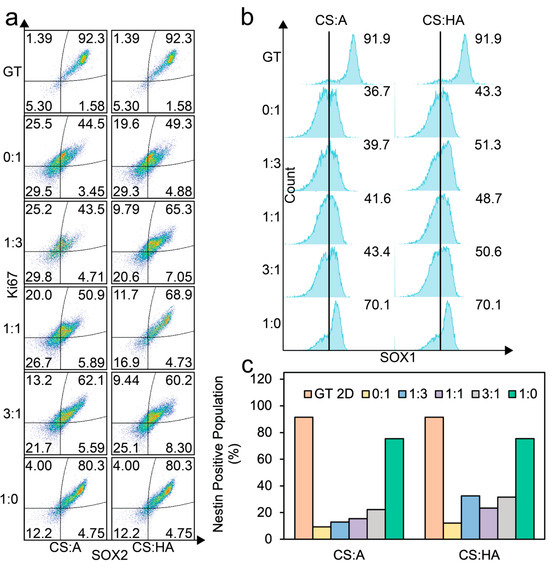
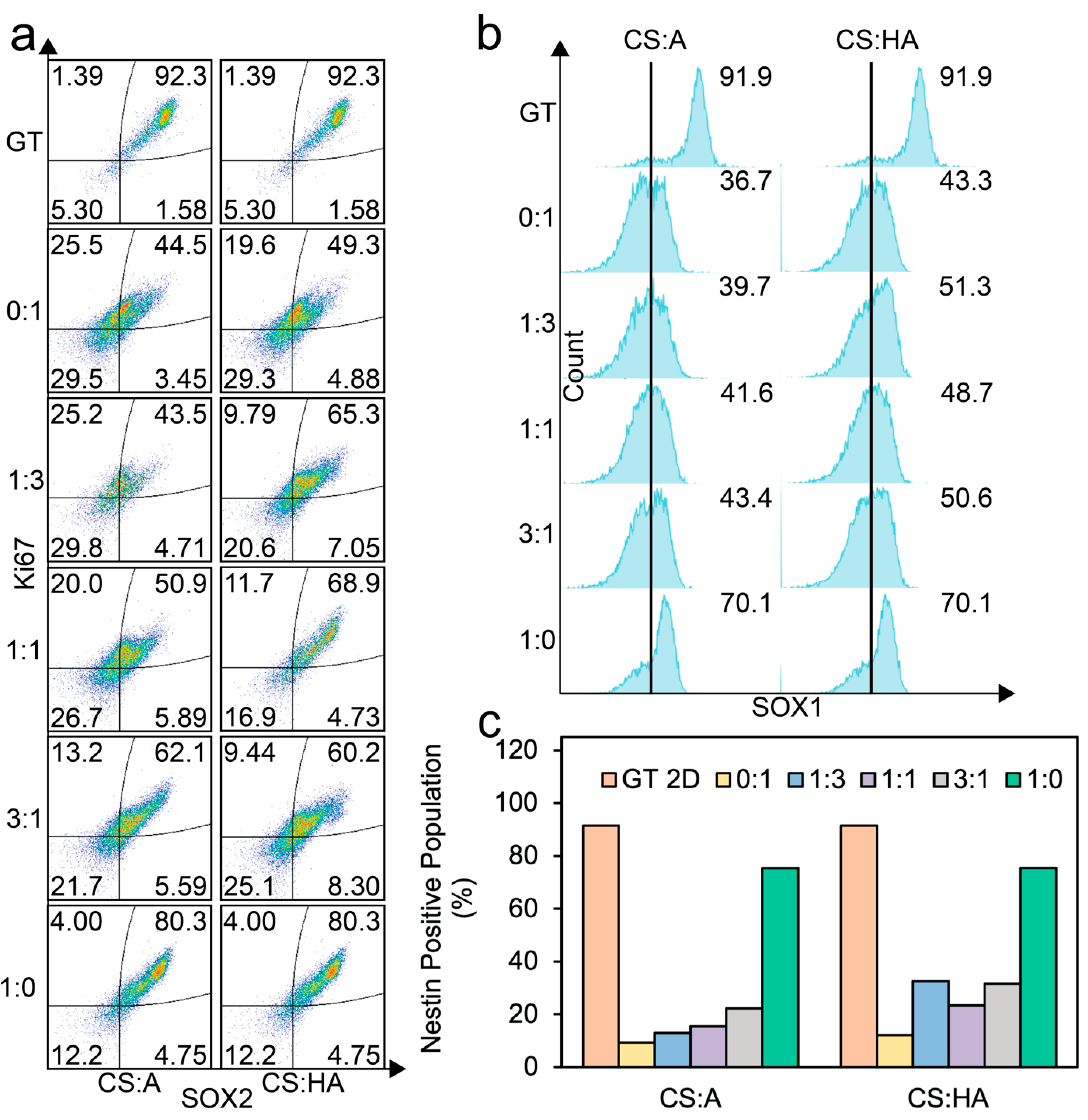
Figure 6.
