A Surface Photovoltage Study of Surface Defects on Co-Doped TiO2 Thin Films Deposited by Spray Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Compositional Analysis
3.2. Modulated Charge Separation
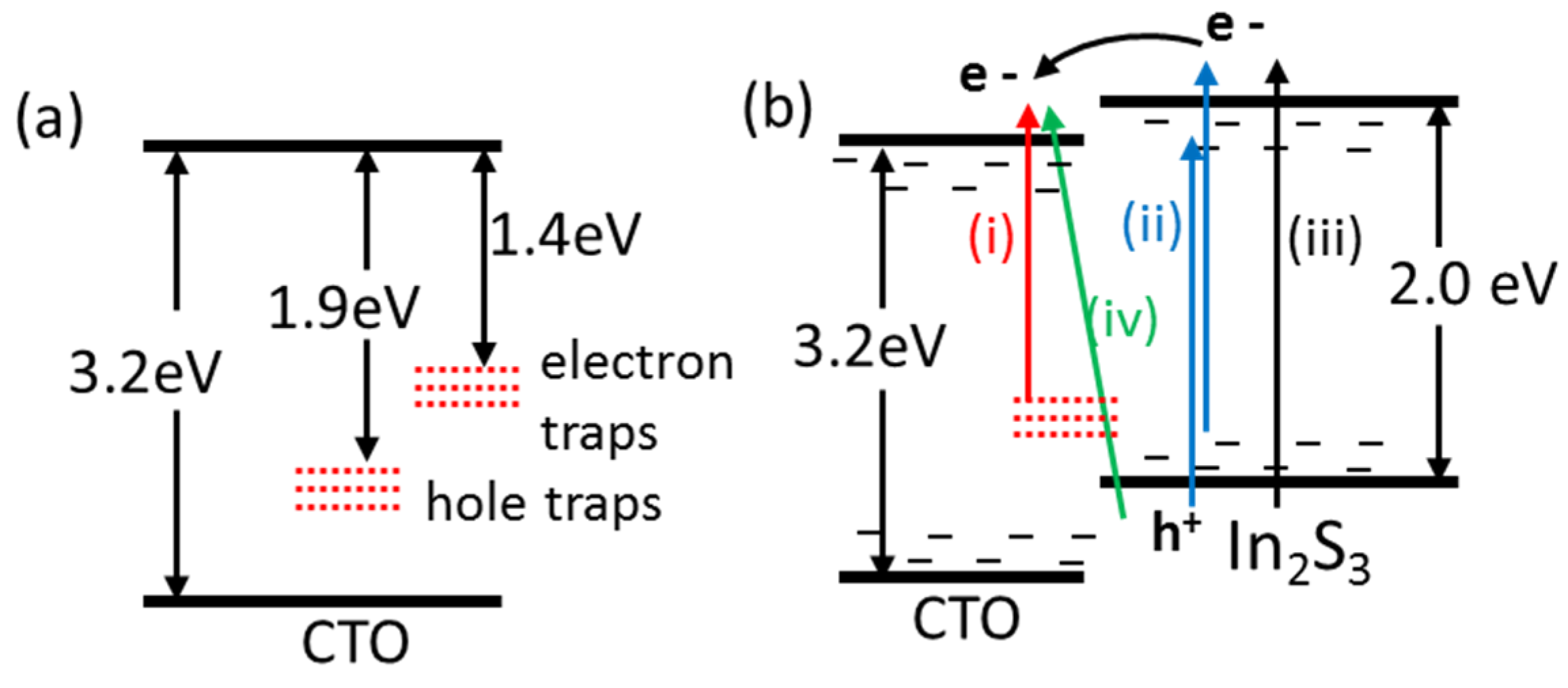
3.3. Band Gap Diagram for CTO/In2S3 Double Layer
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kronik, L.; Shapira, Y. Surface photovoltage phenomena: Theory, experiments and applications. Surf. Sci. Rep. 1999, 37, 1–206. [Google Scholar] [CrossRef]
- Liqianga, J.; Xiaojun, S.; Jing, S.; Weimin, C.; Zili, X.; Yaoguo, D.; Honggang, F. Review of surface photovoltage spectra of nanosized semiconductor and its applications in heterogeneous photocatalysis. Sol. Energy Mater. Sol. Cells 2003, 79, 133–151. [Google Scholar] [CrossRef]
- Zhao, J.; Osterloh, F. Photochemical charge separation in nanocrystal Photocatalyst films: Insights from surface photovoltage spectroscopy. J. Phys. Chem. Lett. 2014, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Rotschild, A.; Levakov, A.; Shapira, Y.; Ashkenasy, N.; Komem, Y. Surface photovoltage spectroscopy study of reduced and oxidized nanocrystalline TiO2 films. Surf. Sci. 2003, 532–535, 456–460. [Google Scholar] [CrossRef]
- Ali, B.; Shah, L.; Ni, C.; Xiao, J.; Shah, S. Interplay of dopant, defects and electronic structure in driving ferromagnetism in Co-doped oxides: TiO2, CeO2 and ZnO. J. Phys. Condens. Mater. 2009, 21, 456005. [Google Scholar] [CrossRef] [PubMed]
- Tatlıdil, I.; Bacaksız, E.; Kurtulus, C.; Buruk, C.; Breen, C.; Sokmen, M. A short literature survey on iron and cobalt ion doped TiO2 thin films and photocatalytic activity of these films against fungi. J. Alloys Compds. 2012, 517, 80–86. [Google Scholar] [CrossRef]
- Mital, G.; Manoj, T. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar]
- García-Mota, M.; Vojvodic, A.; Abild-Pedersen, F.; Nørskov, J. Electronic origin of the surface reactivity of transition-metal-doped TiO2 (110). J. Phys. Chem. 2013, 117, 460–465. [Google Scholar] [CrossRef]
- Barakat, M.; Hayes, G.; Shah, S. Effect of cobalt doping on the phase transformation of TiO2 nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Choudhury, A. Luminescence characteristics of cobalt doped TiO2 nanoparticles. J. Lumin. 2012, 132, 178–184. [Google Scholar] [CrossRef]
