Abstract
We evaluated the applicability of metal spray coating as a waterproofing/corrosion protection method for a concrete structure used for water purification. We carried out an ozone resistance test on four metal sprays and evaluated the water permeability and bond strength of the metals with superior ozone resistance, depending on the surface treatment method. In the ozone resistance test, four metal sprays and an existing ozone-proof paint were considered. In the experiment on the water permeability and bond strength depending on the surface treatment method, the methods of no treatment, surface polishing, and two types of pore sealing agents were considered. The results showed that the sprayed titanium had the best ozone resistance. Applying a pore sealing agent provided the best adhesion performance, of about 3.2 MPa. Applying a pore sealing agent also provided the best waterproofing performance. Scanning electron microscope analysis showed that applying a pore sealing agent resulted in an excellent waterproofing performance because a coating film formed on top of the metal spray coating. Thus, when using a metal spray as waterproofing/corrosion protection for a water treatment concrete structure, applying a pore sealing agent on top of a film formed by spraying titanium was concluded to be the most appropriate method.
1. Introduction
At present, water is purified by using chlorine. Chlorine input processes in the mixing tank, coagulation tank, sedimentation tank, and filtering tank remove harmful materials (e.g., Pb, As, Sn, and Cd) from the water supply and acidic materials from the wastewater, which are otherwise harmful to living things [1,2]. However, some harmful materials cannot be treated by the existing purification method. Thus, the use of an advanced water purification method that uses ozone to effectively remove such harmful materials has recently been increasing. The ozone used in advanced water purification facilities forms a stable oxygen molecule in water and generates one oxygen radical. Then, the oxygen radical generates two hydroxyl radicals (OH−) with a strong oxidizing power by decomposing a polar molecule of water. Organic materials react with ozone directly or with the hydroxyl radical (OH−) generated by ozone decomposition, which changes their properties. This is called oxidation [3,4,5,6,7,8,9]. Advanced water purification uses the properties of ozone to purify wastewater, producing clean water [10]. The oxidation of ozone and strong oxidizing power of harmful acids in wastewater affect the quality of the concrete of the water treatment structure. This can become a cause of deterioration, resulting in the waterproofing/anti-corrosion material of the concrete structure breaking away and causing the concrete to crack [1,2,11,12,13,14]. In 2010, Korea established recommended standards for ozone-proof paint to resolve this problem, and the development of organic/inorganic paint materials has been studied [15]. However, existing waterproofing/anti-corrosion materials (i.e., organic materials based on epoxy resins) cause corrosion, breakaway, exfoliation, and cracks in the finishing material, in an environment with high concentrations of ozone and chemicals; they can even affect the concrete itself and degrade the long-term durability [16,17]. To address this problem of organic/inorganic paint materials, the stainless steel (SUS) panel method has recently been applied to some facilities; this method uses SUS panels with excellent waterproofing/corrosion protection performance, ozone resistance, and chemical resistance [18,19]. However, the SUS panel method has problems with adhesion to concrete structures and corrosion caused by the welding of the joint sections, and it requires very challenging construction technology. In addition, there are enormous costs for the initial construction and maintenance, and there are further costs for waste disposal and construction during repairs because of the complete removal and reconstruction of the paint film [1].
In this study, we evaluated the ozone resistance of metal spray coatings and examined the bond strength, water impermeability, and impact resistance depending on the surface treatment method, in order to develop a finishing method that can fundamentally prevent the deterioration of concrete structures in water treatment facilities. Our hope was to demonstrate the feasibility of metal sprays for constructing metal panels with excellent ozone resistance and chemical resistance more easily than the existing construction method. The basic data from this study should help with the development of a finishing method for water treatment concrete structures.
2. Experimental Plan
2.1. Outline
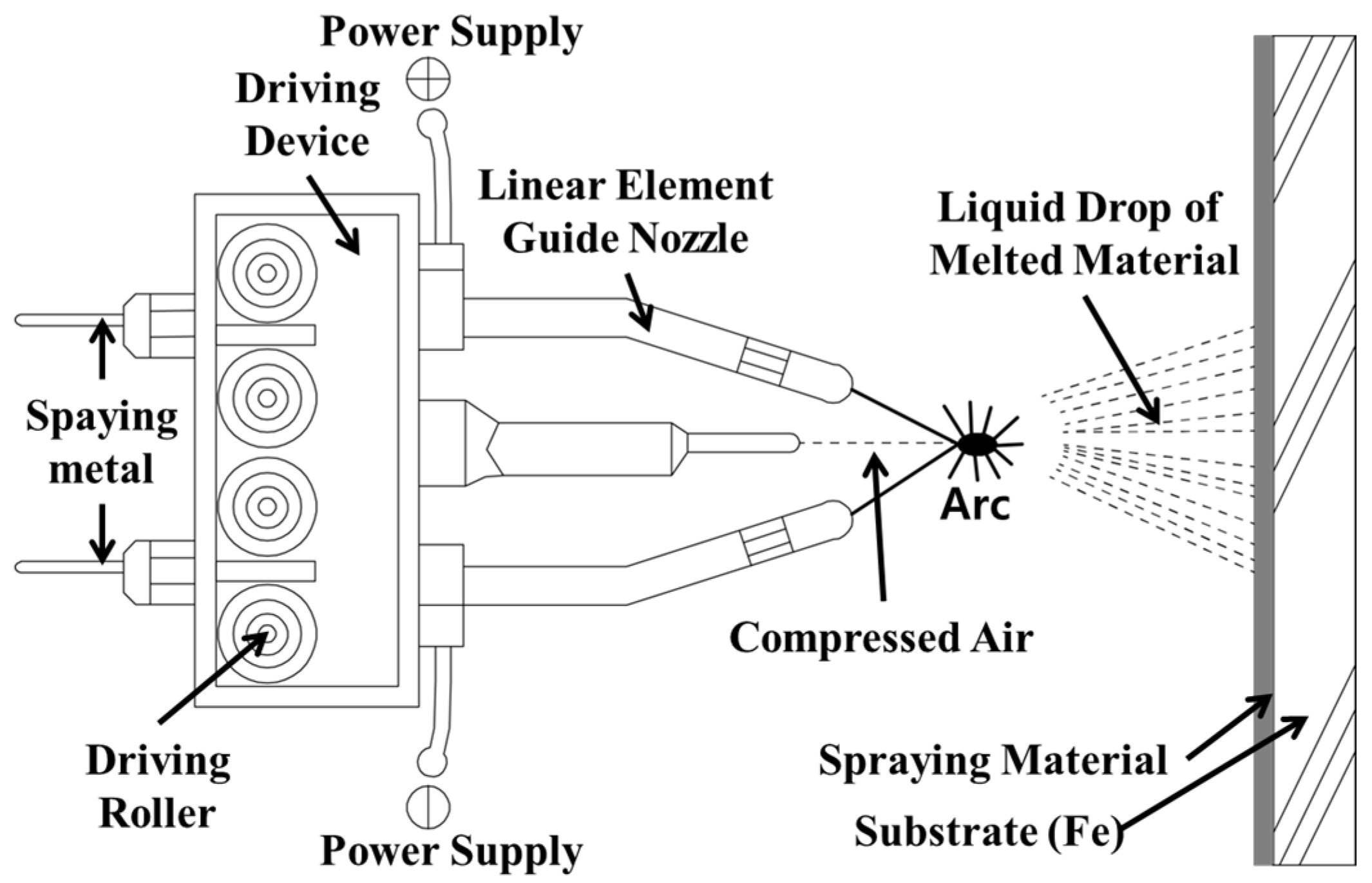
Table 1 outlines the experiment and experimental factors. A basic procedure was conducted to apply the metal spray to a concrete structure for water treatment. Figure 1 shows that the arc thermal spraying process using the coating was applied on the concrete surface with a circular slit of hot and compressed air [20,21]. Four kinds of metal sprays were selected, and their ozone resistance was evaluated. A zinc–aluminum alloy, SUS304 (commonly used structural steel), SUS316L (commonly used with the SUS panel method), and titanium (high corrosion resistance) were considered, along with the existing ozone-proof paint. Then, the adhesion performance of the metal with the best ozone resistance was evaluated. Because water treatment concrete structures are in contact with water at all times, ozonated water or moisture infiltrates the concrete and finishing material, which degrades the adhesion performance and durability. Thus, the water impermeability of the metal spray coating is an important factor. However, because a metal spray forms a film from stacking solid metal particles on the concrete in powder form, many pores exist between particles and on the surface [22,23,24,25,26]. Thus, different surface treatments were performed on the metal spray coating, and their effects on the water impermeability were evaluated. Concrete with a compressive strength of 24 MPa was used as the substrate, and the metal spray coating was 200 µm thick, which is the average thickness used in construction.