Flow cytometry analysis of cells cultured on CS:A and CS:HA films. (a) Dot plots of SOX2 and Ki67 expression. (b) SOX1-positive populations. (c) Nestin-positive populations. At least 5500 cells were analyzed for each population.
The double-positive population for SOX2 and Ki67 generally increased as the chitosan content in the films increased for both the CA and CHA films. A minimal double-positive population was observed at a CS:A ratio of 1:3. In contrast, the CHA films exhibited a local maximum in the SOX2/Ki67 double-positive population at a CS:HA ratio of 1:1, indicating an optimal ratio where the negative impact from increasing HA, which has negative charge, is outweighed by the increased presentation of surface receptors. Notably, the pure alginate film demonstrated the lowest expression of this double-positive population, likely due to poor adhesion of the cells to the film surface.
Figure 6b shows the SOX1-positive population as histograms. For the CA films, SOX1 expression increases as chitosan concentration increases, while for the CHA films, we see an initial increase in SOX1 expression when chitosan is added, and then a large increase can be seen in the pure chitosan condition.
Figure 6c illustrates the expression of nestin, an intermediate filament protein. A significant increase in nestin expression was observed in the pure CS condition compared to other film conditions. In the CA films, nestin expression progressively increased with increasing chitosan content. However, for CHA, the film with the highest nestin expression, other than the pure chitosan film, was observed in the 1:3 ratio condition. The nestin expression decreased at the 1:1 ratio and then increased in the 3:1 and pure CS conditions. This trend contrasts with the observed behavior of the SOX2/Ki67 double-positive populations, where a local maximum was observed at a 1:1 CS:HA ratio. This discrepancy may be attributed to the influence of altered film mechanical properties on cellular cytoskeleton organization.
These findings collectively indicate that increasing chitosan content in the CA and CHA films positively affects cell proliferation and the expression of key hNSC markers. Across all markers, the pure CS films demonstrated superior performance, while the Geltrex control consistently exhibited the highest overall expression levels. While cells growing as spheroids on the films did not have high levels of multipotency expression, they could potentially be beneficial for hNSC differentiation [81,82,83].
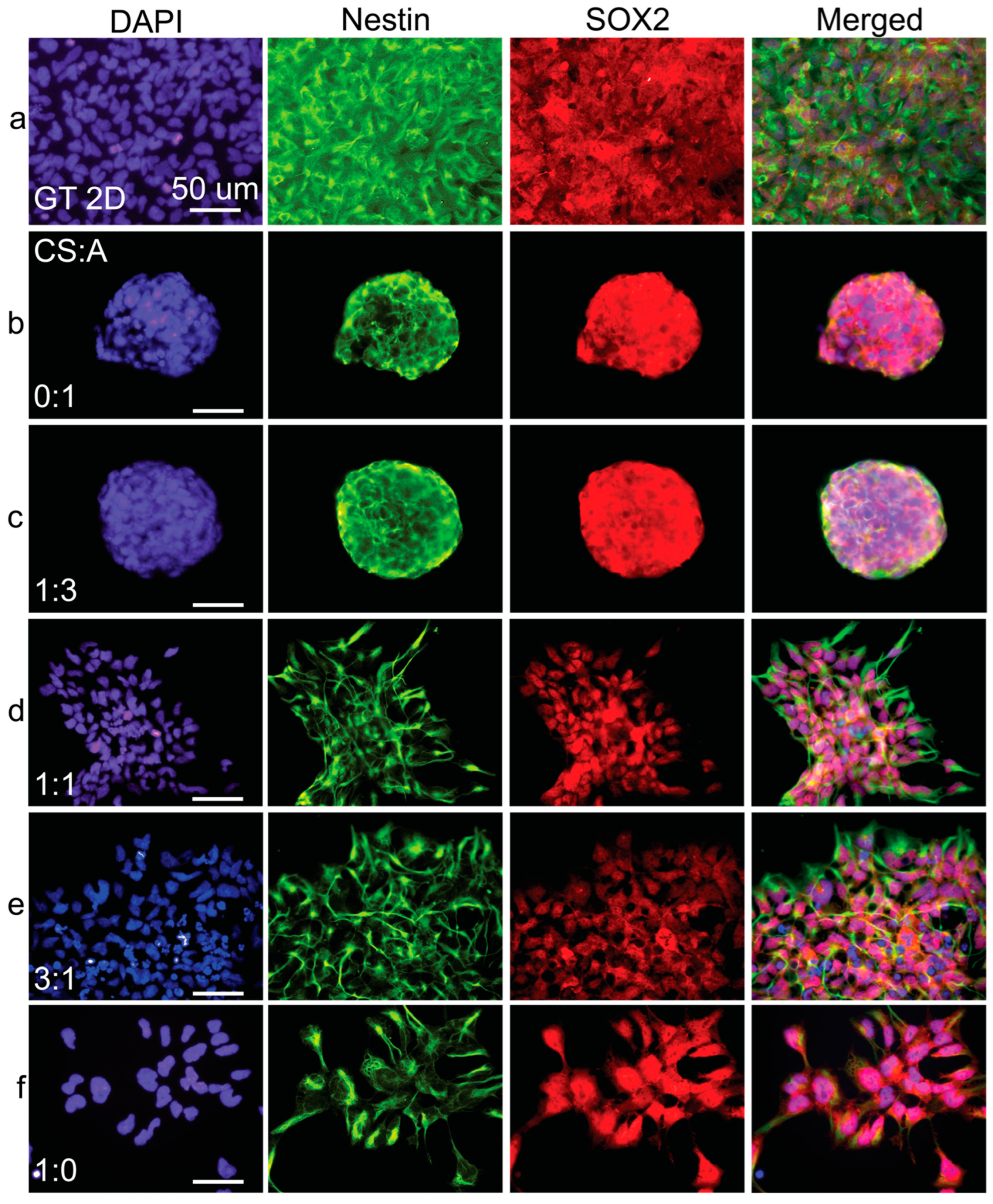
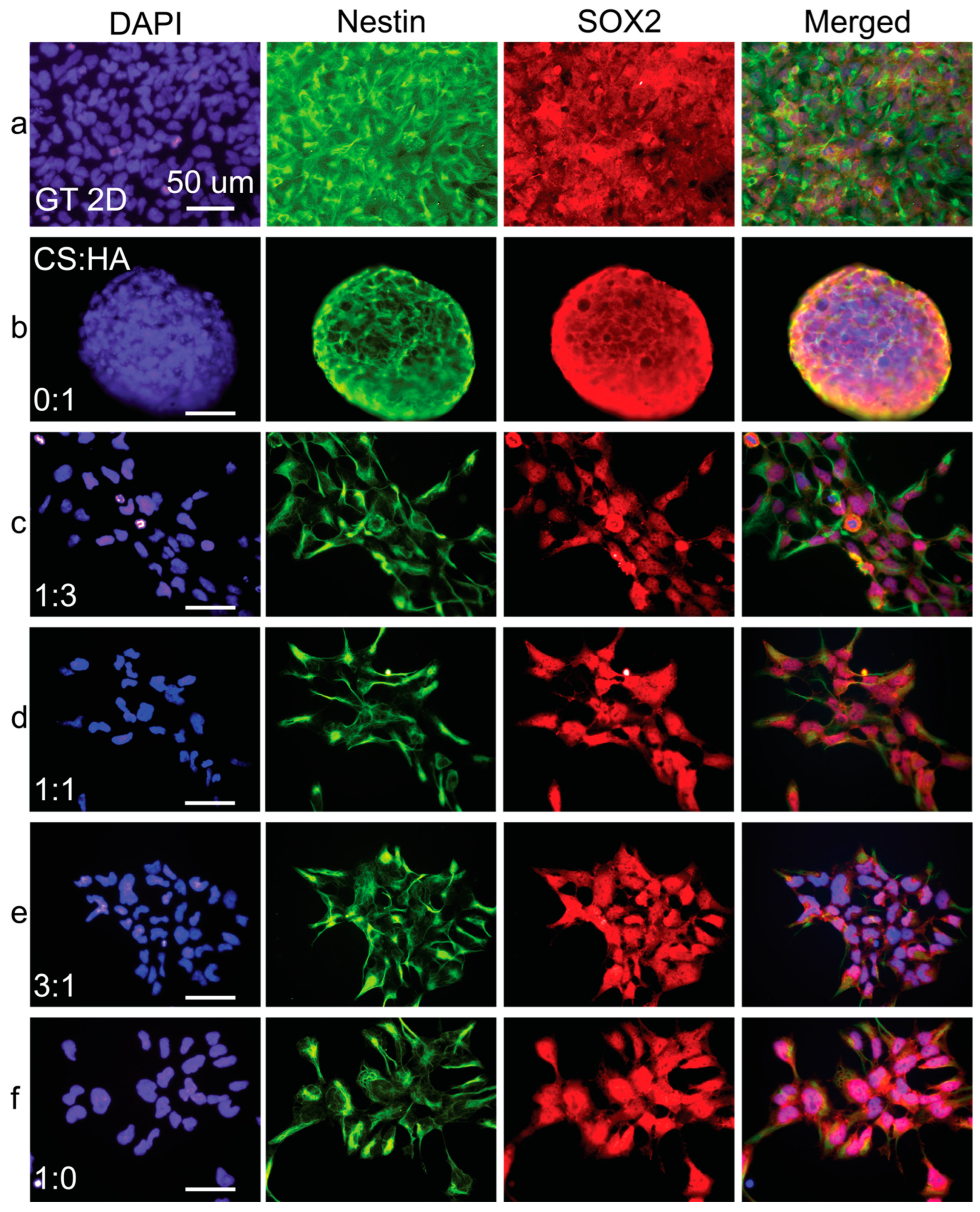
Immunofluorescence imaging revealed a notable trend: as the concentration of either alginate (Figure 7) or HA (Figure 8) increased in the films, nestin expression became progressively less intense and exhibited a more uneven distribution. This downregulation of nestin expression strongly suggests a decline in the overall health and differentiation status of the hNSCs as alginate or HA content increased.