- Roberts, K.; Varela, M.; Rashkeev, S.; Pantelides, S.; Pennycook, S.; Krishnan, K. Defect-mediated ferromagnetism in insulating Co-doped anatase TiO2 thin films. Phys. Rev. B 2008, 78, 014409. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Park, B.; Noh, H.; Oh, S.; Yang, J.; Kim, D.; Bu, S.; Noh, T.; Lin, H.; et al. Ferromagnetism induced by clustered Co in Co-doped anatase TiO2 thin films. Phys. Rev. Lett. 2003, 90, 017401. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, G.; Lalitha, K.; Kumari, V.; Shankar, M.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrog. Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Santara, B.; Pal, B.; Giri, P. Signature of strong ferromagnetism and optical properties of Co-doped TiO2 nanoparticles. J. Appl. Phys. 2011, 110, 114322. [Google Scholar] [CrossRef]
- Das, K.; Sharma, S.; Kumar, M.; De, S. Morphology dependent luminescence properties of Co doped TiO2 nanostructures. J. Phys. Chem. 2009, C113, 14783–14792. [Google Scholar] [CrossRef]
- Karimipour, M.; Mageto, M.; Etefagh, R.; Azhir, E.; Mwamburi, M.; Topalian, Z. Room temperature magnetization in Co-doped anatase phase of TiO2. Eur. Phys. J. Appl. Phys. 2013, 61, 10601. [Google Scholar] [CrossRef]
- Sharma, S.; Chaudhary, S.; Kashyap, S. Observation of room temperature ferromagnetism in spray pyrolyzed polycrystalline Ti1−xCoxO2 thin films. J. Phys. D: Appl. Phys. 2010, 43, 015007. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Pérez-Álvarez, J.; Solís-Casados, D.; Camps, E.; Romero, S.; Jiménez-Becerril, J. Preparation of Co:TiO2 thin films by crossed-beam pulsed laser deposition. Appl. Phys. A 2013, 110, 909–913. [Google Scholar] [CrossRef]
- Nguyen, H.; Prellier, W.; Sakai, J.; Ruyter, A. Substrate effects on room temperature ferromagnetism in Co-doped TiO2 thin films grown by pulsed laser deposition. J. Appl. Phys. 2004, 95, 7378–7380. [Google Scholar] [CrossRef]
- Murakami, M.; Matsumoto, Y.; Hasegawa, T.; Ahmet, P.; Nakajima, K.; Chikyow, T.; Ofuchi, H.; Nakai, I.; Koinuma, H. Cobalt valence states and origins of ferromagnetism in Co doped TiO2 rutile thin films. J. Appl. Phys. 2004, 95, 5330–5333. [Google Scholar] [CrossRef]