Table 1.
Experimental factors and measurement catalogs. Substrate: concrete (24 MPa); Coating thickness: 200 µm.
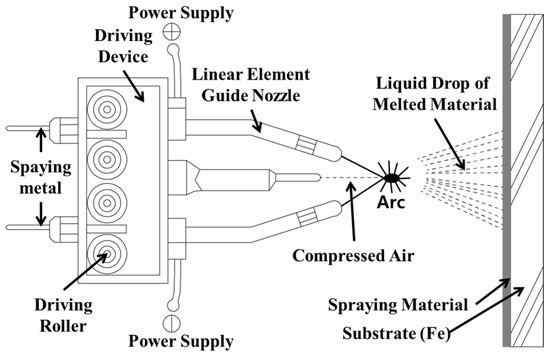
Figure 1.
Schematic of the arc thermal spraying process.
2.2. Ozone Treatment
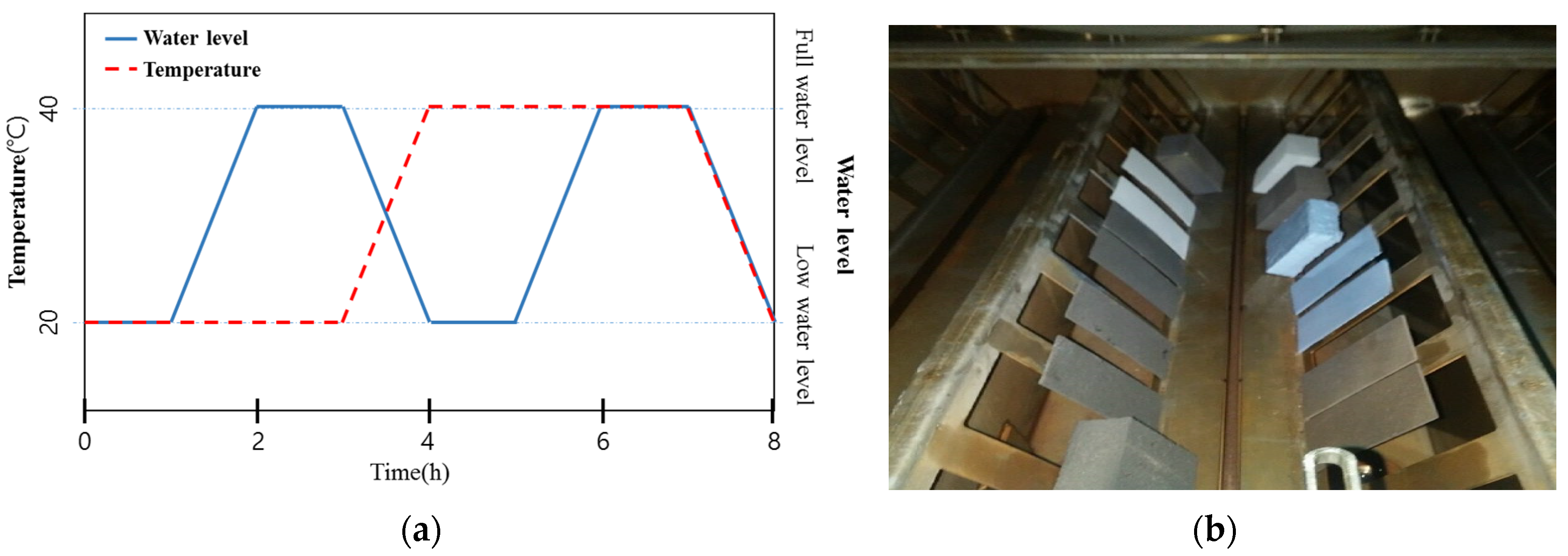
Ozone treatment was carried out in accordance with the SPS KWWA M211 method provided by the Korea Water and Wastewater Works Association [27]. The dissolved ozone concentration of the ozonated water was maintained at 10 ± 1 ppm by using an ozone tester that can be applied to both underwater and airborne settings. The temperature of the chamber was regulated according to an 8-h cycle, comprising 3 h at 20 ± 2 °C, 3 h at 40 ± 2 °C, and 1 h of transition time between each temperature setting. The cycle was repeated 84 times. The ozonated water level was regulated according to an 8-h cycle, comprising a full water level for 1 h and a low water level for 1 h, as shown in Figure 2. This cycle was repeated 84 times and the experiment was then conducted, after the specimens had been washed with clean water.
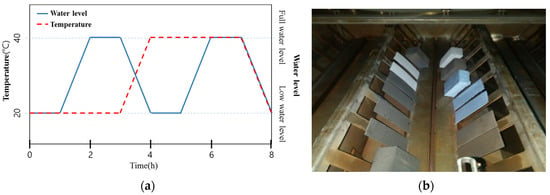
Figure 2.
(a) Cyclic condition of ozone treatment (Ozone concentration: 10 ± 1 ppm) and (b) view of the ozone resistance test.
2.3. Specimen Production
Test pieces were produced with different sizes using 24 MPa concrete, as shown in Figure 3. To secure the bond strength between the metal spray coating and the concrete, the surfaces of the prepared concrete were sand-blasted. To increase the tensile strength of the concrete surfaces after the sand-blasting treatment, a pervious surface hardener was applied. Then, a surface roughness agent was applied, to increase the roughness. The different surface treatments were then applied to the test pieces.

Figure 3.
Specimen production process.
2.4. Method
2.4.1. Weight Reduction after Ozone Treatment
The weight of each specimen was measured before ozone treatment in accordance with SPS KWWA M211 [27], by using a scale with a precision of one decimal place. After ozone treatment, each specimen was cleaned using running distilled water and dried in a 60 °C chamber for 24 h or longer. Then, the specimen was stored under standard conditions for 30 min, before the weight was measured with the same method used before ozone treatment. The weight reduction rate was calculated as follows:
where g is the weight reduction (g/m2), g1 is the weight of the specimen before ozone treatment (g), g2 is the weight of the specimen after ozone treatment (g), and A is the area of the specimen (m2).
2.4.2. Appearance after Ozone Treatment
The appearance was evaluated by visual observation in accordance with SPS KWWA M211 [27]. The test pieces produced by spraying metal on all six faces were put through 84 cycles of preprocessing in accordance with the ozone treatment method and were then visually observed for phenomena such as cracks, swelling, pinholes, rust, or discoloration on the surface.