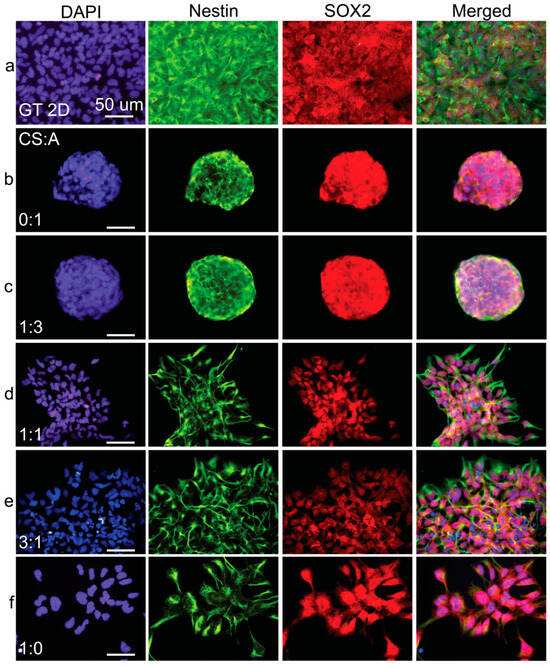
Figure 7.
Immunostaining analysis of cells cultured on CA films. Fluorescent images of (a) Geltrex control and films with CS:A ratios of (b) 0:1, (c) 1:3, (d) 1:1, (e) 3:1, and (f) 1:0. hNSC-H14 cells were stained for nestin (green), SOX2 (red), and nuclei (DAPI, blue). The scale bars are 50 µm.
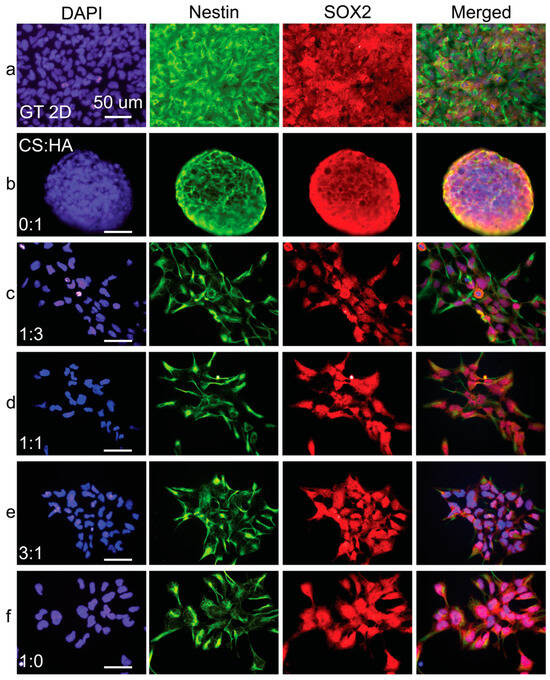
Figure 8.
Immunostaining analysis of hNSC-H14 cells cultured on CHA films. Fluorescent images of (a) Geltrex control and films with CS:HA ratios of (b) 0:1, (c) 1:3, (d) 1:1, (e) 3:1, and (f) 1:0. Cells were stained for nestin (green), SOX2 (red), and nuclei (DAPI, blue). The scale bars are 50 µm.
In contrast, immunofluorescence micrographs of pure CS films demonstrated the prominent nuclear translocation of SOX2, a critical transcription factor supporting hNSC multipotency and self-renewal. However, in the film conditions with increased alginate or HA ratios, SOX2 expression appeared to be predominantly localized in the cytoplasm rather than the nucleus. This cytoplasmic localization suggests a potential loss of long-term self-renewal and multipotency capabilities in hNSCs cultured on these substrates. Supplementary Figures S1 and S2 show PAX6 and Ki67 expression in cells grown on CS:A and CS:HA films, respectively. The results seen here corroborate the previously described trends.
4. Conclusions
This study investigated the influence of varying chitosan (CS) ratios within chitosan–alginate (CA) and chitosan–hyaluronic acid (CHA) films on human neural stem cell (hNSC) fate. The fabricated films exhibited consistent topography, favorable hydrophilicity, and a wide range of mechanical properties, enabling a systematic exploration of the impact of polymer composition on hNSC behavior. The 2D nature of these films allows for the clear visualization and imaging of the cultured hNSCs. Our findings demonstrated that pure CS films provided the most conducive environment for hNSC adhesion and proliferation, and the maintenance of multipotency, as evidenced by light optical imaging, live/dead staining, alamarBlue assays, flow cytometry, and immunostaining. This superior performance was observed despite the absence of any exogenous extracellular matrix proteins like laminin or fibronectin in the pure CS films.
