Biodegradable Ceramics Consisting of Hydroxyapatite for Orthopaedic Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Coatings
2.2. Elemental Composition, Chemical Binding, Morphology and Mechanical Properties
2.3. In Vitro Corrosion Behaviour
2.4. In Vitro Biological Assessment
3. Results and Discussion
3.1. Chemical Binding and Elemental Composition
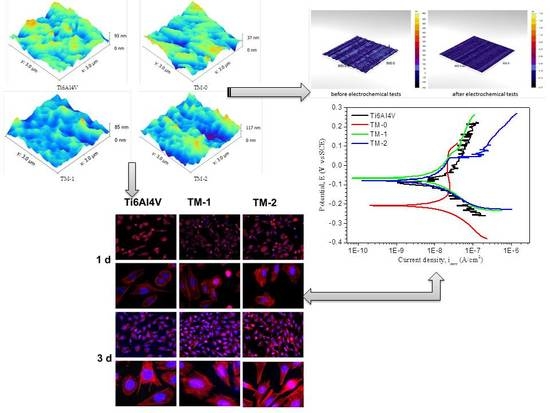
3.2. Morphology
3.3. Mechanical Properties
3.4. In Vitro Electrochemical Behaviour
3.5. In Vitro Biological Properties
4. Conclusions
- Surface roughness of the coatings increases with Mg incorporation into a Ti-doped HAP structure. Additionally, with increasing RF power fed to the MgO cathode there is intensification in the roughness of the coating surface.
- The decrease in the surface roughness plays an important role in increasing the corrosion resistance.
- Corrosion results show that coating obtained at low RF power fed to the MgO target led to a decrease in the current density compared with other coatings and bare alloy, suggesting a high corrosion performance.
- Mg- and Ti-doped HAP coatings obtained at low RF power fed to the MgO target provided the material with high protection efficiency (78.1%) to the corrosive SBF attack, indicating a higher corrosion resistance.
- It can be concluded that the elastic modulus of the coated samples decreases with Mg addition in the HAP structure, especially in the case of the coatings with high Mg concentration (53 GPa).
- All tested surfaces are non-cytotoxic, as they support the adhesion and growth of bone cells.
- Mg ions seem to accelerate initial osteoblast adhesion and proliferation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sedelnikova, M.B.; Sharkeev, Y.P.; Komarova, E.G.; Khlusov, I.A.; Chebodaeva, V.V. Structure and properties of the wollastonite calcium phosphate coatings deposited on titanium and titanium niobium alloy using microarc oxidation method. Surf. Coat. Technol. 2016, 307, 1274–1283. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tanaka, Y.; Ide-Ektessabi, A. Fabrication of hydroxyapatite thin films for biomedical applications using RF magnetron sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 249, 723–725. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Pardun, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Destri, G.L.; Marletta, G.; Rezwan, K. Mixed zirconia calcium phosphate coatings for dental implants: Tailoring coating stability and bioactivity potential. Mater. Sci. Eng. C 2015, 48, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.R.; Rutledge, L.; Randolph, L.D.; Meenan, B.J. Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater. Sci. Eng. C 2015, 46, 290–300. [Google Scholar] [CrossRef] [PubMed]
- López, E.O.; Rossi, A.L.; Archanjo, B.S.; Ospina, R.O.; Mello, A.; Rossi, A.M. Crystalline nano-coatings of fluorine-substituted hydroxyapatite produced by magnetron sputtering with high plasma confinement. Surf. Coat. Technol. 2015, 264, 163–174. [Google Scholar] [CrossRef]
- DeGroot, K.; DePutter, C.; Smitt, P.; Driessen, A. Mechanical failure of artificial teeth made of dense calcium hydroxyapatite. Sci. Ceram. 1981, 11, 433–437. [Google Scholar]
- Revilla-López, G.; Bertran, O.; Casanovas, J.; Turon, P.; Puiggalí, J.; Alemán, C. Effects of hydroxyapatite (0001) Ca2+/Mg2+ substitution on adsorbed d-ribose ring puckering. RSC Adv. 2016, 6, 69634–69640. [Google Scholar] [CrossRef]