2.4.3. Bond Strength Depending on the Surface Treatment Method
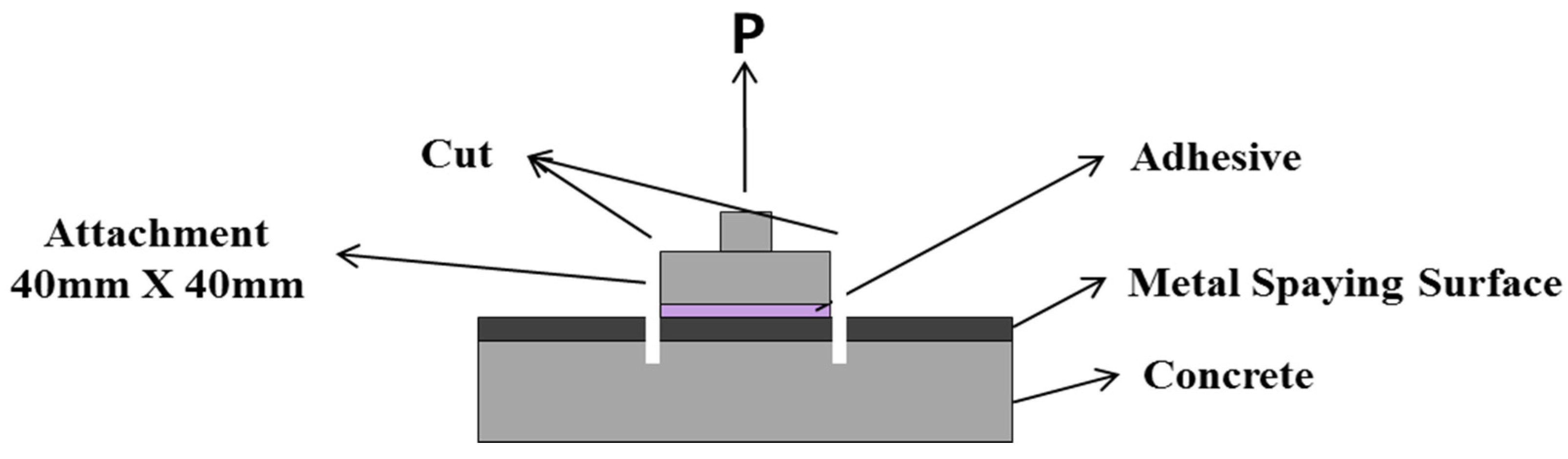
The bond strength depending on the surface treatment method was evaluated in accordance with KS F 4716 [28]. For the tensile bond strength test, a square attachment measuring 40 mm × 40 mm was glued onto each surface-treated specimen by using an epoxy adhesive, as shown in Figure 4. After 24 h, the perimeter of the attachment was cut to the concrete surface, and the maximum load was measured. Then, the bond strength was calculated with Equation (2). An average of nine measurements was regarded as sufficient for calculating the bond strength, and the adhesion failure behavior was visually checked.
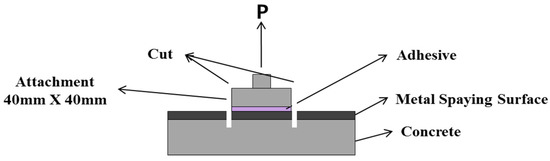
Figure 4.
Outline of the bond strength test.
Here, T is the maximum tensile load (N) and A is the attachment area (1600 mm2).
2.4.4. Impermeability Depending on the Surface Treatment Method
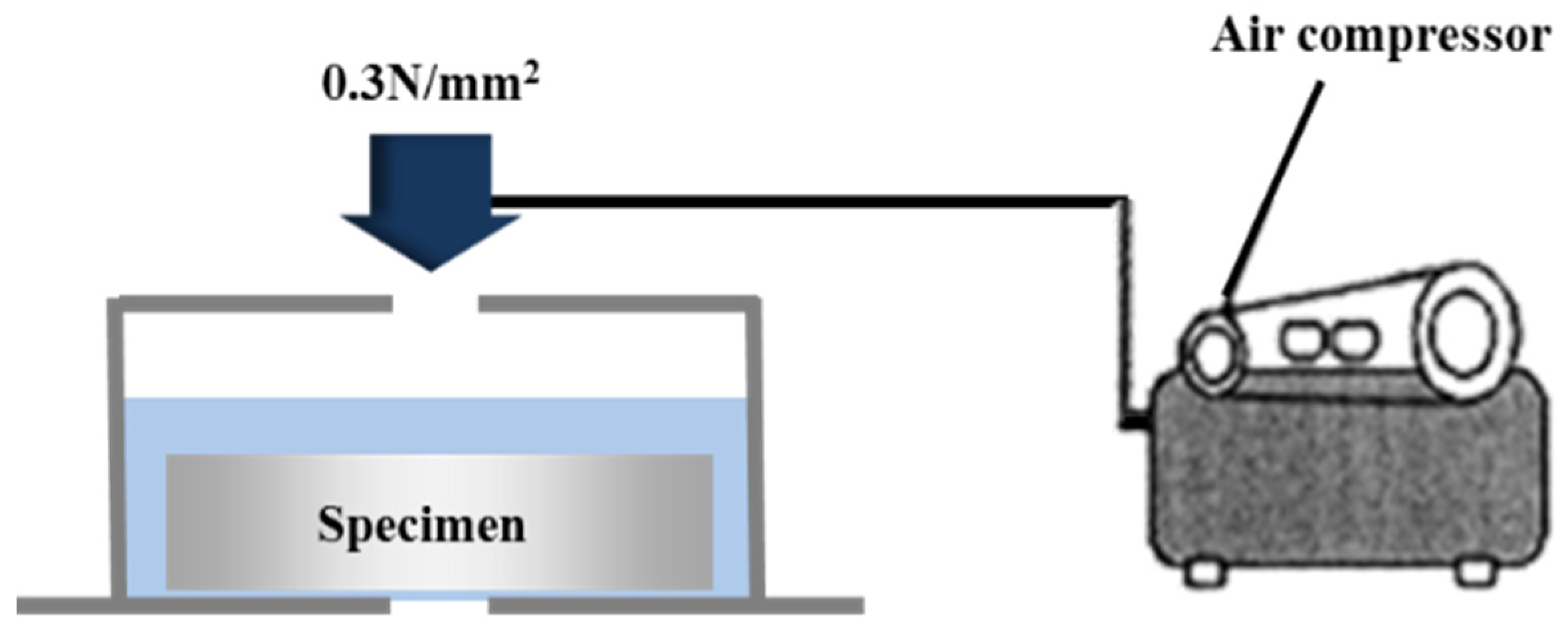
The water impermeability was evaluated in accordance with KS F 4919 [29]. The lateral faces, excluding the surface on which the metal was sprayed and the opposite face, were completely sealed with an epoxy resin. A water pressure of 0.3 N/mm2 was applied to the specimen for 3 h by using the equipment shown in Figure 5. Then, moisture was lightly removed for about 10 s by using a piece of filter paper [30], as per KS M 7602. The mortar bottom plate beneath the sprayed metal surface at the center of the specimen was visually checked to see if it was wet with water. The difference between the weight before the water pressure was applied (W1) and the weight after the water pressure was applied (W2) was set as the amount of water permeation based on KS F 4930 [31], and the permeability ratio was calculated as follows:
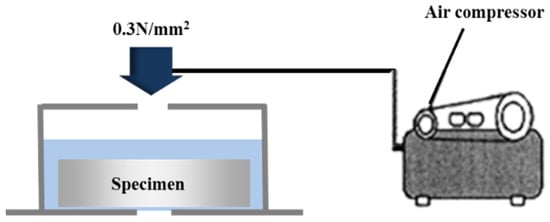
Figure 5.
Outline of the impermeability test.