The incorporation of chitosan into both alginate and HA films consistently resulted in enhanced cell attachment, proliferation, and maintenance of multipotency compared to films composed solely of alginate or HA. Conversely, pure alginate or HA films predominantly induced the formation of cell spheroids. While most chitosan-containing films supported adherent cell populations for up to 4 days, except for the CA 1:3 ratio film, the HA films generally exhibited a higher degree of cell adhesion.
This study represents the first systemic comparison of varying ratios of chitosan to alginate and chitosan to hyaluronic acid and their effect on the multipotency of hNSCs. Collectively, our findings indicate that pure CS surfaces offer a more favorable microenvironment for hNSCs compared to hybrid CA and CHA materials in terms of cell adhesion, proliferation, and the preservation of multipotency. Furthermore, this study establishes a xeno-free and chemically defined 2D culture substrate that possesses significant potential for migrating to 3D systems for future culture applications, specifically bioprinting. The results presented here can be used to identify optimal properties based on applications in bioprinting. For applications where hNSC adhesion is desirable, pure chitosan or some of the higher ratios (3:1, 1:1, and 1:3 for CHA and 3:1 and 1:1 for CA) are beneficial. For nonadherent or weakly adherent applications, pure HA or alginate, or the 1:3 ratio for CA, could be used. For nonadherent applications, the pore size of the 3D substrate will be important, as if the pore size is too large, it will allow the spheroids to grow to too large a size and they will suffer from necrotic cores due to the inability of nutrients to reach the core. Pore sizes will need to be tailored to prevent spheroids from becoming too large.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/coatings15040473/s1, Figure S1: DAPI, PAX6, and Ki67 staining on cells grown on CA thin films; Figure S2: DAPI, PAX6, and Ki67 staining on cells grown on CHA thin films.
Author Contributions
Conceptualization, M.Z.; methodology, M.Z., M.J., Y.Z., and F.-C.C.; software, M.J.; validation, M.J. and Y.Z.; formal analysis, M.J. and Y.Z.; investigation, M.J. and Y.Z.; resources, M.Z.; data curation, M.J. and Y.Z.; writing—original draft preparation, M.J.; writing—review and editing, M.Z., M.J. and Y.Z.; visualization, M.J. and Y.Z.; supervision, M.Z.; project administration, M.Z. and F.-C.C.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided through NIHR56DE032720. We also express our sincere appreciation for access to state-of-the-art equipment at Nanoengineering & Science Institute and Molecular Engineering & Science Institute, facilitated by the NSF grant NNCI-1542101.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, C.-L.; Huang, T.; Wu, B.-L.; He, W.-X.; Liu, D. Stem cells in cancer therapy: Opportunities and challenges. Oncotarget 2017, 8, 75756. [Google Scholar] [CrossRef]
- Benmelouka, A.Y.; Munir, M.; Sayed, A.; Attia, M.S.; Ali, M.M.; Negida, A.; Alghamdi, B.S.; Kamal, M.A.; Barreto, G.E.; Ashraf, G.M. Neural stem cell-based therapies and glioblastoma management: Current evidence and clinical challenges. Int. J. Mol. Sci. 2021, 22, 2258. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- McGinley, L.M.; Kashlan, O.N.; Bruno, E.S.; Chen, K.S.; Hayes, J.M.; Kashlan, S.R.; Raykin, J.; Johe, K.; Murphy, G.G.; Feldman, E.L. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer’s disease. Sci. Rep. 2018, 8, 14776. [Google Scholar] [CrossRef]
- Fleifel, D.; Rahmoon, M.A.; AlOkda, A.; Nasr, M.; Elserafy, M.; El-Khamisy, S.F. Recent advances in stem cells therapy: A focus on cancer, Parkinson’s and Alzheimer’s. J. Genet. Eng. Biotechnol. 2018, 16, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-H.; Ji, W.-L.; Chen, H.; Sun, Y.-Y.; Zhao, X.-Y.; Wang, F.; Shi, Y.; Hu, Y.-N.; Liu, B.-X.; Wu, J.-w. Intranasal transplantation of human neural stem cells ameliorates Alzheimer’s disease-like pathology in a mouse model. Front. Aging Neurosci. 2021, 13, 650103. [Google Scholar] [CrossRef] [PubMed]