- Park, W.; Ortega-Hertogs, R.; Moodera, J.; Punnoose, A.; Seehra, M. Semiconducting and ferromagnetic behavior of sputtered Co-doped TiO2 thin films above room temperature. J. Appl. Phys. 2002, 91, 8093–8095. [Google Scholar] [CrossRef]
- Pore, V.; Dimri, M.; Khanduri, H.; Stern, R.; Lu, J.; Hultman, L.; Kukli, K.; Ritala, M.; Leskelä, M. Atomic layer deposition of ferromagnetic cobalt doped titanium oxide thin films. Thin Solid Films 2011, 519, 3318–3324. [Google Scholar] [CrossRef]
- Juma, A.O.; Azarpira, A.; Steigert, A.; Pomaska, M.; Fischer, C.-H.; Lauermann, I.; Dittrich, T. Role of chlorine in In2S3 for band alignment at nanoporous-TiO2/In2S3 interfaces. J. Appl. Phys. 2013, 114, 053711. [Google Scholar] [CrossRef]
- Andreas, K.; Robin, H.; Michael, G. Artificial Photosynthesis Investigation on the Mechanism of Photosensitization of Nanocrystalline TiO2 Solar cells by Chlorophyll Derivatives. J. Phys. Chem. 1994, 98, 952–959. [Google Scholar]
- Duzhko, V.; Timoshenko, V.; Koch, F.; Dittrich, T. Photovoltage in nanocrystalline porous TiO2. Phys. Rev. B 2001, 64, 075204. [Google Scholar] [CrossRef]
- Prajongtat, P.; Dittrich, T. Precipitation of CH3NH3PbCl3 in CH3NH3PbI3 and its impact on modulated charge separation. J. Phys. Chem. C 2015, 119, 9926–9933. [Google Scholar] [CrossRef]
- Chu, W.; Mayer, J.; Nicolet, M. Rutherford Backscattering Spectrometry; Academic Press Inc.: San Diego, CA, USA, 1978. [Google Scholar]
- Jeynes, C.; Barradas, N.P.; Rafla-Yuan, H.; Hichwa, B.P.; Close, R. Accurate depth profiling of complex optical coatings. Surf. Interface Anal. 2000, 30, 237–242. [Google Scholar] [CrossRef]
- Juma, A.O. Copper diffusion in In2S3 and charge separation at In2S3/CuSCN and TiO2/In2S3 interfaces. Ph.D. Thesis, Freie Universitaet, Berlin, Germany, 2013. [Google Scholar]
- Kaushik, A.; Dalela, B.; Kumar, S.; Alvi, P.A.; Dalela, S. Role of Co doping on structural, optical and magnetic properties of TiO2. J. Alloys Compds. 2013, 552, 274–278. [Google Scholar] [CrossRef]
- Kykyneshi, R.; McIntyre, D.H.; Tate, J.; Park, C.H.; Keszler, D.A. Electrical and optical properties of epitaxial transparent conductive BaCuTeF thin films deposited by pulsed laser deposition. Solid State Sci. 2008, 10, 921–927. [Google Scholar] [CrossRef]
