Suppressing Grain Growth on Cu Foil Using Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- The pressure in the chamber was pumped down to 5 mTorr using a mechanical pump;
- Hydrogen (H2) gas was injected into the chamber at 40 sccm, the temperature of the Cu foil then raised to 1000 °C over 60 min;
- CH4 gas was injected into the chamber at 10 sccm for 10 min, for Samples A and B; for Sample C, there was no CH4 injection;
- Finally, the furnace was opened and rapidly cooled for about 20 min to 200 °C.
2.2. Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beaudoin, A.J.; Acharya, A.; Chen, S.R.; Korzekwa, D.A.; Stout, M.G. Consideration of grain-size effect and kinetics in the plastic deformation of metal polycrystals. Acta Mater. 2000, 48, 3409–3423. [Google Scholar] [CrossRef]
- Saida, K.; Zhou, Y.; North, T.H. The influence of base metal grain size on isothermal solidification during transient liquid-phase brazing of nickel. J. Mater. Sci. 1993, 28, 6427–6432. [Google Scholar] [CrossRef]
- Wu, P.D.; MacEwen, S.R.; Lloyd, D.J.; Neale, K.W. Effect of cube texture on sheet metal formability. Mater. Sci. Eng. A 2004, 364, 182–187. [Google Scholar] [CrossRef]
- Stanford, N.; Barnett, M. Effect of composition on the texture and deformation behaviour of wrought Mg alloys. Scr. Mater. 2008, 58, 179–182. [Google Scholar] [CrossRef]
- Koike, J.; Wada, M.; Sanada, M.; Maruyama, K. Effects of crystallographic texture on stress-migration resistance in copper thin films. Appl. Phys. Lett. 2002, 81, 1017–1019. [Google Scholar] [CrossRef]
- Hassan, A.M. The effects of ball- and roller-burnishing on the surface roughness and hardness of some non-ferrous metals. J. Mater. Process. Technol. 1997, 72, 385–391. [Google Scholar] [CrossRef]
- Kim, S.H.; Na, S.W.; Lee, N.E.; Nam, Y.W.; Kim, Y.H. Effect of surface roughness on the adhesion properties of Cu/Cr films on polyimide substrate treated by inductively coupled oxygen plasma. Surf. Coat. Technol. 2005, 200, 2072–2079. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behavior. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Harper, J.M.E.; Cabral Jr, C.; Andricacos, P.C.; Gignac, L.; Noyan, I.C.; Rodbell, K.P.; Hu, C.K. Mechanisms for microstructure evolution in electroplated copper thin films near room temperature. J. Appl. Phys. 1999, 86, 2516–2525. [Google Scholar] [CrossRef]
- Koo, J.B.; Yoon, D.Y. Abnormal grain growth in bulk Cu—The dependence on initial grain size and annealing temperature. Metall. Mater. Trans. A 2001, 32, 1911–1926. [Google Scholar] [CrossRef]
- Weihnacht, V.; Brückner, W. Abnormal grain growth in {111} textured Cu thin films. Thin Solid Films 2002, 418, 136–144. [Google Scholar] [CrossRef]
- Huang, J.S.; Zhang, J.; Cuevas, A.; Tu, K.N. Recrystallization and grain growth in bulk Cu and Cu(Sn) alloy. Mater. Chem. Phys. 1997, 49, 33–41. [Google Scholar] [CrossRef]
- Hibbard, G.D.; McCrea, J.L.; Palumbo, G.; Aust, K.T.; Erb, U. An initial analysis of mechanisms leading to late stage abnormal grain growth in nanocrystalline Ni. Scr. Mater. 2002, 47, 83–87. [Google Scholar] [CrossRef]
- Afshar, A.; Simchi, A. Abnormal grain growth in alumina dispersion-strengthened copper produced by an internal oxidation process. Scr. Mater. 2008, 58, 966–969. [Google Scholar] [CrossRef]