- Apodaca, L.A.; Baddour, A.A.D.; Garcia, C.; Alikhani, L.; Giedzinski, E.; Ru, N.; Agrawal, A.; Acharya, M.M.; Baulch, J.E. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Kim, S.W.; Woo, H.-J.; Kim, E.H.; Kim, H.S.; Suh, H.N.; Kim, S.-h.; Song, J.-J.; Wulansari, N.; Kang, M.; Choi, S.-Y. Neural stem cells derived from human midbrain organoids as a stable source for treating Parkinson’s disease: Midbrain organoid-NSCs (Og-NSC) as a stable source for PD treatment. Prog. Neurobiol. 2021, 204, 102086. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, Y.; Lee, H.J.; Hwang, D.W.; Lee, D.S. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 2022, 20, 198. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Xu, Z.; Fang, F.; Xu, R.; Wang, Y.; Hu, X.; Fan, L.; Liu, H. Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J. Transl. Med. 2013, 11, 21. [Google Scholar] [CrossRef]
- Lv, Z.; Li, Y.; Wang, Y.; Cong, F.; Li, X.; Cui, W.; Han, C.; Wei, Y.; Hong, X.; Liu, Y. Safety and efficacy outcomes after intranasal administration of neural stem cells in cerebral palsy: A randomized phase 1/2 controlled trial. Stem Cell Res. Ther. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, J.; Li, X.; Xu, H.; Wang, W.; Wang, L.; Zhao, X.; Li, W.; Jiao, J.; Hu, B. Treatment of multiple sclerosis by transplantation of neural stem cells derived from induced pluripotent stem cells. Sci. China Life Sci. 2016, 59, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Halassy, S.; Kojan, S.; Feinstein, D.L.; Chaudhry, G.R. Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Stem Cell Res. Ther. 2021, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Genchi, A.; Brambilla, E.; Sangalli, F.; Radaelli, M.; Bacigaluppi, M.; Furlan, R.; Andolfo, A.; Drago, D.; Magagnotti, C.; Scotti, G.M. Neural stem cell transplantation in patients with progressive multiple sclerosis: An open-label, phase 1 study. Nat. Med. 2023, 29, 75–85. [Google Scholar] [CrossRef]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sgaravizzi, G.; Projetti Pensi, M.; Muzi, G.; Ricciolini, C.; Rota Nodari, L.; Carletti, S.; Giorgi, C. Human neural stem cell transplantation in ALS: Initial results from a phase I trial. J. Transl. Med. 2015, 13, 17. [Google Scholar] [CrossRef]
- Kassi, A.A.Y.; Mahavadi, A.K.; Clavijo, A.; Caliz, D.; Lee, S.W.; Ahmed, A.I.; Yokobori, S.; Hu, Z.; Spurlock, M.S.; Wasserman, J.M. Enduring neuroprotective effect of subacute neural stem cell transplantation after penetrating TBI. Front. Neurol. 2019, 9, 1097. [Google Scholar] [CrossRef]
- Hayashi, Y.; Lin, H.-T.; Lee, C.-C.; Tsai, K.-J. Effects of neural stem cell transplantation in Alzheimer’s disease models. J. Biomed. Sci. 2020, 27, 29. [Google Scholar] [CrossRef]
- Pluchino, S.; Smith, J.A.; Peruzzotti-Jametti, L. Promises and limitations of neural stem cell therapies for progressive multiple sclerosis. Trends Mol. Med. 2020, 26, 898–912. [Google Scholar] [CrossRef]
- Smith, M.J.; Paton, M.C.B.; Fahey, M.C.; Jenkin, G.; Miller, S.L.; Finch-Edmondson, M.; McDonald, C.A. Neural stem cell treatment for perinatal brain injury: A systematic review and meta-analysis of preclinical studies. Stem Cells Transl. Med. 2021, 10, 1621–1636. [Google Scholar] [CrossRef]
- Sterneckert, J.L.; Reinhardt, P.; Schöler, H.R. Investigating human disease using stem cell models. Nat. Rev. Genet. 2014, 15, 625–639. [Google Scholar] [CrossRef]
- Ghourichaee, S.S.; Leach, J.B. The effect of hypoxia and laminin-rich substrates on the proliferative behavior of human neural stem cells. J. Mater. Chem. B 2016, 4, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative evaluation of chitosan, cellulose acetate, and polyethersulfone nanofiber scaffolds for neural differentiation. Carbohydr. Polym. 2014, 99, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Chang, F.-C.; James, M.; Zhou, Y.; Zhang, M. Chitosan Scaffolds as Microcarriers for Dynamic Culture of Human Neural Stem Cells. Pharmaceutics 2023, 15, 1957. [Google Scholar] [CrossRef]
- Bai, T.; Duan, H.; Zhang, B.; Hao, P.; Zhao, W.; Gao, Y.; Yang, Z.; Li, X. Neuronal differentiation and functional maturation of neurons from neural stem cells induced by bFGF-chitosan controlled release system. Drug Deliv. Transl. Res. 2023, 13, 2378–2393. [Google Scholar] [CrossRef]
- Chang, F.-C.; James, M.M.; Qassab, A.M.; Zhou, Y.; Ando, Y.; Shi, M.; Zhang, M. 3D chitosan scaffolds support expansion of human neural stem cells in chemically defined condition. Matter 2023, 6, 3631–3660. [Google Scholar] [CrossRef]
- Chang, F.C.; Zhou, Y.; James, M.M.; Zareie, H.M.; Ando, Y.; Yang, J.; Zhang, M. Effect of degree of deacetylation of chitosan/chitin on human neural stem cell culture. Macromol. Biosci. 2023, 23, 2200389. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Busilacchi, A.; Gigante, A.; Mattioli-Belmonte, M.; Manzotti, S.; Muzzarelli, R.A.A. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr. Polym. 2013, 98, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Sivashankari, P.R.; Prabaharan, M. Prospects of chitosan-based scaffolds for growth factor release in tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.T.; Di Lisa, D.; Massobrio, P.; Colistra, N.; Pesce, M.; Catelani, T.; Dellacasa, E.; Raiteri, R.; Martinoia, S.; Pastorino, L. Soft chitosan microbeads scaffold for 3D functional neuronal networks. Biomaterials 2018, 156, 159–171. [Google Scholar] [CrossRef]