- Juma, A.O.; Pistor, P.; Fengler, S.; Dittrich, T.; Wendler, E. Copper diffusion in thin In2S3 layers investigated by Rutherford backscattering spectroscopy. Thin Solid Films 2012, 520, 6740–6743. [Google Scholar] [CrossRef]
- Jin, C.; Liu, B.; Lei, Z.; Sun, J. Structure and photoluminescence of the TiO2 films grown by atomic layer deposition using tetrakis-dimethylamino titanium and ozone. Nano. Res. Lett. 2015, 10, 1–9. [Google Scholar]
- Mercado, C.; Knorr, J.; McHale, L.; Usmani, M.; Ichimura, S.; Saraf, V. Location of Hole and Electron Traps on Nanocrystalline Anatase TiO2. J. Phys. Chem. C. 2012, 116, 10796–10804. [Google Scholar] [CrossRef]
- Strunk, J.; Vining, C.; Bell, T. A study of oxygen vacancy formation and annihilation in sub monolayer coverages of TiO2 Dispersed on MCM-48. J. Phys. Chem. C. 2010, 114, 16937–16945. [Google Scholar] [CrossRef]
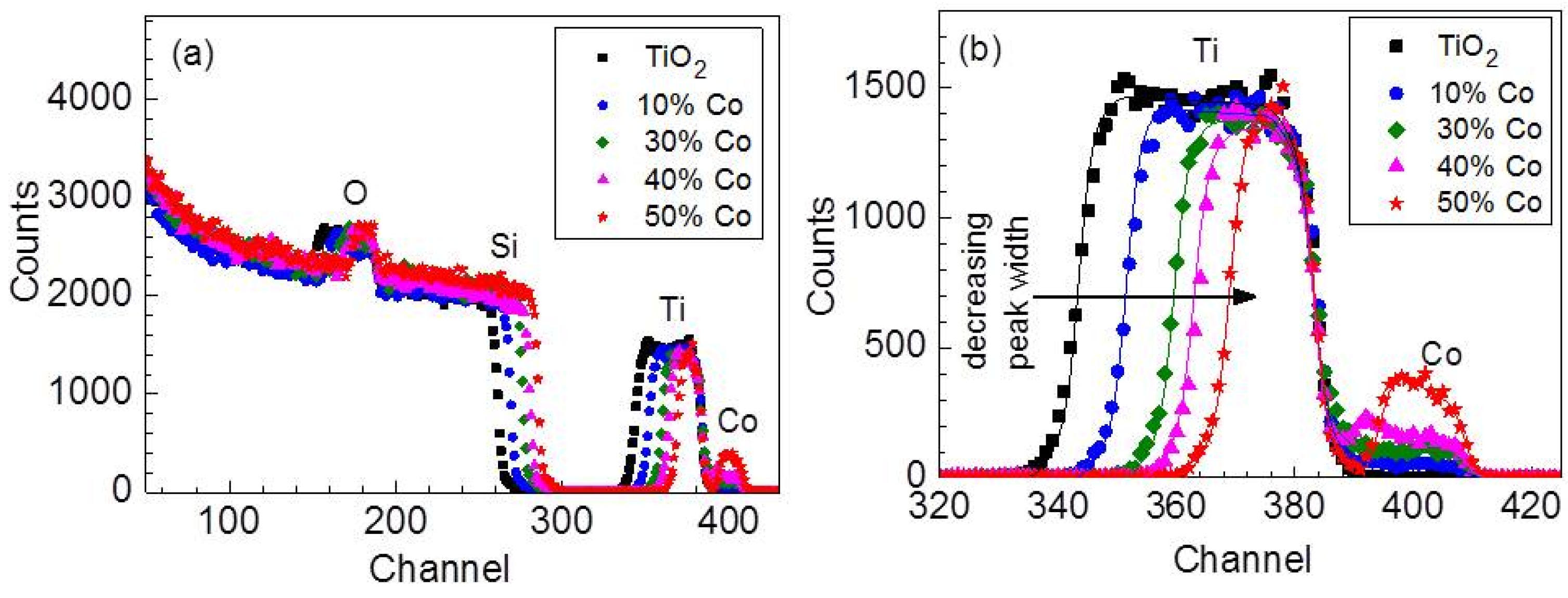


| CoCl2 vol % | Thickness (nm) | Co (at %) |
|---|---|---|
| 0 | 175 | 0 |
| 5 | 175 | 0.43 |
| 10 | 140 | 0.60 |
| 20 | 140 | 1.25 |
| 30 | 105 | 1.50 |
| 40 | 90 | 2.40 |
| 50 | 60 | 4.51 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wafula, H.; Juma, A.; Sakwa, T.; Musembi, R.; Simiyu, J. A Surface Photovoltage Study of Surface Defects on Co-Doped TiO2 Thin Films Deposited by Spray Pyrolysis. Coatings 2016, 6, 30. https://doi.org/10.3390/coatings6030030
Wafula H, Juma A, Sakwa T, Musembi R, Simiyu J. A Surface Photovoltage Study of Surface Defects on Co-Doped TiO2 Thin Films Deposited by Spray Pyrolysis. Coatings. 2016; 6(3):30. https://doi.org/10.3390/coatings6030030
Chicago/Turabian StyleWafula, Henry, Albert Juma, Thomas Sakwa, Robinson Musembi, and Justus Simiyu. 2016. "A Surface Photovoltage Study of Surface Defects on Co-Doped TiO2 Thin Films Deposited by Spray Pyrolysis" Coatings 6, no. 3: 30. https://doi.org/10.3390/coatings6030030
APA StyleWafula, H., Juma, A., Sakwa, T., Musembi, R., & Simiyu, J. (2016). A Surface Photovoltage Study of Surface Defects on Co-Doped TiO2 Thin Films Deposited by Spray Pyrolysis. Coatings, 6(3), 30. https://doi.org/10.3390/coatings6030030