- Gopi, D.; Karthika, A.; Nithiya, S.; Kavitha, L. In vitro biological performance of minerals substituted hydroxyapatite coating by pulsed electrodeposition method. Mater. Chem. Phys. 2014, 144, 75–85. [Google Scholar] [CrossRef]
- Vijayalakshmi, V.; Dhanasekaran, P. Synthesis and structural properties characterization of Ap/MgO nanocomposites for biomedical applications. Biol. Med. Case Rep. 2017, 1, 1–6. [Google Scholar]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Magnesium incorporated hydroxyapatite: Synthesis and structural properties characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef]
- Vladescu, A.; Padmanabhan, S.C.; Ak Azem, F.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.A.; Braic, V. Mechanical properties and biocompatibility of the sputtered Ti doped hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Lósarczyk, A.; Ima, A.Z.; Aszkiewicz, Z.P.; Zczepaniak, J.S.; Za, A.H.D.E.A.; Hróĝcicka, A.C. The Influence of Titanium on Physicochemical Properties of Ti-modified Hydroxyapatite Materials. Mater. Ceram. 2010, 62, 369–375. [Google Scholar]
- Pichugin, V.F.; Surmenev, R.A.; Shesterikov, E.V.; Ryabtseva, M.A.; Eshenko, E.V.; Tverdokhlebov, S.I.; Prymak, O.; Epple, M. The preparation of calcium phosphate coatings on titanium and nickel-titanium by rf-magnetron-sputtered deposition: Composition, structure and micromechanical properties. Surf. Coat. Technol. 2008, 202, 3913–3920. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I.N. Microstructure and mechanical properties of hydroxyapatite thin films grown by RF magnetron sputtering. Surf. Coat. Technol. 2003, 173, 315–322. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Vladescu, A.; Surmenev, R.A.; Pantilimon, C.M.; Braic, M.; Cotrut, C.M. Study on a hydrophobic Ti-doped hydroxyapatite coating for corrosion protection of a titanium based alloy. RSC Adv. 2016, 6, 87665–87674. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Rameshbabu, N.; Venkateswarlu, K.; Ravisankar, K.V.; Ashok, M.; Anandan, S. Photocatalytic and Antibacterial Activity of Titanium, Fluorine and Silver Co-Substituted Hydroxyapatite. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 268–277. [Google Scholar] [CrossRef]
- Wakamura, M. Photocatalysis by calcium hydroxyapatite modified with Ti (IV). Fujitsu Sci. Tech. J. 2005, 41, 181–190. [Google Scholar]
- Wakamura, M.; Hashimoto, K.; Watanabe, T. Photocatalysis by calcium hydroxyapatite modified with Ti(IV): Albumin decomposition and bactericidal effect. Langmuir 2003, 19, 3428–3431. [Google Scholar] [CrossRef]
- Tsukada, M.; Wakamura, M.; Yoshida, N.; Watanabe, T. Band gap and photocatalytic properties of Ti-substituted hydroxyapatite: Comparison with anatase-TiO2. J. Mol. Catal. A Chem. 2011, 338, 18–23. [Google Scholar] [CrossRef]
- Vladescu, A.; Braic, M.; Azem, F.A.; Titorencu, I.; Braic, V.; Pruna, V.; Kiss, A.; Parau, A.C.; Birlik, I. Effect of the deposition temperature on corrosion resistance and biocompatibility of the hydroxyapatite coatings. Appl. Surf. Sci. 2015, 354, 373–379. [Google Scholar] [CrossRef]
- Bull, S.J. Nanoindentation of coatings. J. Phys. D Appl. Phys. 2005, 38, 393–413. [Google Scholar] [CrossRef]
- Dudin, S.; Cotrut, C.M.; Dinu, M.; Zykova, A.; Parau, A.C.; Yakovin, S.; Vladescu, A. Comparative study of the hydroxyapatite coatings prepared with/without substrate bias. Ceram. Int. 2017, 43, 14968–14975. [Google Scholar] [CrossRef]
- ASTM G5-94 Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; ASTM: West Conshohocken, PA, USA, 1999.
