Effects of Chitosan-Based Coatings Enriched with Cinnamaldehyde on Mandarin Fruit cv. Ponkan during Room-Temperature Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. Preparation of Coating
2.3. Fruit Treatments
2.4. Quality Determinations
2.4.1. Weight Loss
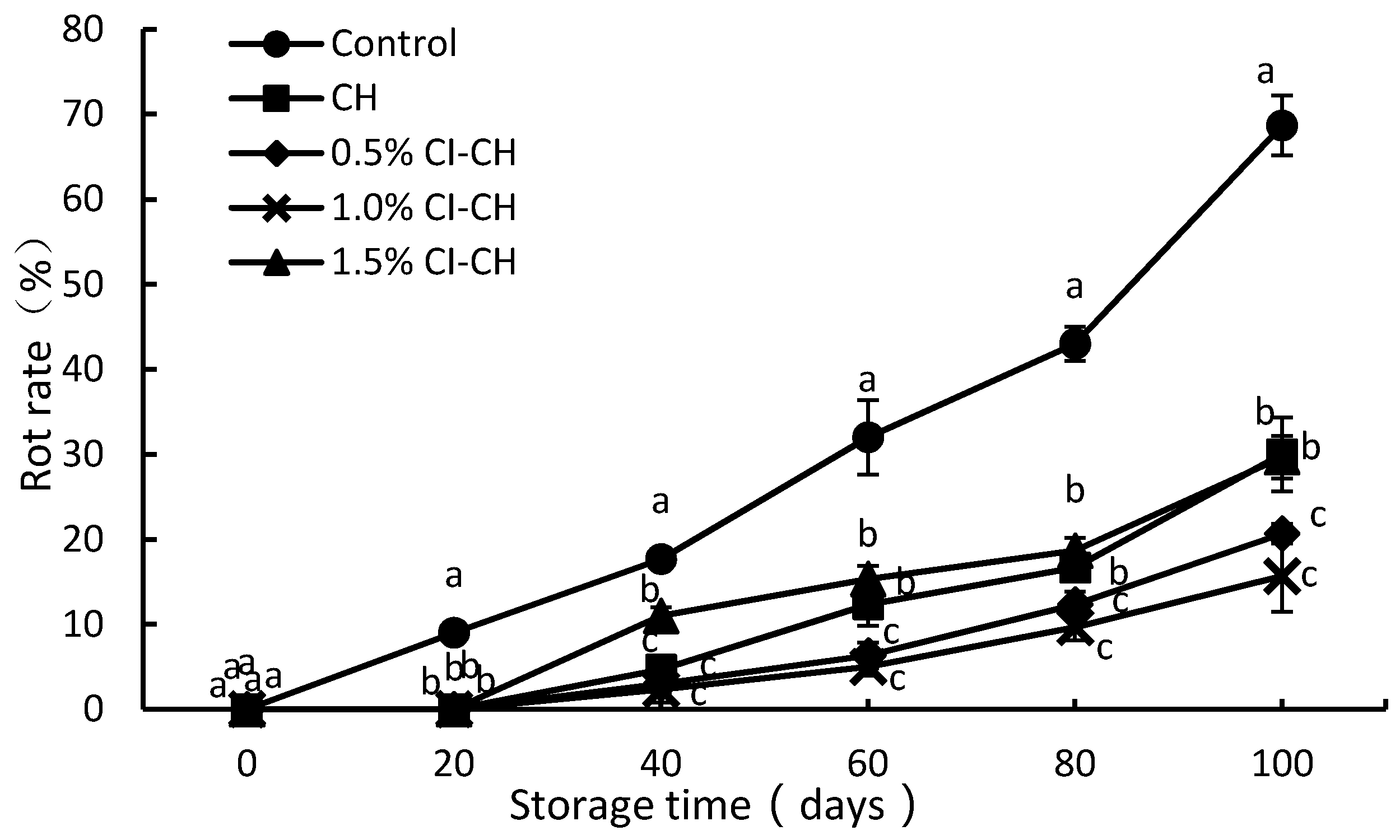
2.4.2. Decay Rate
2.4.3. Total Soluble Solids, Titratable Acidity, and Vitamin C
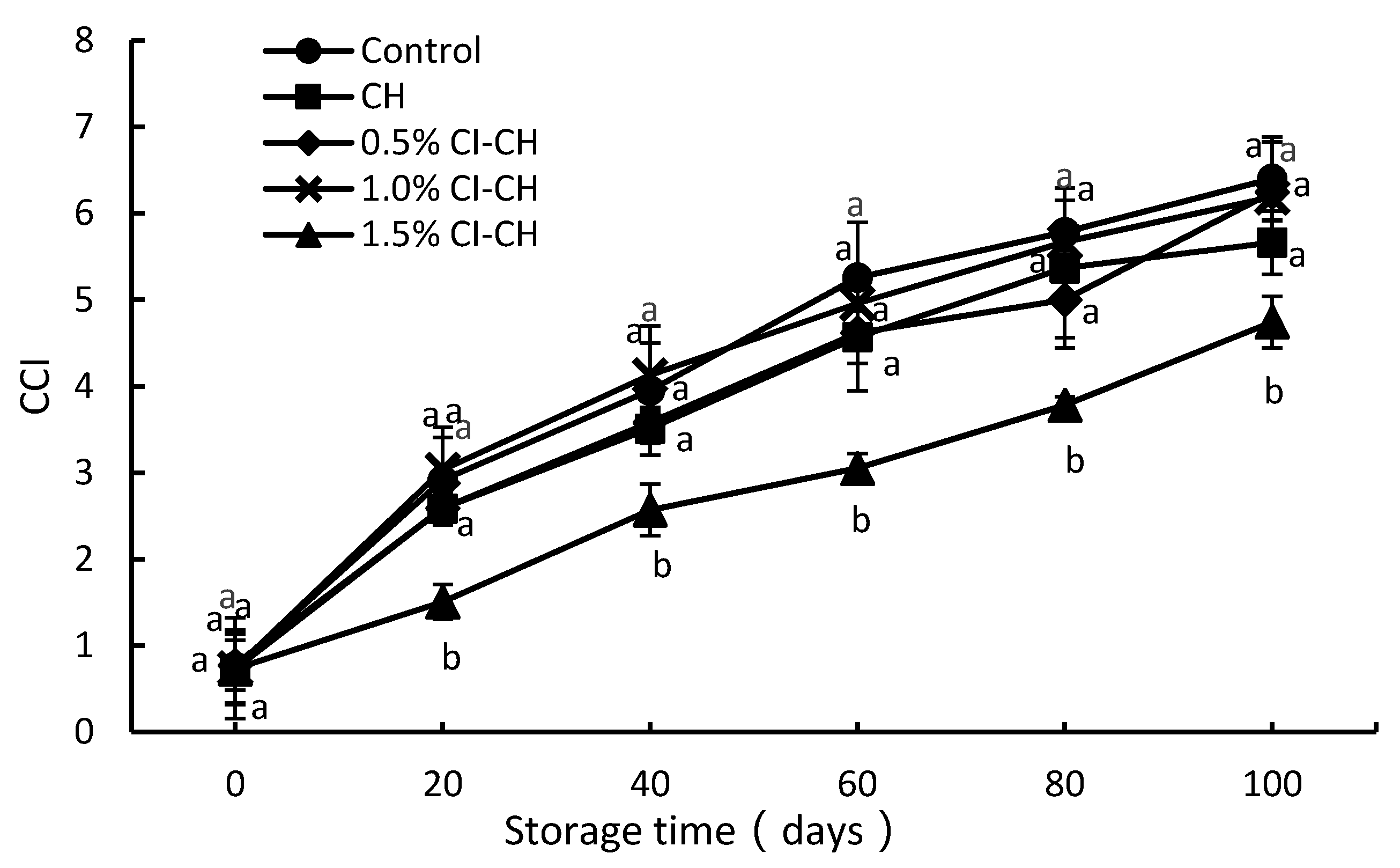
2.4.4. Color Index
2.4.5. Content of Malondialdehyde of Fruit Peel
2.4.6. Enzyme Measurements of Fruit Peel
2.5. Data Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Decay Rate
3.3. Total Soluble Solids, Titratable Acidity, and Vitamin C
3.4. Color Index
3.5. Malondialdehyde
3.6. Activities of Catalase, Peroxidase, and Superoxide Dismutase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roongruangsri, W.; Rattanapanone, N.; Leksawasdi, N.; Boonyakiat, D. Changes in organic acid contents and related metabolic enzyme activities at different stages of growth of two tangerine cultivars. J. Agric. Sci. 2012, 4, 276–284. [Google Scholar] [CrossRef]
- Tang, D.; Lin, Q.; Lin, J.; Wang, D.; Liu, C.; Wu, W.; Sun, C.; Chen, K. Effects of combined heat and preservative treatment on storability of Ponkan fruit (Citrus reticulata Blanco cv. Ponkan) during postharvest storage. J. Food Qual. 2017, 2017, 5871756. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Ping, L.; Liu, S.; Tao, L.; Shuai, J.; Qiang, X.; Xu, J.; Cheng, Y.; Deng, X. Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC. Plant Biol. 2013, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R. Effects of temperature on fruit thinning with ethephon in ‘Golden Delicious’ apples. Sci. Hortic. 2007, 113, 8–12. [Google Scholar] [CrossRef]