3. Results and Discussion
3.1. Weight Reduction after Ozone Treatment
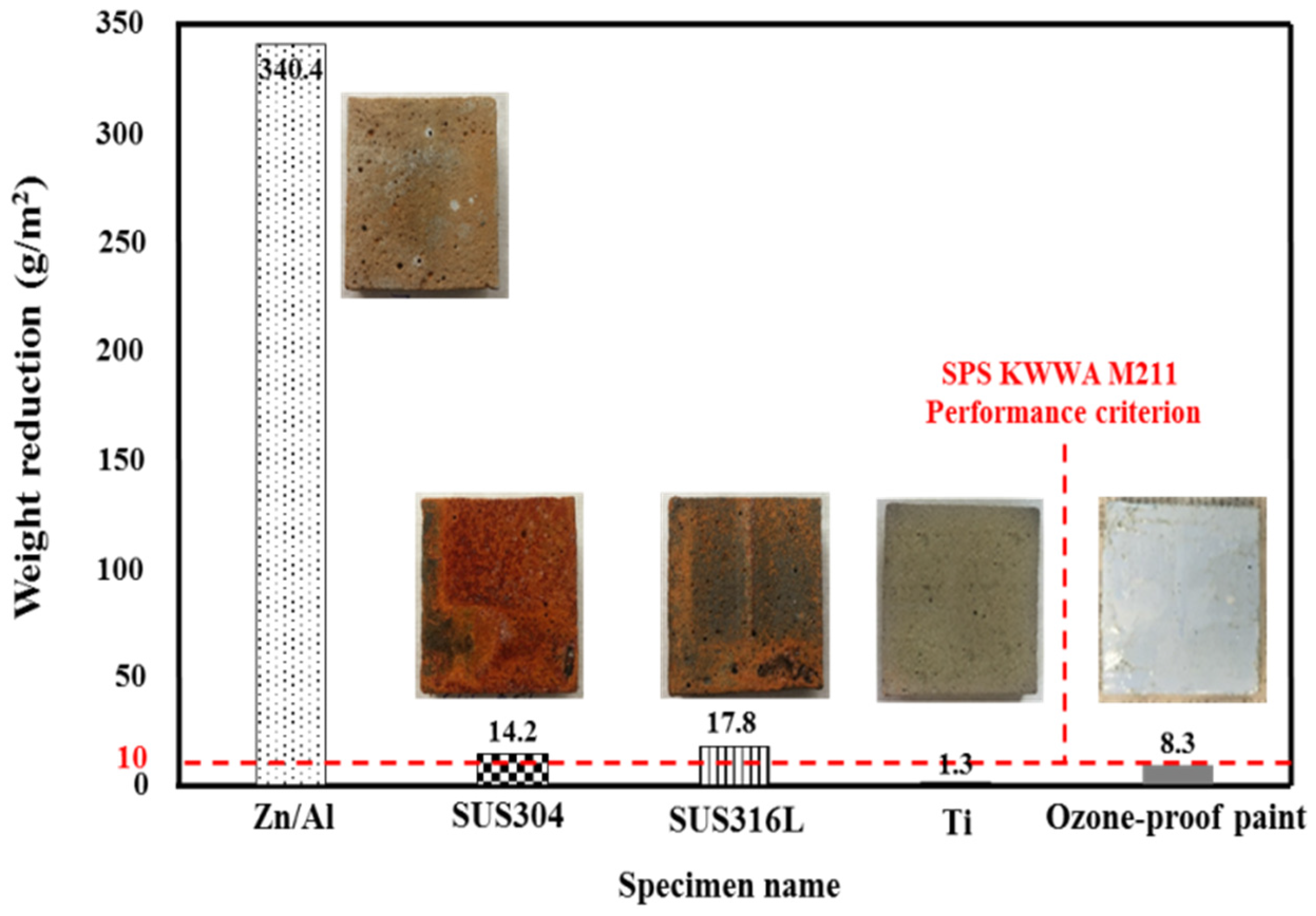
To determine the metal spray with the highest ozone resistance, the weight reduction after ozone treatment was evaluated. Figure 6 shows the results. The Zn/Al alloy showed the largest weight reduction of 340.4 g/m2, which confirmed that it is most vulnerable to ozone. SUS304 and SUS316L, which are known to have a strong chemical resistance, showed reductions of 14.2 and 17.8 g/m2, respectively. These materials are vulnerable to ozone because the metal is arc-discharged at a high temperature when sprayed, and the Fe–Ni–Cr structure of stainless steel is destroyed when heat-treated at a high temperature. This causes the Fe component to move to the surface of the sprayed metal in large quantities [32]. The ozone-proof paint showed a weight reduction of 8.3 g/m2. Ti showed almost no weight reduction, with a value of 1.3 g/m2. Thus, only Ti and the ozone-proof paint satisfied the performance criterion of SPS KWWA M211 (i.e., 10 g/m2).
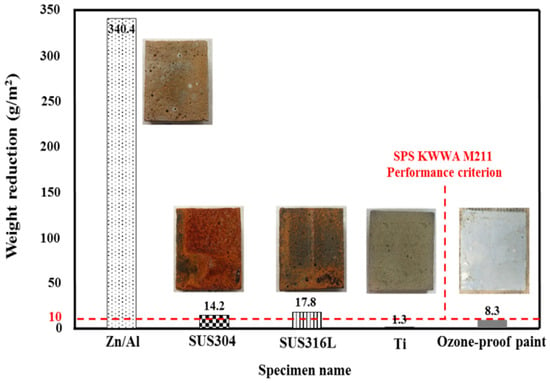
Figure 6.
Weight reduction after ozone treatment depending on the sprayed metal.
3.2. Change in Appearance after Ozone Treatment
Table 2 presents the changes in appearance of the specimens before and after ozone treatment. The Zn/Al specimen showed the most serious deterioration. The SUS304 specimen was partially exfoliated and heavily rusted. The SUS316L specimen was also visually confirmed to have rusted. The specimen may have failed to withstand the ozone because the Fe–Ni–Cr structure was destroyed by heat treatment, similar to the case of the weight reduction, which increased the intergranular corrosion susceptibility [32]. In the case of the ozone-proof paint, although there was no exfoliation, cracks, or rust, the specimen was discolored. Thus, the ozone-proof paint may be unable to withstand high concentrations of ozone for a long time. The Ti specimen showed no exfoliation, cracks, rust, or discoloration. Thus, it had the highest ozone resistance.

Table 2.
Change in appearance after ozone treatment depending on the metal spray.
3.3. Adhesion Performance of the Metal Spray Depending on the Surface Treatment Method
After the ozone resistance of different metal sprays had been evaluated, the bond strength depending on the surface treatment method was evaluated. Ti was used to test the bond strength because it had the best ozone resistance. Figure 7 presents the results depending on the surface treatment method.
