Effect of Surface Passivation on Electrical Properties of Pd-F:SnO2 Thin Films Prepared by Spray Pyrolysis Technique
Abstract
:1. Introduction
2. Materials and Method
2.1. Sample Preparation
2.2. Thin Film Deposition

| S/N | Deposition parameters | Optimized condition |
|---|---|---|
| 1 | Pressure of carrier gas | 1.5 bar |
| 2 | Substrate temperature | 450 ± 10 °C |
| 3 | Flow rate | 4 mL/min |
| 4 | Quantity of spraying solution | 30 cm3 |
| 5 | Nozzle to substrate distant | 33 ± 3 cm |
2.3. Thin Film Annealing

2.4. Thin Film Passivation
2.5. Electrical Characterization

3. Results and Discussion
3.1. Sheet Resistivity


3.2. Comparison of Doped Thin Films

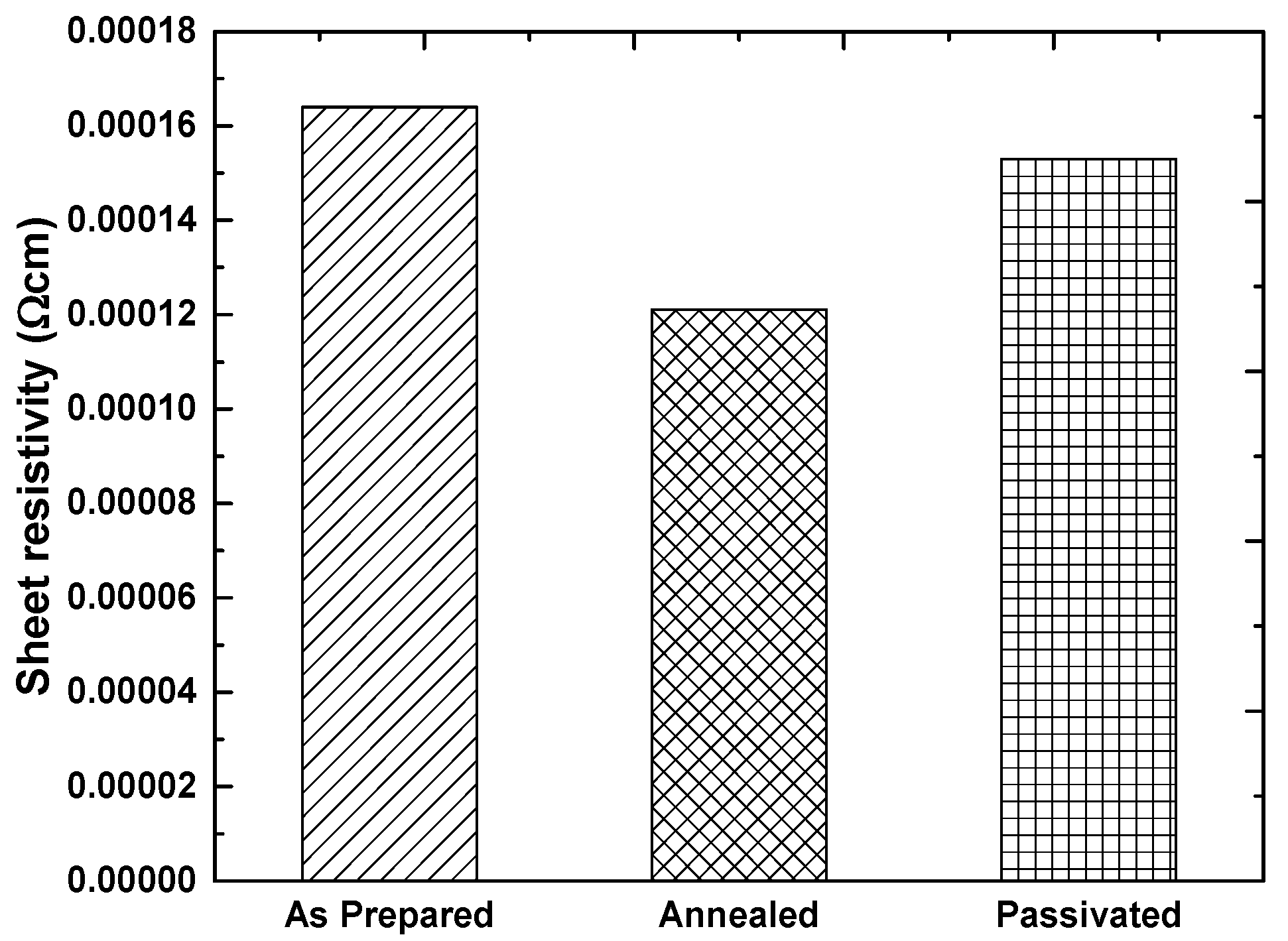
3.3. Post Deposition Treatment of Pd-F:SnO2 Thin Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Young, S.K.; Ansari, S.G.; Ansari, Z.A.; Rizwan, W.; Hyung-Shik, S. A simple method to deposit palladium doped SnO2 thin films using plasma enhanced chemical vapor deposition technique. Rev. Sci. Instrum. 2010, 81. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Chowdhury, S.; Hasan, F.; Begum, T. Optical properties of undoped and indium doped tin oxide thin films. J. Bangladesh Acad. Sci. 2011, 35, 99–111. [Google Scholar] [CrossRef]
- Salehi, H.; Aryadoust, M.; Farbod, M. Electronic and structural properties of tin dioxide in cubic phase. Iran. J. Sci. Technol. Trans. A 2010, 34, 131–138. [Google Scholar]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. Effect of processing on the electrical properties of spray-deposited SnO2:F thin films. Am. J. Appl. Sci. 2008, 5, 672–677. [Google Scholar] [CrossRef]
- Mishra, R.L.; Mishra, S.K.; Prakash, S.G. Optical and gas sensing characteristics of tin-dioxide nano-crystalline thin films. J. Ovonic Res. 2009, 5, 77–85. [Google Scholar]
- Díaz, R. Tin oxide thin films: Electronic properties and growth mechanism under electrochemical control. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2002. [Google Scholar]
- Shamala, K.S.; Murthy, L.C.S.; Rao, K.N. Studies on tin oxide films prepared by electron beam evaporation and spray pyrolysis methods. Bull. Mater. Sci. 2004, 27, 295–301. [Google Scholar] [CrossRef]