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.; Belton, D.J.; Kaplan, D.L.; Perry, C.C. Quantifying the efficiency of Hydroxyapatite Mineralising Peptides. Sci. Rep. 2017, 7, 7681. [Google Scholar] [CrossRef] [PubMed]
- Bala, Y.; Farlay, D.; Delmas, P.D.; Meunier, P.J.; Boivin, G. Time sequence of secondary mineralization and microhardness in cortical and cancellous bone from ewes. Bone 2010, 46, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Tampieri, A.; Prat, M.; Gómez-Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Tank, K.P.; Sharma, P.; Kanchan, D.K.; Joshi, M.J. FTIR, powder XRD, TEM and dielectric studies of pure and zinc doped nano-hydroxyapatite. Cryst. Res. Technol. 2011, 46, 1309–1316. [Google Scholar] [CrossRef]
- Han, J.K.; Song, H.Y.; Saito, F.; Lee, B.T. Synthesis of high purity nano-sized hydroxyapatite powder by microwave-hydrothermal method. Mater. Chem. Phys. 2006, 99, 235–239. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Sedlaček, M.; Vilhena, L.M.S.; Podgornik, B.; Vižintin, J. Surface topography modelling for reduced friction. Stroj. Vestnik J. Mech. Eng. 2011, 57, 674–680. [Google Scholar] [CrossRef]
- Evgeny, B.; Hughes, T.; Eskin, D. Effect of surface roughness on corrosion behaviour of low carbon steel in inhibited 4M hydrochloric acid under laminar and turbulent flow conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- Mejias, A.; Candidato, R.T.; Pawowski, L.; Chicot, D. Mechanical properties by instrumented indentation of solution precursor plasma sprayed hydroxyapatite coatings: Analysis of microstructural effect. Surf. Coat. Technol. 2016, 298, 93–102. [Google Scholar] [CrossRef]
- Tang, C.Y.; Uskokovic, P.S.; Tsui, C.P.; Veljovic, D.; Petrovic, R.; Janackovic, D. Influence of microstructure and phase composition on the nanoindentation characterization of bioceramic materials based on hydroxyapatite. Ceram. Int. 2009, 35, 2171–2178. [Google Scholar] [CrossRef]
- Batory, D.; Gawroński, J.; Kaczorowski, W.; Niedzielska, A. C-HAp composite layers deposited onto AISI 316L austenitic steel. Surf. Coat. Technol. 2012, 206, 2110–2114. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hussein, K.H.; Woo, H.M.; Park, C.H.; Kim, C.S. One-step anodization deposition of anticorrosive bioceramic compounds on AZ31B magnesium alloy for biomedical application. Ceram. Int. 2015, 41, 10861–10870. [Google Scholar] [CrossRef]
- Pintaude, G. Introduction of the Ratio of the Hardness to the Reduced Elastic Modulus for Abrasion. In Tribology—Fundamentals and Advancements; Jürgen, G., Ed.; InTech: Rijeka, Croatia, 2013; pp. 217–230. [Google Scholar]
- Elsener, B.; Rota, A.; Bohni, H. Impedance study on the corrosion of PVD and CVD titanium nitride coatings. Mater. Sci. Forum 1989, 29, 44–45. [Google Scholar] [CrossRef]
- Mansfeld, F. The Polarization Resistance Technique for Measuring Corrosion Currents. Adv. Corros. Sci. Technol. 1976, 6, 163–262. [Google Scholar]
- Scully, J.R. Polarization resistance method for determination of instantaneous corrosion rates. Corrosion 2000, 56, 199–217. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Adamek, G.; Jurczyk, M.U.; Jurczyk, M. 3D surface topography study of the biofunctionalized nanocrystalline Ti-6Zr-4Nb/Ca-P. Mater. Charact. 2012, 70, 55–62. [Google Scholar] [CrossRef]
- Radin, S.R.; Ducheyne, P. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. J. Biomed. Mater. Res. 1993, 27, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.T.; Wong, P.K.; Cheng, F.T.; Man, H.C. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Appl. Surf. Sci. 2009, 255, 6736–6744. [Google Scholar] [CrossRef]