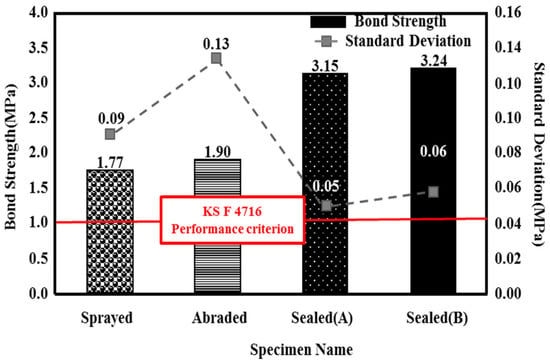
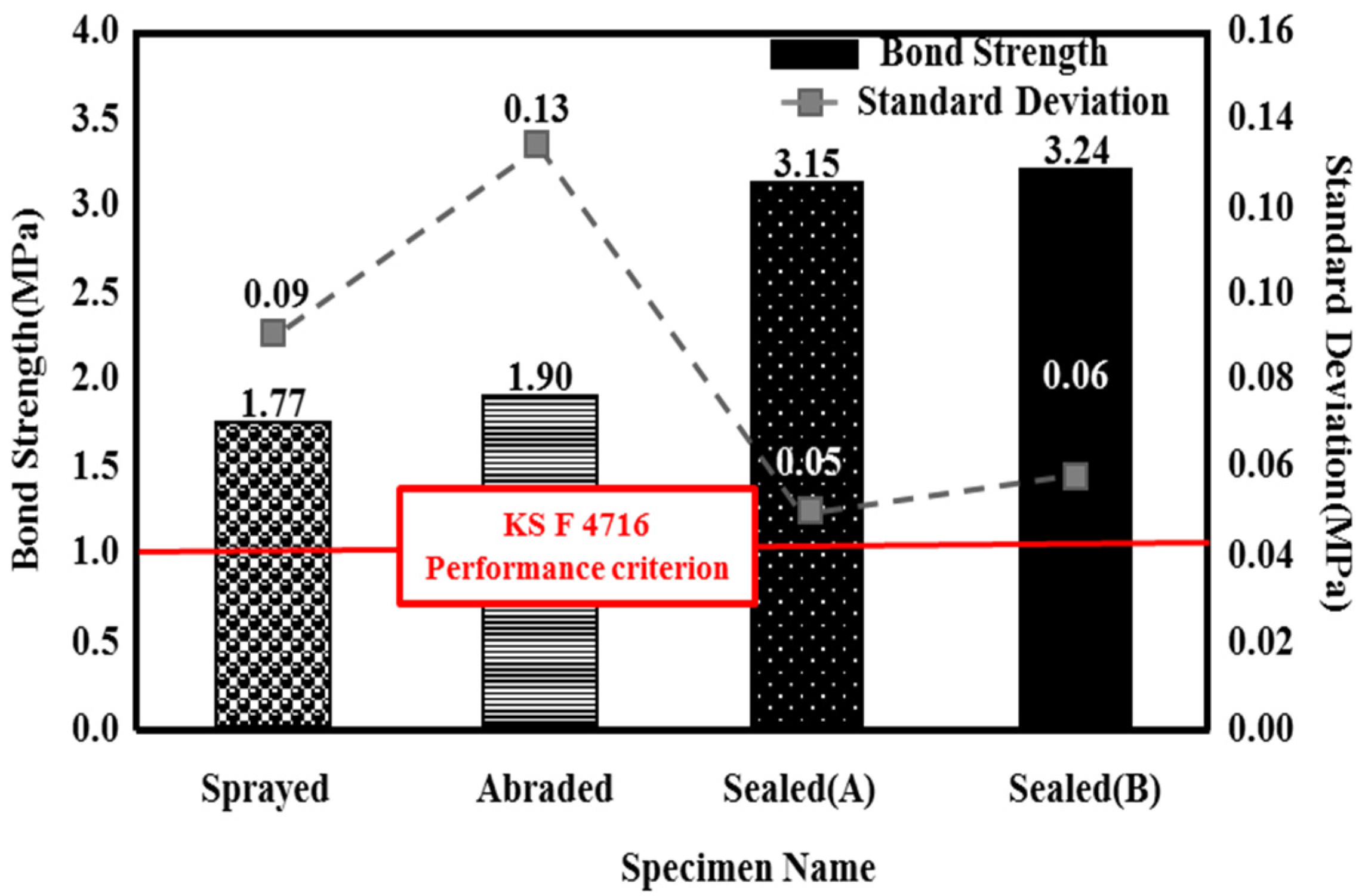
Figure 7.
Bond strength depending on the surface treatment method
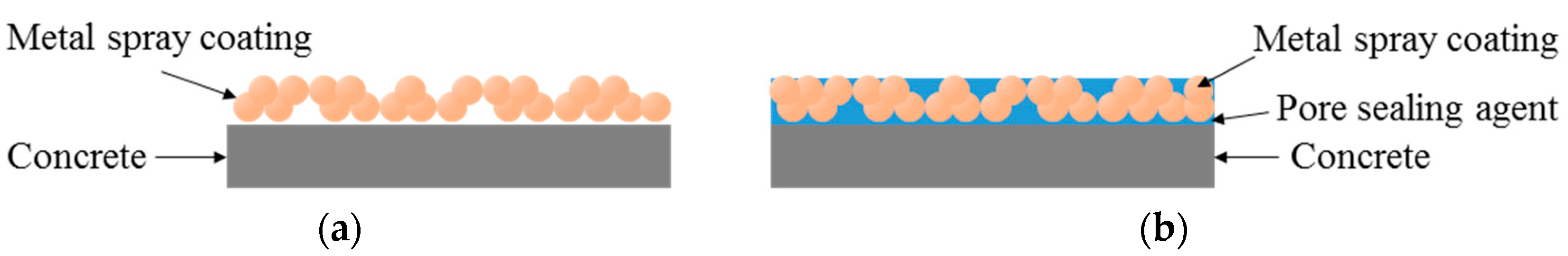
The sealed (A) and sealed (B) specimens showed a superior bond performance to the sprayed specimen, by about 1.8 times (3.15 and 3.24 MPa, respectively), regardless of the type of pore sealing agent. This may be because many pores are generated on the film by the metal spray method [25]; when a pore sealing agent is applied to such a film, it permeates and plays the role of an adhesive between the metal spray coating and concrete, as shown in Figure 8. This enhances the adhesion performance. The sprayed specimen showed the lowest bond strength of 1.77 MPa. However, it satisfied the bond strength performance criterion of 1.1 MPa, as stated in in KS F 4716. Although the abraded specimen showed a bond strength of 1.90 MPa, which was higher than that of the sprayed specimen, the standard deviation was relatively high, at about 0.13. This may be because the error range of the experimental values increased due to the impact applied to the film during the surface polishing process.
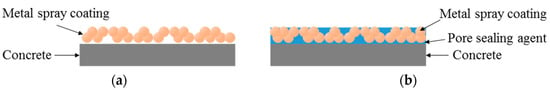
Figure 8.
Mimetic diagram of the pore sealing agent. (a) Sprayed mimetic diagram; (b) Sealed mimetic diagram.
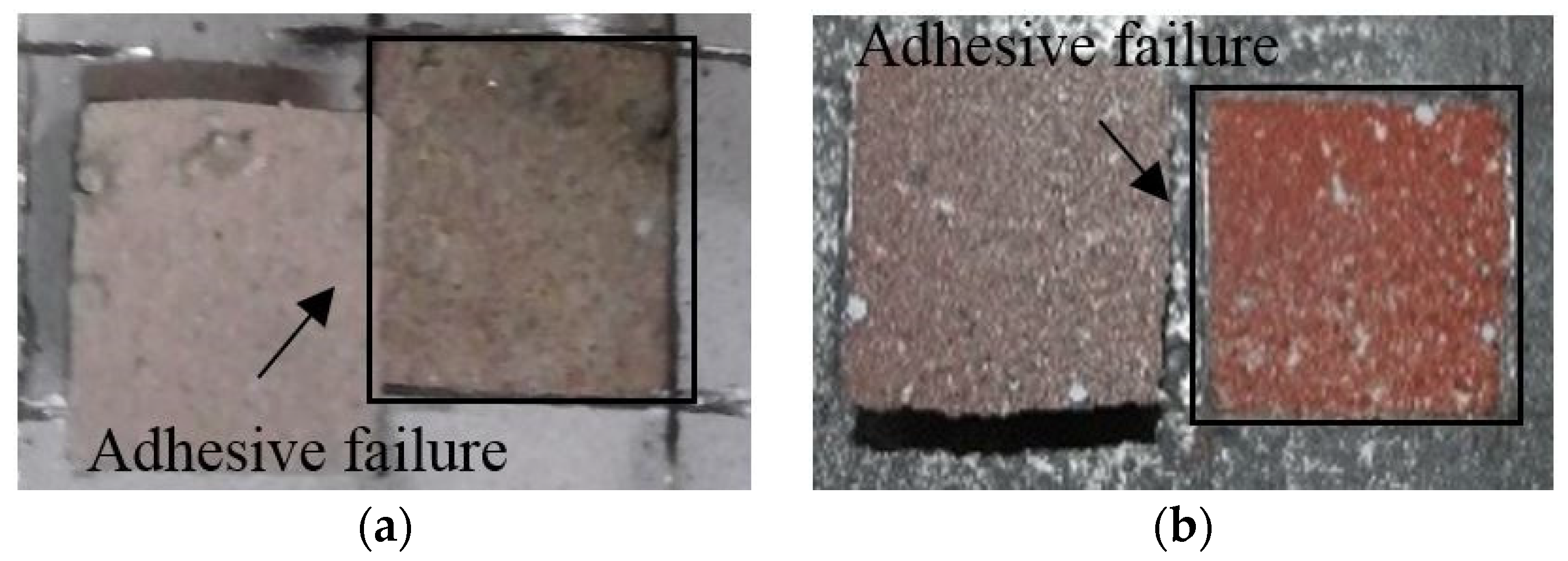
Figure 9 shows the failure modes of the specimens after the bond strength test. The sprayed specimen with a bond strength of 1.77 MPa showed failure between the metal spray coating and concrete. The abraded specimen showed the same failure mode. However, both the sealed (A) and sealed (B) specimens showed non-interfacial failure, where the failure occurred in the concrete. Thus, within the scope of this study, applying a pore sealing agent was found to be very effective at securing the bond strength, regardless of the agent type.