- Revkova, V.A.; Grebenik, E.A.; Kalsin, V.A.; Demina, T.S.; Bardakova, K.N.; Shavkuta, B.S.; Melnikov, P.A.; Samoilova, E.M.; Konoplyannikov, M.A.; Efremov, Y.M. Chitosan-g-oligo (L, L-lactide) copolymer hydrogel potential for neural stem cell differentiation. Tissue Eng. Part A 2020, 26, 953–963. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef]
- Majid; Bagher, Z.; Kamrava, S.K.; Ehterami, A.; Alizadeh, R.; Farhadi, M.; Falah, M.; Komeili, A. Alginate/chitosan hydrogel containing olfactory ectomesenchymal stem cells for sciatic nerve tissue engineering. J. Cell. Physiol. 2019, 234, 15357–15368. [Google Scholar] [CrossRef]
- Mattotti, M.; Alvarez, Z.; Delgado, L.; Mateos-Timoneda, M.A.; Aparicio, C.; Planell, J.A.; Alcántara, S.; Engel, E. Differential neuronal and glial behavior on flat and micro patterned chitosan films. Colloids Surf. B Biointerfaces 2017, 158, 569–577. [Google Scholar] [CrossRef]
- Yan, X.L.; Khor, E.; Lim, L.Y. Chitosan-alginate films prepared with chitosans of different molecular weights. J. Biomed. Mater. Res. 2001, 58, 358–365. [Google Scholar] [CrossRef]
- Verma, D.; Desai, M.S.; Kulkarni, N.; Langrana, N. Characterization of surface charge and mechanical properties of chitosan/alginate based biomaterials. Mater. Sci. Eng. C 2011, 31, 1741–1747. [Google Scholar] [CrossRef]
- Dennaoui, H.; Chouery, E.; Rammal, H.; Abdel-Razzak, Z.; Harmouch, C. Chitosan/hyaluronic acid multilayer films are biocompatible substrate for Wharton’s jelly derived stem cells. Stem Cell Investig. 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Ren, L.; Yin, S.; Ge, J.; Gao, Q.; Luxbacher, T.; Luo, S. A study on the performance of hyaluronic acid immobilized chitosan film. Biomed. Mater. 2009, 4, 035009. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guan, T.; Zhang, X.; Wang, Z.; Wang, M.; Zhong, W.; Feng, H.; Xing, M.; Kong, J. The effect of layer-by-layer assembly coating on the proliferation and differentiation of neural stem cells. ACS Appl. Mater. Interfaces 2015, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Moattari, M.; Kouchesfehani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M.; Mansouri, K. Chitosan-film associated with mesenchymal stem cells enhanced regeneration of peripheral nerves: A rat sciatic nerve model. J. Chem. Neuroanat. 2018, 88, 46–54. [Google Scholar] [CrossRef]
- Arulmoli, J.; Wright, H.J.; Phan, D.T.T.; Sheth, U.; Que, R.A.; Botten, G.A.; Keating, M.; Botvinick, E.L.; Pathak, M.M.; Zarembinski, T.I. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 43, 122–138. [Google Scholar] [CrossRef]
- Niu, X.; Wei, Y.; Liu, Q.; Yang, B.; Ma, N.; Li, Z.; Zhao, L.; Chen, W.; Huang, D. Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur. Polym. J. 2020, 134, 109861. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Erickson, A.E.; Lan Levengood, S.K.; Sun, J.; Chang, F.C.; Zhang, M. Fabrication and characterization of chitosan–hyaluronic acid scaffolds with varying stiffness for glioblastoma cell culture. Adv. Healthc. Mater. 2018, 7, 1800295. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Jańczewski, D.; Neoh, K.G. Parallel Control over Surface Charge and Wettability Using Polyelectrolyte Architecture: Effect on Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 30552–30563. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Xu, K.; Ganapathy, K.; Andl, T.; Wang, Z.; Copland, J.A.; Chakrabarti, R.; Florczyk, S.J. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 2019, 217, 119311. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. Part A 2004, 71A, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bozza, A.; Coates, E.E.; Incitti, T.; Ferlin, K.M.; Messina, A.; Menna, E.; Bozzi, Y.; Fisher, J.P.; Casarosa, S. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials 2014, 35, 4636–4645. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chian, C.-H. Plasma surface modification effects on biodegradability and protein adsorption properties of chitosan films. Appl. Surf. Sci. 2013, 282, 735–740. [Google Scholar] [CrossRef]