- Jebbari, N.; Kamoun, N.; Bennaceur, R. Effect of SnCl4 concentration on F:SnO2, deposited by chemical spray pyrolysis. In the Proceedings of International Renewable Energy Congress, Souse, Tunisia, 5–7 November 2010; pp. 276–279.
- Yousaf, S.A.; Ali, S. The effect of fluorine doping on optoelectronic properties of tin-dioxide (F:SnO2) thin films. Coden. Jnsmac. 2008, 48, 43–50. [Google Scholar]
- Odari, B.V.; Musembi, R.J.; Mageto, M.J.; Othieno, H.; Gaitho, F.; Mghendi, M.; Muramba, V. Optoelectronic properties of F-co-doped PTO thin films deposited by spray pyrolysis. Am. J. Mater. Sci. 2013, 3, 91–99. [Google Scholar] [CrossRef]
- Baco, S.; Chik, A.; Tassin, F.Md. Study on optical properties of tin oxide thin films at different annealing temperatures. J. Sci. Technol. 2012, 4, 61–72. [Google Scholar]
- Miller, T.A.; Bakrania, S.D.; Perez, C.; Woondridge, M.S. Nanostructured Tin Dioxide Materials for Gas Sensor Applications; American Scientific Publishers: Valencia, CA, USA, 2006; pp. 1–24. [Google Scholar]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, Selectivity, and Stability of Gas-Sensitive Metal-Oxide Nanostructures and Their Applications; American Scientific Publishers: Valencia, CA, USA, 2010; pp. 31–52. [Google Scholar]
- Vaezi, M.R. Effects of surface modification on the recovery time and stability of nano structured tin oxide thick films gas sensors. Int. J. Electron. Trans. B Appl. 2007, 20, 1–8. [Google Scholar]
- Adamyan, A.Z.; Adamyan, Z.N.; Aroutiounian, V.M.; Schietbaum, K.D.; Han, S.-D. Improvement and stabilization of thin-film hydrogen sensors parameters. Am. J. Phys. 2009, 2, 200–212. [Google Scholar]
- De Moure-Flores, F.; Guillen-Gervauntes, A.; Nieto-Zapeda, K.E.; Quinones-Galvan, J.G.; Hernandez-Hernandez, A.; Olvera, M.G.; Melendez-Lira, M. SnO2 thin films deposited by RF magnetron sputtering: Effect of the SnF2 amount in the target on the physical properties. Rev. Mex. Fis. 2013, 59, 335–338. [Google Scholar]
- Al-Delaimy, S.M.; Basheer, R.J. Annealing effects on structural and optcal properties of SnO2 thin films. J. Edu. Sci. 2008, 21, 99–109. [Google Scholar]