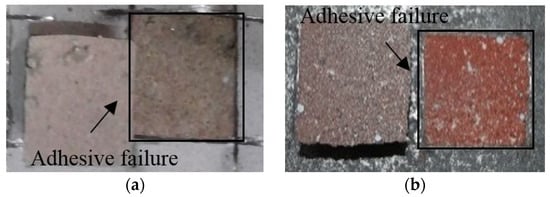

Figure 9.
Failure mode depending on the surface treatment method: (a) sprayed, (b) abraded (c) sealed (A), and (d) sealed (B).
3.4. Impermeability of the Metal Spray Depending on the Surface Treatment Method
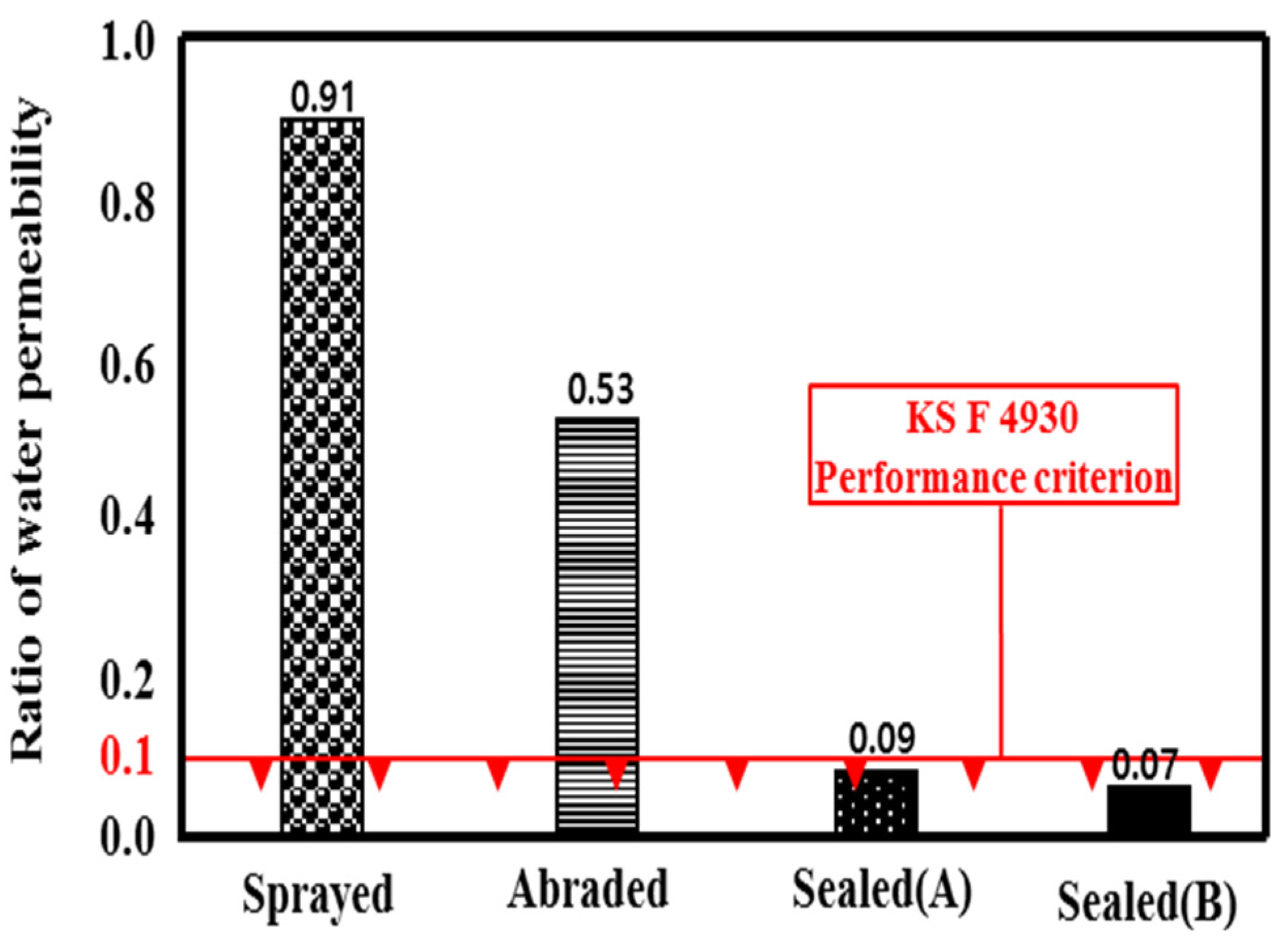
Because a water treatment concrete structure is in contact with water at all times, the water impermeability of the metal spray coating is an important factor. Figure 10 shows the related ratio of water permeability. The water permeation of the mortar specimen was about 12.1 g, and the sprayed specimen showed the highest water permeation, at about 11.0 g. This may be because water permeated through the pores in the film formed by the metal spray method, as shown in Figure 7a [25]. The water permeation of the sealed (B) specimen was about 0.8 g, which showed a 92.6% improvement in the water impermeability compared to the sprayed specimen. The water permeation of the sealed (B) specimen was 1.0 g, which showed a 91.1% improvement. The water permeation of the abraded specimen was about 6.4 g, which revealed a 42.9% improvement.
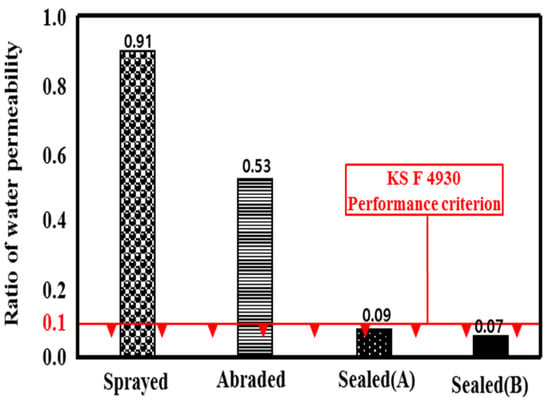
Figure 10.
Permeability ratio depending on the surface treatment method.