- Wagener, V.; Schilling, A.; Mainka, A.; Hennig, D.; Gerum, R.; Kelch, M.L.; Keim, S.; Fabry, B.; Virtanen, S. Cell Adhesion on Surface-Functionalized Magnesium. ACS Appl. Mater. Interfaces 2016, 8, 11998–12006. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Moreau, R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007, 40, 849–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeichi, M.; Okada, T.S. Roles of magnesium and calcium ions in cell-to-substrate adhesion. Exp. Cell Res. 1972, 74, 51–60. [Google Scholar] [CrossRef]
- Rubin, H. The membrane, magnesium, mitosis ( MMM ) model of cell proliferation control. Magnes. Res. 2005, 18, 268–274. [Google Scholar] [PubMed]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High magnesium inhibits human osteoblast differentiation in vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [PubMed]









| Identification | Base Pressure (×10−4 Pa) | Ar Pressure (×10−1 Pa) | RF Powers Fed (W) | Substrate Bias Voltage (V) | Deposition Temperature (°C) | ||
|---|---|---|---|---|---|---|---|
| HAP Cathode | TiO2 Cathode | MgO Cathode | |||||
| TM-0 | 1.3 | 6.6 | 50 | 25 | – | −60 | 700 |
| TM-1 | 50 | 25 | 25 | ||||
| TM-2 | 50 | 25 | 50 | ||||
| Coatings | Ca | P | Ti | Mg | O | Ca/P |
|---|---|---|---|---|---|---|
| TM-0 | 5.9 ± 0.2 | 3.6 ± 0.2 | 8.9 ± 0.3 | – | 81.6 ± 2.8 | 1.64 |
| TM-1 | 5.8 ± 0.2 | 3.5 ± 0.1 | 8.3 ± 0.3 | 3.1 ± 0.03 | 79.3 ± 2.4 | 1.66 |
| TM-2 | 6.2 ± 0.2 | 3.7 ± 0.1 | 8.1 ± 0.2 | 9.2 ± 0.1 | 72.8 ± 2.1 | 1.68 |
| Coating | Load (mN) | Eaverage (GPa) | Haverage (GPa) | H/E | H2/E2 | H3/E2 (GPa) | H2/2E (GPa) |
|---|---|---|---|---|---|---|---|
| TM-0 | 1.0 | 87.156 ± 1.83 | 8.809 ± 0.23 | 0.1011 | 0.0102 | 0.0899 | 3382 |
| TM-1 | 83.171 ± 1.77 | 8.670 ± 0.20 | 0.1042 | 0.0108 | 0.0942 | 3126 | |
| TM-2 | 52.993 ± 1.46 | 5.764 ± 0.18 | 0.1088 | 0.0118 | 0.0681 | 880 |
| Sample | Ei=0 (mV) | icorr (nA/cm2) | Rp (kΩ) | P | Pe (%) |
|---|---|---|---|---|---|
| Ti6Al4V | −94 | 45.76 | 2345 | – | – |
| TM-0 | −209 | 27.36 | 1825 | 1.14 | 40.2 |
| TM-1 | −67 | 10.02 | 3294 | 0.69 | 78.1 |
| TM-2 | −81 | 26.48 | 3092 | 0.75 | 42.1 |
| Samples | Before Corrosion Tests | After Corrosion Tests | ||||
|---|---|---|---|---|---|---|
| Ra (nm) | rms (nm) | Sk | Ra (nm) | rms (nm) | Sk | |
| Ti6Al4V | 52.2 | 87.4 | 31.110 | 72.8 | 98.6 | 0.218 |
| TM-0 | 57.9 | 84.7 | 0.201 | 68.4 | 97.1 | 0.140 |
| TM-1 | 28.2 | 35.9 | 0.179 | 27.5 | 31.7 | −0.012 |
| TM-2 | 29.8 | 40.2 | 1.731 | 33.5 | 41.4 | 0.218 |
| Surface | 1 Day | 3 Days |
|---|---|---|
| Ti6Al4V | 42.33 ± 6.15 a | 81.83 ± 13.35 a |
| TM-1 | 57.5 ± 6.98 b | 78.0 ± 18.3 a |
| TM-2 | 52.67 ± 5.64 b | 68.33 ± 18.17 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monsees, T.K.; Ak Azem, F.; Cotrut, C.M.; Braic, M.; Abdulgader, R.; Pana, I.; Birlik, I.; Kiss, A.; Booysen, R.; Vladescu, A. Biodegradable Ceramics Consisting of Hydroxyapatite for Orthopaedic Implants. Coatings 2017, 7, 184. https://doi.org/10.3390/coatings7110184
Monsees TK, Ak Azem F, Cotrut CM, Braic M, Abdulgader R, Pana I, Birlik I, Kiss A, Booysen R, Vladescu A. Biodegradable Ceramics Consisting of Hydroxyapatite for Orthopaedic Implants. Coatings. 2017; 7(11):184. https://doi.org/10.3390/coatings7110184
Chicago/Turabian StyleMonsees, Thomas K., Funda Ak Azem, Cosmin Mihai Cotrut, Mariana Braic, Radwan Abdulgader, Iulian Pana, Isil Birlik, Adrian Kiss, Robin Booysen, and Alina Vladescu. 2017. "Biodegradable Ceramics Consisting of Hydroxyapatite for Orthopaedic Implants" Coatings 7, no. 11: 184. https://doi.org/10.3390/coatings7110184
APA StyleMonsees, T. K., Ak Azem, F., Cotrut, C. M., Braic, M., Abdulgader, R., Pana, I., Birlik, I., Kiss, A., Booysen, R., & Vladescu, A. (2017). Biodegradable Ceramics Consisting of Hydroxyapatite for Orthopaedic Implants. Coatings, 7(11), 184. https://doi.org/10.3390/coatings7110184








.jpg)