- Barmak, K.; Gungor, A.; Cabral, C.; Harper, J.M.E. Annealing behavior of Cu and dillute Cu-alloy films: Precipitation, grain growth, and resistivity. J. Appl. Phys. 2003, 94, 1605–1616. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Cheng, X.; Li, Z.; Liu, Y.; Dong, C. Enhanced thermal stability of Cu alloy films by strong interaction between Ni and Zr (or Fe). J. Phys. D Appl. Phys. 2018, 51, 135304. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.G.; Park, H.J.; Ahn, S.H.; Guo, L.J. Transparent Cu nanowire mesh electrode on flexible substrates fabricated by transfer printing and its application in organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1179–1184. [Google Scholar] [CrossRef]
- Zhuang, Z.; Su, X.; Yuan, H.; Sun, Q.; Xiao, D.; Choi, M.M. An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 2008, 133, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Rossnagel, S.M.; Kuan, T.S. Alteration of Cu conductivity in the size effect regime. J. Vac. Sci. Technol. B 2004, 22, 240–247. [Google Scholar] [CrossRef]
- Takata, N.; Lee, S.H.; Tsuji, N. Ultrafine grained copper alloy sheets having both high strength and high electric conductivity. Mater. Lett. 2009, 63, 1757–1760. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Chen, D.R.; Chiang, W.Y.; Chen, K.J.; Hofmann, M. Recrystallization of copper at a solid interface for improved CVD graphene growth. RSC Adv. 2017, 7, 3736–3740. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Rim, K.T.; Zhou, H.; He, R.; Heinz, T.F.; Pinczuk, A.; Flynn, G.W.; Pasupathy, A.N. Influence of copper crystal surface on the CVD growth of alrge area monolayer graphene. Solid State Commun. 2011, 151, 509–513. [Google Scholar] [CrossRef]
- Lu, A.Y.; Wei, S.Y.; Wu, C.Y.; Hernandez, Y.; Chen, T.Y.; Liu, T.H.; Pao, C.W.; Chen, F.R.; Li, L.J.; Juang, Z.Y. Decoupling of CVD graphene by controlled oxidation of recrystallized Cu. RSC Adv. 2012, 2, 3008–3013. [Google Scholar] [CrossRef]
- Giovannetti, G.A.K.P.A.; Khomyakov, P.A.; Brocks, G.; Karpan, V.V.; van den Brink, J.; Kelly, P.J. Doping graphene with metal contacts. Phys. Rev. Lett. 2008, 101, 026803. [Google Scholar] [CrossRef] [PubMed]
- Vanin, M.; Mortensen, J.J.; Kelkkanen, A.K.; Garcia-Lastra, J.M.; Thygesen, K.S.; Jacobsen, K.W. Graphene on metals: A van der Waals density functional study. Phys. Rev. B 2010, 81, 081408. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Li, M.; Chen, F.; Eklund, P.; Xue, J.; Huang, F.; Du, S.; Huang, Q. Effect of carbide interlayers on the microstructure and properties of graphene-nanoplatelet-reinforced copper matrix composites. Mat. Sci. Eng. A 2017, 708, 311–318. [Google Scholar] [CrossRef]
- Chu, K.; Wang, F.; Wang, X.H.; Huang, D.J. Anisotropic mechanical properties of graphene/copper composites with aligned graphene. Mat. Sci. Eng. A 2018, 713, 269–277. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, M.; Crimp, M.A.; Wang, Y.; Qu, Z. Anisotropic thermal expansion coefficient of multilayer graphene reinforced copper matrix composites. J. Alloy. Compd. 2018, 755, 114–122. [Google Scholar] [CrossRef]
- Wofford, J.M.; Nie, S.; McCarty, K.F.; Bartelt, N.C.; Dubon, O.D. Graphene island on Cu foils: The interplay between shape, orientation, and defects. Nano Lett. 2010, 10, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Goli, P.; Ning, H.; Li, X.; Lu, C.Y.; Novoselov, K.S.; Balandin, A.A. Thermal properties of graphene−copper−graphene heterogeneous films. Nano Lett. 2014, 14, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, E.M.; Vinci, R.P.; Bravman, J.C. Effects of barrier layer and annealing on abnormal grain growth in copper thin films. J. Appl. Phys. 1994, 76, 4516–4523. [Google Scholar] [CrossRef]
- ASTM E112-13 Standard Test Methods for Determining Average Grain Size; ASTM International: West Conshohocken, PA, USA, 2013.