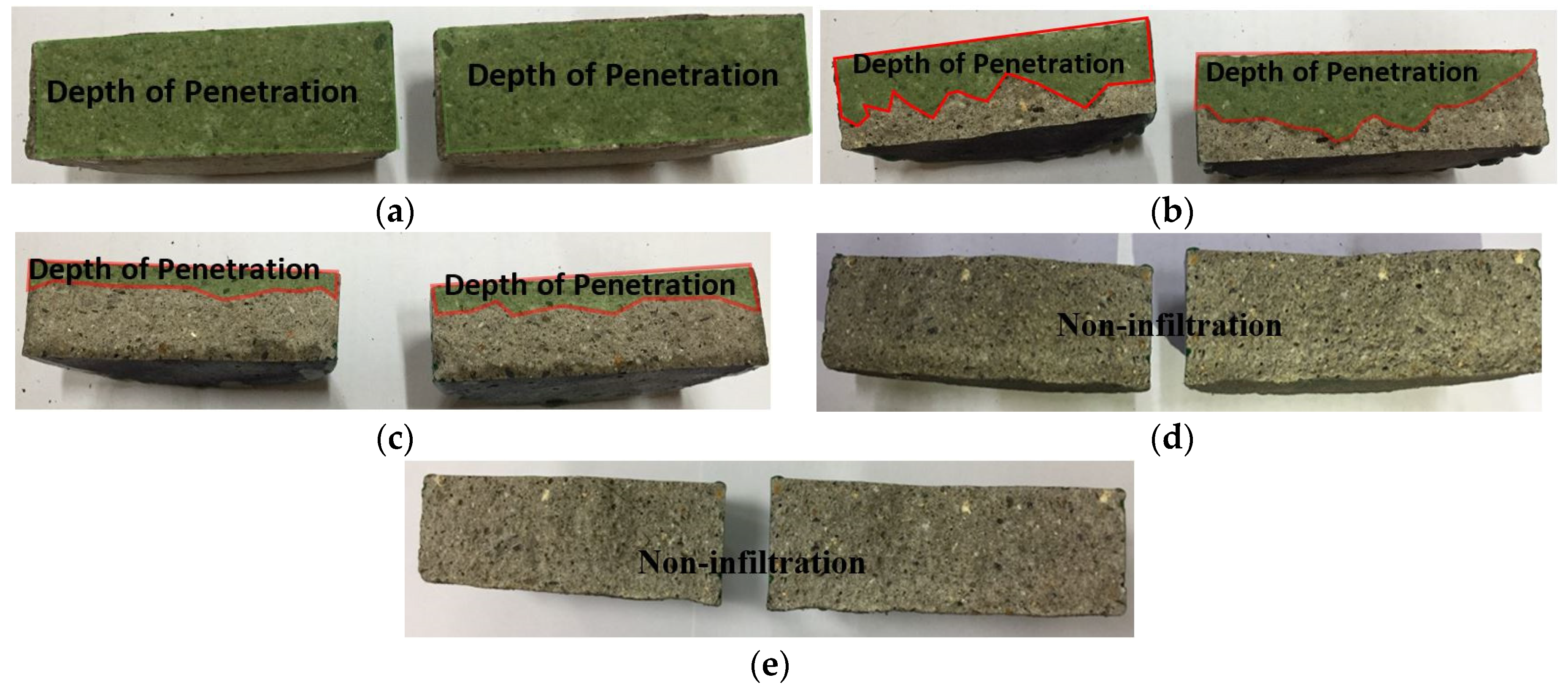
Based on the experimental results, the permeability ratios were in the order of sprayed > abraded > sealed (A) > sealed (B). Both of the sealed specimens satisfied the KS F 4930 performance criterion, exhibiting a ratio of less than 0.1. A visual check showed that water permeated the whole area of the mortar specimen, as shown in Figure 11. In the case of the sprayed specimen, water permeated to a depth of about 22.5 mm. Thus, its permeability was 25% of that of the mortar specimen. In the case of the abraded specimen, water permeated to a depth of about 7.5 mm, so the water permeability was about 66.6% of that of the sprayed specimen and about 75% of that of the mortar specimen. In contrast, although the sealed (A) and sealed (B) specimens showed changes in weight after the experiment, the visual check showed that no water permeated the specimens. Thus, the impermeability depending on the surface treatment method of the sprayed metal was in the order of sealed (A) = sealed (B) > abraded > sprayed. No difference was found regarding the type of pore sealing agent. This may be because the pore sealing agents formed a thin film on the metal spray coating to prevent water from permeating the specimens.
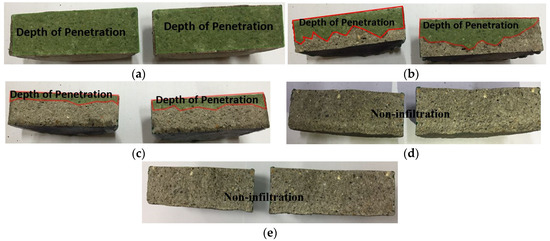
Figure 11.
Results of the impermeability test: (a) mortar; (b) sprayed; (c) abraded; (d) sealed (A); and (e) sealed (B).
3.5. Analysis of the Surface and Cross-Section of the Sprayed Metal Depending on the Surface Treatment Method
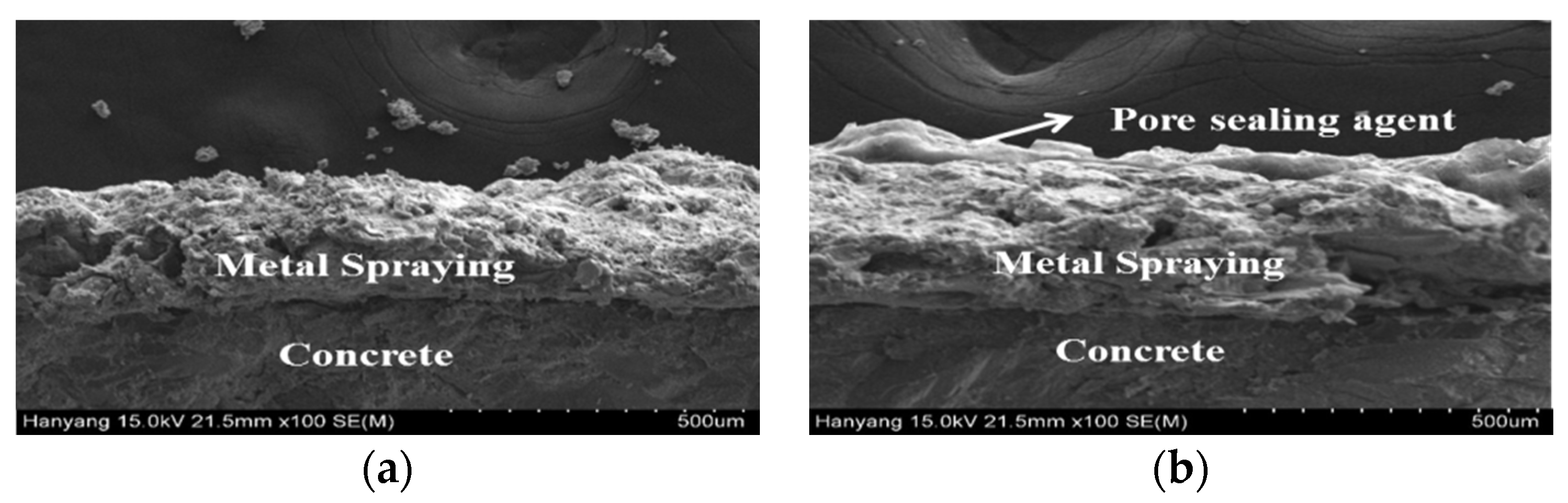
Based on the results of the impermeability test, the specimens to which a pore sealing agent was applied were found to be superior. The sprayed metal surfaces of each specimen were compared by using a scanning electron microscope (SEM) and digital optical microscope. Figure 12 shows the surface images taken with the digital optical microscope, and Figure 13 and Figure 14 show the surface and cross-sectional images taken with the SEM.

Figure 12.
Surface analysis using a digital optical microscope (X160): (a) sprayed; (b) abraded; and (c) sealed.

Figure 13.
Surface analysis using an SEM: (a) sprayed; (b) abraded; and (c) sealed.
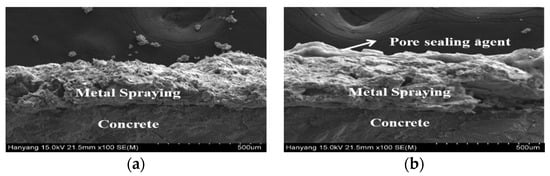
Figure 14.
Cross-sectional analysis using an SEM: (a) sprayed and (b) sealed.
4. Conclusions
The following conclusions were derived after evaluating the ozone resistance, bond strength, and impermeability of metal sprays for their applicability to finishing water treatment concrete structures:
- With regard to the ozone resistance, Ti showed the smallest weight reduction of 1.3 g/m2 among the metal sprays and no observable deterioration.
- The stainless steel family (SUS304 and SUS316L) showed large weight reductions and deterioration phenomena, such as rust and exfoliation, in contrast to Ti. These materials may have failed against ozone because the Fe–Ni–Cr structure was destroyed when they were heat-treated, due to the characteristics of the metal spray method. This caused the Fe component to move to the surface. Thus, Ti is the most suitable metal spray for finishing a water treatment facility.