- Murguía-Flores, D.A.; Bonilla-Ríos, J.; Canales-Fiscal, M.R.; Sánchez-Fernández, A. Protein adsorption through Chitosan–Alginate membranes for potential applications. Chem. Cent. J. 2016, 10, 26. [Google Scholar] [CrossRef]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Reed, S.; Wu, B.M. Biological and mechanical characterization of chitosan-alginate scaffolds for growth factor delivery and chondrogenesis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 272–282. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Lopez-Martinez, M.J.; Villasante, A.; Introna, C.; Tornero, D.; Canals, J.M.; Samitier, J. Hyaluronic acid-based bioink improves the differentiation and network formation of neural progenitor cells. Front. Bioeng. Biotechnol. 2023, 11, 1110547. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, S.; Li, Y.; Li, M.; Sun, X.; An, J.; Xu, Q.; Chen, Z.; Wang, Y. Impact of hydrogel stiffness on the induced neural stem cells modulation. Ann. Transl. Med. 2021, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, M.G.; Demina, T.S.; Dregval, O.A.; Gaidar, A.I.; Andreeva, E.R.; Zelenetskii, A.N.; Akopova, T.A.; Markvicheva, E. Macroporous Hyaluronic Acid/Chitosan Polyelectrolyte Complex-Based Hydrogels Loaded with Hydroxyapatite Nanoparticles: Preparation, Characterization and In Vitro Evaluation. Polysaccharides 2022, 3, 745–760. [Google Scholar] [CrossRef]
- Meng, X.; Lu, Y.; Gao, Y.; Cheng, S.; Tian, F.; Xiao, Y.; Li, F. Chitosan/alginate/hyaluronic acid polyelectrolyte composite sponges crosslinked with genipin for wound dressing application. Int. J. Biol. Macromol. 2021, 182, 512–523. [Google Scholar] [CrossRef]
- Yamanlar, S.; Sant, S.; Boudou, T.; Picart, C.; Khademhosseini, A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011, 32, 5590–5599. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Wu, H.; Huang, Y.; Weitz, D.A. Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells. Biomaterials 2020, 248, 120014. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Wysotzki, P.; Sancho, A.; Gimsa, J.; Groll, J. A comparative analysis of detachment forces and energies in initial and mature cell-material interaction. Colloids Surf. B Biointerfaces 2020, 190, 110894. [Google Scholar] [CrossRef]
- Sarker, B.; Rompf, J.; Silva, R.; Lang, N.; Detsch, R.; Kaschta, J.; Fabry, B.; Boccaccini, A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int. J. Biol. Macromol. 2015, 78, 72–78. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Stukel, J.M.; Willits, R.K. Mechanotransduction of neural cells through cell–substrate interactions. Tissue Eng. Part B Rev. 2016, 22, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, M.; Willits, R.K. Mechanotransductive N-cadherin binding induces differentiation in human neural stem cells. Mechanobiol. Med. 2025, 3, 100099. [Google Scholar] [CrossRef]
- Marinval, N.; Chew, S.Y. Mechanotransduction assays for neural regeneration strategies: A focus on glial cells. APL Bioeng. 2021, 5, 1784. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Guo, Z.; Li, D. Effect of molecular weight and concentration of hyaluronan on cell proliferation and osteogenic differentiation in vitro. Biochem. Biophys. Res. Commun. 2015, 465, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Wrobel, S.; Serra, S.C.; Ribeiro-Samy, S.; Sousa, N.; Heimann, C.; Barwig, C.; Grothe, C.; Salgado, A.J.; Haastert-Talini, K. In vitro evaluation of cell-seeded chitosan films for peripheral nerve tissue engineering. Tissue Eng. Part A 2014, 20, 2339–2349. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Laghari, Z.A.; Novoa, C.A.; Hughes, J.; Webster, J.R.; Goodwin, P.E.; Wheatley, S.P.; Scotting, P.J. Sox2 acts as a transcriptional repressor in neural stem cells. BMC Neurosci. 2014, 15, 95. [Google Scholar] [CrossRef]
- Lee, H.-K.; Velazquez Sanchez, C.; Chen, M.; Morin, P.J.; Wells, J.M.; Hanlon, E.B.; Xia, W. Three dimensional human neuro-spheroid model of Alzheimer’s disease based on differentiated induced pluripotent stem cells. PloS ONE 2016, 11, e0163072. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tsai, A.-C.; Yuan, X.; Bejoy, J.; Sart, S.; Ma, T.; Li, Y. Neural differentiation of spheroids derived from human induced pluripotent stem cells–mesenchymal stem cells coculture. Tissue Eng. Part A 2018, 24, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Shang, Y.; Chen, X.; Li, H.; Xu, X.; Liu, W.; Yang, G.; Wang, T.; Gao, X.; Chai, R. Oriented neural spheroid formation and differentiation of neural stem cells guided by anisotropic inverse opals. Front. Bioeng. Biotechnol. 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).