- Malard, L.M.; Pimenta, M.A.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Park, J.S.; Reina, A.; Saito, R.; Kong, J.; Dresselhaus, G.; Dresselhaus, M.S. G’ band Raman spectra of single, double and triple layer graphene. Carbon 2009, 47, 1303–1310. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, Y.H.; Jeong, H.S.; Jung, H.T. Direct visualization of large-area graphene domains and boundaries by optical birefringency. Nat. Nanotechnol. 2012, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Simoes, S.; Calinas, R.; Vieira, M.T.; Vieira, M.F.; Ferreira, P.J. In situ TEM study of grain growth in nanocrystalline copper thin films. Nanotechnology 2010, 21, 145701. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.V. Overview no.65: Theories of normal grain growth in pure single phase systems. Acta Metall. 1988, 36, 469–491. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Aaron, H.B.; Aaronson, H.I. Growth of grain boundary precipitates in Al-4% Cu by interfacial diffusion. Acta Metall. 1968, 16, 789–798. [Google Scholar] [CrossRef]
- Speight, M.V. Growth kinetics of grain-boundary precipitates. Acta Metall. 1968, 16, 133–135. [Google Scholar] [CrossRef]

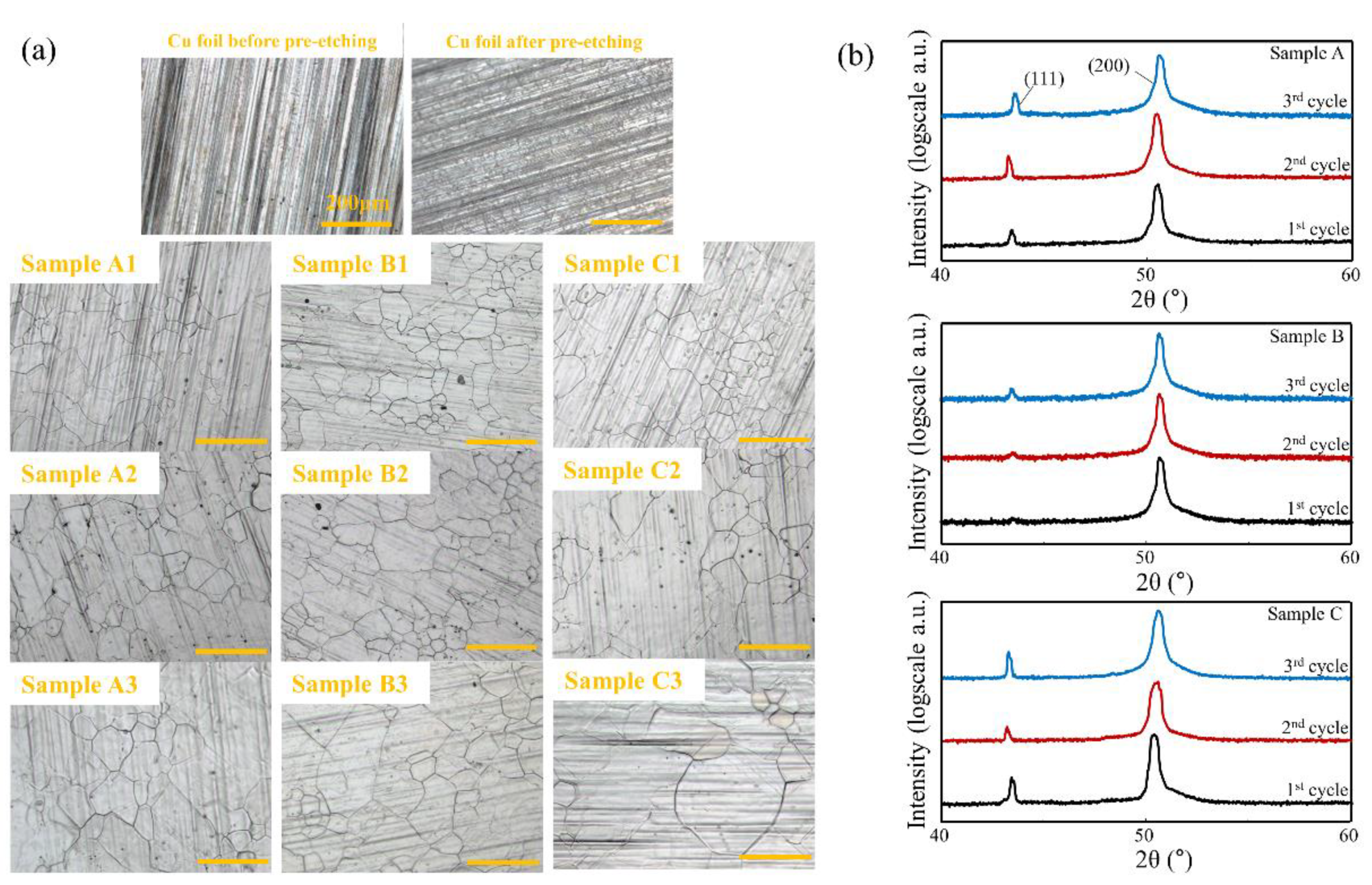
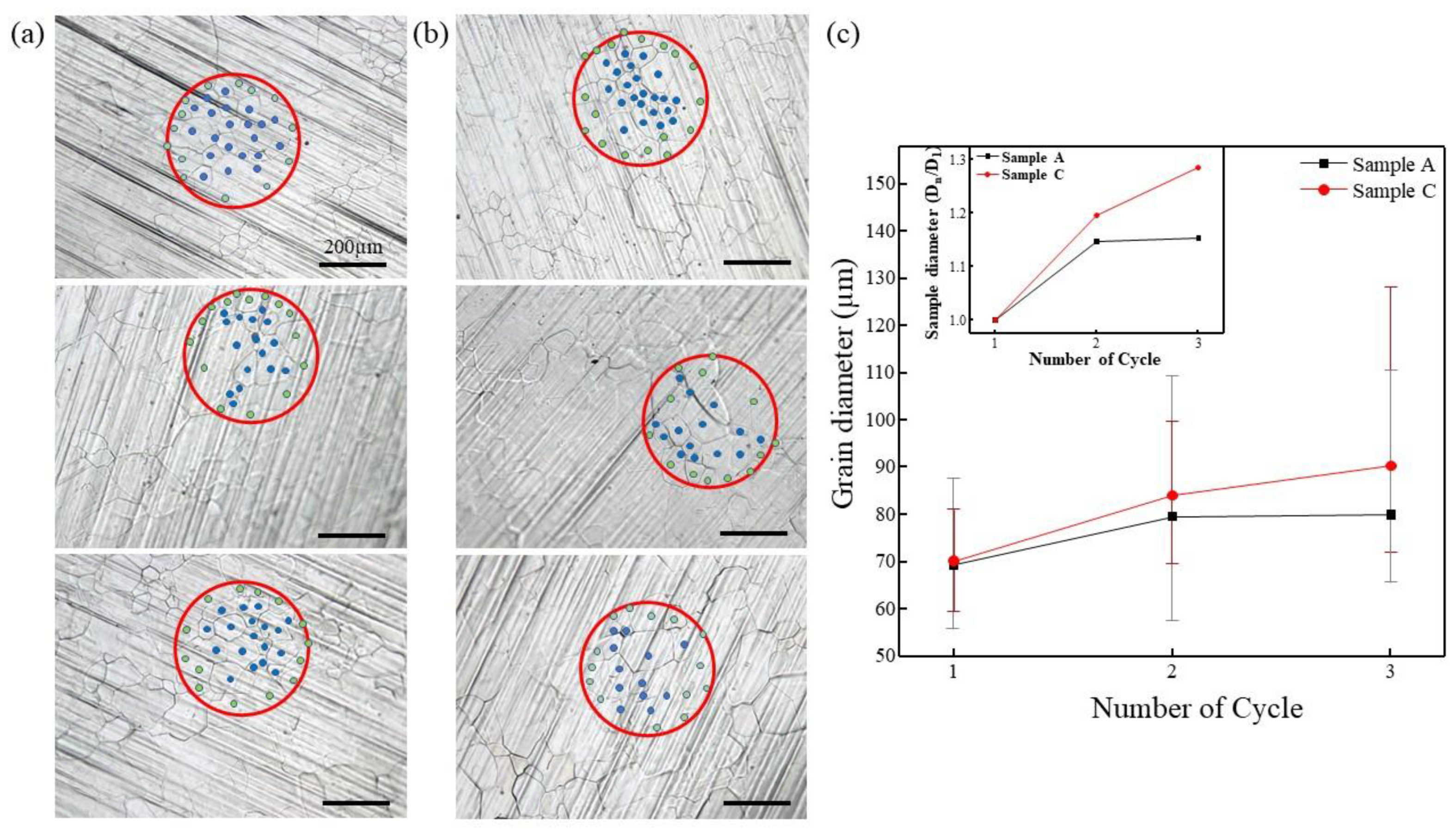

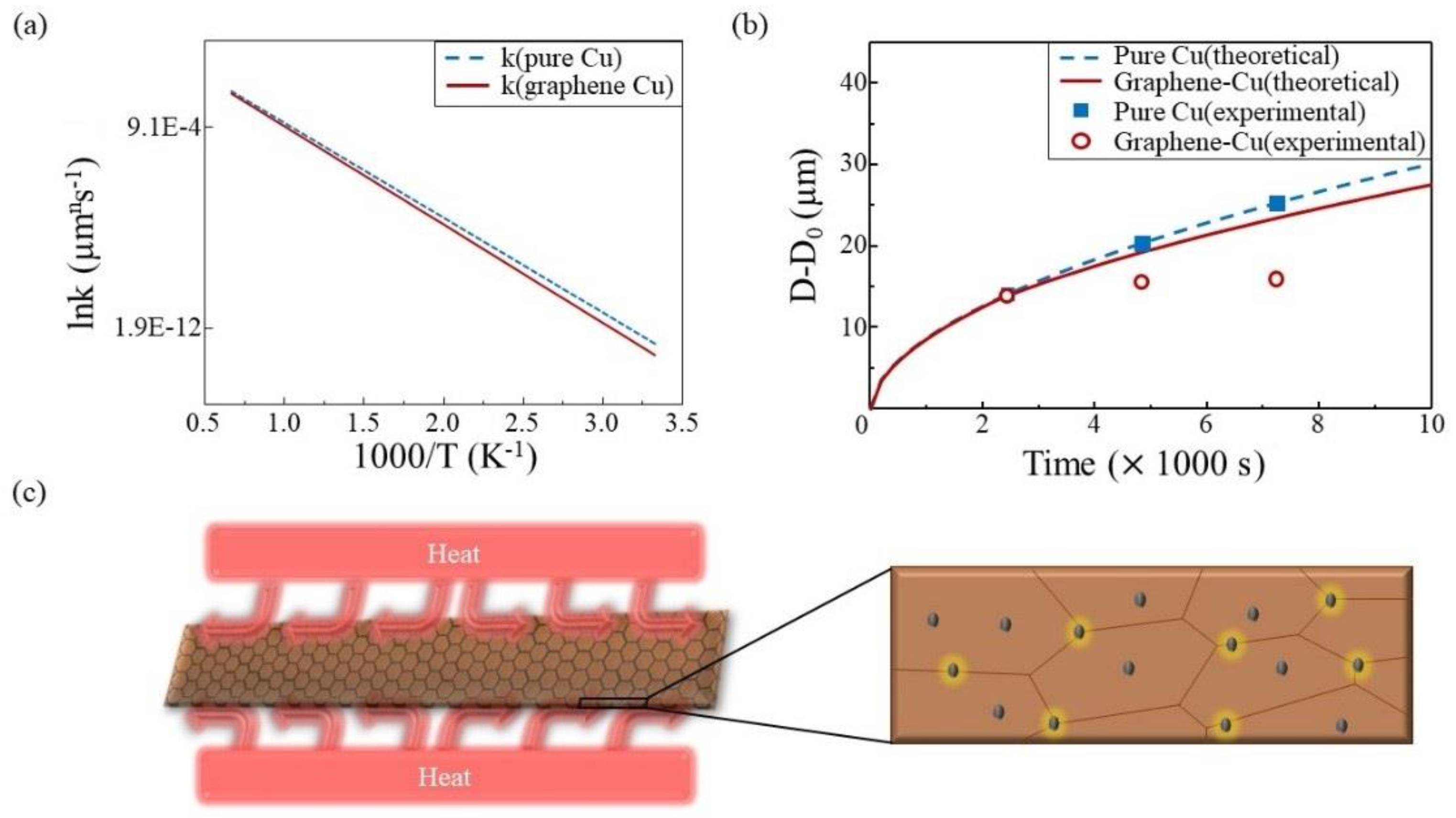
| Injected Gas | 1st Cycle | 2nd Cycle | 3rd Cycle |
|---|---|---|---|
| Sample A | CH4 | CH4 | CH4 |
| Sample B | CH4 | X | X |
| Sample C | X | X | X |
| FWHM | I(111)/I(200) Ratio | FWHM (200) | FWHM (111) |
|---|---|---|---|
| Sample A1 | 0.0183 | 0.26 | 0.137 |
| Sample A2 | 0.0308 | 0.31 | 0.105 |
| Sample A3 | 0.0216 | 0.28 | 0.127 |
| Sample C1 | 0.0312 | 0.27 | 0.101 |
| Sample C2 | 0.0230 | 0.275 | 0.117 |
| Sample C3 | 0.0421 | 0.27 | 0.097 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Shin, H.; Choi, J.-Y.; Yu, H.K. Suppressing Grain Growth on Cu Foil Using Graphene. Coatings 2018, 8, 334. https://doi.org/10.3390/coatings8100334
Lee J, Shin H, Choi J-Y, Yu HK. Suppressing Grain Growth on Cu Foil Using Graphene. Coatings. 2018; 8(10):334. https://doi.org/10.3390/coatings8100334
Chicago/Turabian StyleLee, Jaeyeong, Hojun Shin, Jae-Young Choi, and Hak Ki Yu. 2018. "Suppressing Grain Growth on Cu Foil Using Graphene" Coatings 8, no. 10: 334. https://doi.org/10.3390/coatings8100334
APA StyleLee, J., Shin, H., Choi, J.-Y., & Yu, H. K. (2018). Suppressing Grain Growth on Cu Foil Using Graphene. Coatings, 8(10), 334. https://doi.org/10.3390/coatings8100334