- With regard to the bond strength depending on the surface treatment method, all of the specimens satisfied the KS standard. Using a pore sealing agent (i.e., sealed (A) and sealed (B)) produced the best adhesion performance. This may be because the pore sealing agent permeates the pores generated by the metal spray method and acts as an adhesive to enhance the adhesion performance.
- When the failure modes were compared, while the sprayed and abraded specimens had a relatively low bond strength and showed interfacial failure between the metal spray coating and concrete, the sealed (A) and sealed (B) specimens exhibited a high bond strength and showed non-interfacial failure that occurred in the concrete.
- With regard to the impermeability depending on the surface treatment method, the sprayed specimen showed the lowest impermeability, similar to the case of the bond strength. This may be because water permeates through the pores generated by the metal spray method.
- The sealed (A) and sealed (B) specimens showed that no water permeated the structure. This may be because the pore sealing agents formed a thin film on the metal spray coating that prevented water permeation.
- Surface analysis confirmed many pores on the surface of the sprayed specimen, while the pores were filled up to some extent, in the case of the abraded specimen, from polishing of the metal spray coating. However, polishing could not fill up the pores across the whole area and reduced the bond strength. Thus, applying a pore sealing agent is the most efficient method.
- Ti should be used with the proposed metal spray method to finish water treatment concrete structures, and a pore sealing agent is the most suitable surface treatment method to prevent water from permeating the concrete. However, because the main ingredient of the pore sealing agent of the sealed (A) specimen is epoxy, similar to paint, deterioration by ozonation is expected [17]. Accordingly, using a Teflon-based pore sealing agent is the most appropriate and efficient surface treatment method.
Acknowledgments
This research was supported by Korea Ministry of Environment (MOE) as Public Technology Program based on Environmental Policy (No. 2015000700002) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2015R1A5A1037548).
Author Contributions
Jin-Ho Park is the main author, who planned and conducted the experiments and created this paper. Han-Seung Lee is a corresponding author, who managed the general control of the entire paper. Jitendra Kumar Singh performed the experiments of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, H.S.; Park, J.H.; Singh, J.K.; Ismail, M.A. Protection of reinforced concrete structures of waste water treatment reservoirs with stainless steel coating using arc thermal spraying technique in acidified water. Materials 2016, 9, 753. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Krol, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard. Mater. 2008, 160, 47–255. [Google Scholar] [CrossRef] [PubMed]
- Dodo, M.C.; Buffle, M.O.; Ginten, U.V. Oxidation of antibacterial molecules by aqueous ozone: Moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef]
- Hirsch, R.; Ternes, T.A.; Haberer, K.; Kratz, K.L. Occurrence of antibiotics in the environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; Mcardell, C.S.; Joss, A.; Giger, W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Eichorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Hanson, M.L.; Sanderson, H.; Lam, M.W.; Young, C.; Mabury, S.A.; Sibley, P.K.; Solomon, K.R. Microcosm evaluation of the effects of an eight pharmaceutical mixture to the aquatic macrophytes Lemna gibba and Myriophyllum sibiricum. Aquat. Toxicol. 2004, 70, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Brain, R.A.; Sanderson, H.; Johnson, D.J.; Bestari, K.T.; Sibley, P.K.; Solomon, K.R. Structural and functional responses of plankton to a mixture of four tetracyclines in aquatic microcosms. Environ. Sci. Technol. 2004, 38, 6430–6439. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.S.; Shin, J.H. An experimental study on evaluation of bond strength of arc thermal metal spraying according to treatment method of water facilities concrete surface. J. Korea Inst. Build. Construct. 2016, 16, 107–115. [Google Scholar] [CrossRef]
- Lupsea, M.; Tiruta-Barna, L.; Schiopu, N. Leaching of hazardous substances from a composite construction product—An experimental and modelling approach for fibre-cement sheets. J. Hazard. Mater. 2014, 264, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q. Increases of lead and chromium in drinking water from using cement-mortar-lined pipes: Initial modeling and assessment. J. Hazard. Mater. 1997, 56, 181–213. [Google Scholar] [CrossRef]
- Jensen, H.S.; Lens, P.N.L.; Nielsen, J.L.; Bester, K.; Nielsen, A.; Haaning, H.-J.; Thorkild, V.J. Growth kinetics of hydrogen sulfide oxidizing bacteria in corroded concrete from sewers. J. Hazard. Mater. 2011, 189, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Owaki, E.; Okamoto, R.; Nagashio, D. Deterioration of concrete in an advanced water treatment plant. In Concrete Under Severe Condition: Environment and Loading; Gjorv, E., Odd, E., Sakai, K., Banthia, N., Eds.; E & FN Spon: London, UK, 1998; p. 438. [Google Scholar]
- Jang, B.H. A study on the ozone resistance test of concrete. Korea Soc. Civ. Eng. 2014, 10, 327–328. [Google Scholar]
- Swamy, R.N.; Tanikawa, S. An external surface coating to protect the concrete and steel from aggressive environments. Mater. Struct. 1993, 26, 465–478. [Google Scholar] [CrossRef]
- Vera, R.; Apablaza, J.; Carvajal, A.M.; Vera, E. Effect of surface coatings in the corrosion of reinforced concrete in acid environments. Int. J. Electrochem. Sci. 2013, 8, 11832–11846. [Google Scholar]
- Crowe, D.; Nixon, R. Corrosion of stainless steels in waste water applications. Available online: http://www.hwea.org/wp-content/uploads/2015/07/150204_Corrosion_of_Stainless_Steels_in_Wastewater_Applications.pdf (accessed on 19 January 2016).
- Bhalerao, B.B.; Arceivala, S.J. Application of corrosion control techniques in municipal water and waste water engineering. Available online: http://eprints.nmlindia.org/ 5825/1/129--139.PDF (accessed on 9 January 2016).
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Bettridge, D.F.; Ubank, R.G. Quality control of high-temperature protective coatings. Mater. Sci. Technol. 1986, 2, 232–242. [Google Scholar] [CrossRef]
- Jandin, G.; Liao, H.; Feng, Z.Q.; Coddet, C. Correlations between operating conditions, microstructure and mechanical properties of twin wire arc sprayed steel coatings. Mater. Sci. Eng. A 2003, 349, 298–305. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Vourlias, G.; Pavlidou, E.; Stergioudis, G.; Skolianos, S.; Chrissafis, K. High temperature oxidation and corrosion in marine environments of thermal spray deposited coatings. Appl. Surf. Sci. 2008, 255, 3104–3111. [Google Scholar] [CrossRef]
- Choi, H.-B.; Lee, H.-S.; Shin, J.-H. Experimental study on the electrochemical anti-corrosion properties of steel structures applying the arc thermal metal spraying method. Materials 2014, 7, 7722–7736. [Google Scholar] [CrossRef]
- Paredes, R.S.C.; Amico, S.C.; d’Oliveira, A.S.C.M. The effect of roughness and pre-heating of the substrate on the morphology of aluminium coatings deposited by thermal spraying. Surf. Coat. Technol. 2006, 200, 3049–3055. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C. Corrosion resistance properties of aluminum coating applied by arc thermal metal spray in SAE J2334 solution with exposure periods. Metals 2016, 6, 55. [Google Scholar] [CrossRef]
- Ozone Resistance Test Method of Material for Waterproof and Anticorrosion; SPS KWWA M211; Korea Water and Wastewater Works Association (KWWA): Seoul, Korea, 2015.
- Cement Filling Compound for Surface Preparation; Korean Standard KS K 4716; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2016.
- Cement-Polymer Modified Waterproof Coatings; Korean Standard KS K 4919; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2008.
- Filter Paper (for Chemical Analysis); Korean Standard KS M 7602; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2007.
- Penetrating Water Repellency of Liquid Type for Concrete Surface Application; Korean Standard KS F 4930; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2012.
- Lai, J.K.L. A review of precipitation behaviour in AISI type 316 stainless steel. Mater. Sci. Eng. 1983, 61, 101–109. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).