- Senthilkumara, V.; Vickramana, P.; Joseph Princeb, J.; Jayachandranc, M.; Sanjeevirajad, C. Effects of annealing temperature on structural, optical, and electrical properties of antimony-doped tin oxide thin films. Philos. Mag. Lett. 2010, 90, 337–347. [Google Scholar] [CrossRef]
- Jain, V.K.; Kumar, P.; Vijay, Y.K. Preparation of nanostructure ZnO-SnO2 thin films for optoelectronic properties and post annealing influence. World Acad. Sci. Eng. Technol. 2012, 72, 1575–1577. [Google Scholar]
- Yadav, A.A.; Masumdar, E.U.; Moholkar, A.V.; Rajpure, K.Y.; Bhosale, C.H. Gas sensing of fluorine doped tin oxide thin films prepared by spray pyrolysis. J. Sens. Transducers 2008, 92, 55–60. [Google Scholar]
- Radecka, M.; Zakrzewska, K.; Rekas, M. SnO2-TiO2 solid solutions for gas sensors. Sens. Actuators B 1998, 47, 194–204. [Google Scholar] [CrossRef]
- Ozaki, Y.; Suzuki, S.; Morimitsu, M.; Matsunaga, M. Enhanced long-term stability of SnO2-based CO gas sensors modified by sulfuric acid treatment. Sens. Actuators B 2000, 62, 220–225. [Google Scholar] [CrossRef]
- Scout Thin Film Analysis Software Handbook. W.Theiss Hard- and Software Website. Available online: http://www.wtheiss.com/ (accessed on 14 November 2014).
- Mohammad, T.M. Performance and characteristics of Al-PbS/SnO2: F selective coating system for photothermal energy conversion. Solar Energy Mater. 1990, 20, 297–305. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwathe, P.; Musembi, R.; Munji, M.; Odari, V.; Munguti, L.; Ntilakigwa, A.; Nguu, J.; Muthoka, B. Effect of Surface Passivation on Electrical Properties of Pd-F:SnO2 Thin Films Prepared by Spray Pyrolysis Technique. Coatings 2014, 4, 747-755. https://doi.org/10.3390/coatings4040747
Mwathe P, Musembi R, Munji M, Odari V, Munguti L, Ntilakigwa A, Nguu J, Muthoka B. Effect of Surface Passivation on Electrical Properties of Pd-F:SnO2 Thin Films Prepared by Spray Pyrolysis Technique. Coatings. 2014; 4(4):747-755. https://doi.org/10.3390/coatings4040747
Chicago/Turabian StyleMwathe, Patrick, Robinson Musembi, Mathew Munji, Victor Odari, Lawrence Munguti, Alex Ntilakigwa, John Nguu, and Boniface Muthoka. 2014. "Effect of Surface Passivation on Electrical Properties of Pd-F:SnO2 Thin Films Prepared by Spray Pyrolysis Technique" Coatings 4, no. 4: 747-755. https://doi.org/10.3390/coatings4040747