- Rab, A.; Sajid, M.; Bibi, F.; Jan, I.; Nabi, G.; Nawab, K. Quality changes in heat treated sweet orange fruit during storage at low temperature. J. Anim. Plant Sci. 2015, 25, 661–668. [Google Scholar]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait, B.A.A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, K.; Ligorio, A.; Nigro, F.; Ippolito, A. Activity of salts incorporated in wax in controlling postharvest diseases of citrus fruit. Postharvest Biol. Technol. 2012, 65, 39–43. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.; Pérez-Gago, M. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Tunc, S.; Chollet, E.; Chalier, P.; Preziosi-Belloy, L.; Gontard, N. Combined effect of volatile antimicrobial agents on the growth of Penicillium notatum. Int. J. Mol. Sci. 2007, 113, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B.; Lieu, F.Y.; Seier, S.A. Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol. J. Food Sci. 1977, 42, 1107–1109. [Google Scholar] [CrossRef]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, P.; Chen, C.; Peng, X.; Li, M.; Chen, M.; Wang, J.; Chen, J. Antifungal activity of Ramulus cinnamomi explored by ¹H-NMR based metabolomics approach. Molecules 2017, 22, 2237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wan, C.P.; Peng, X.; Chen, J.Y. Study on the antifungal mechanisms of the main active ingredients of Ramulus cinnamomi against Penicillium italicum. Modern Food Sci. Technol. 2016, 32, 45–51, 65. [Google Scholar]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Duan, X.; Ouyang, Q.; Tao, N. Effect of applying cinnamaldehyde incorporated in wax on green mould decay in citrus fruits. J. Sci. Food Agric. 2018, 98, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, X.; Jing, G.; Ouyang, Q.; Tao, N. Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Ali, A.; Mahmud Tengku, M.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2010, 124, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.K.; Shipra, T.; Mehrotra, G.K.; Joydeep, D. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication and testing of pva/chitosan bilayer films for strawberry packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]
- Egusa, M.; Iwamoto, R.; Izawa, H.; Morimoto, M.; Saimoto, H.; Kaminaka, H.; Ifuku, S. Characterization of chitosan nanofiber sheets for antifungal application. Int. J. Mol. Sci. 2015, 16, 26202–26210. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-based coating with antimicrobial agents: Preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Fortunati, E.; Giovanale, G.; Luzi, F.; Mazzaglia, A.; Kenny, J.; Torre, L.; Balestra, G. Effective postharvest preservation of kiwifruit and romaine lettuce with a chitosan hydrochloride coating. Coatings 2017, 7, 196. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1989, 186, 421. [Google Scholar]
- Lo’ay, A.A.; Taher, M.A. Effectiveness salicylic acid blending in chitosan/pvp biopolymer coating on antioxidant enzyme activities under low storage temperature stress of ‘Banati’ guava fruit. Sci. Hortic. 2018, 238, 343–349. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Wan, C.; Chen, M.; Chen, J. Physiological and biochemical responses in cold-stored citrus fruits to carboxymethyl cellulose coating containing ethanol extract of Impatiens balsamina L. Stems. J. Food Process Pres. 2016, 41, e12999. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X.; Lin, Q.; Chen, J.; Grierson, D.; Yin, X.; Sun, C.; Chen, K. Differential expression of organic acid degradation-related genes during fruit development of navel oranges (Citrus sinensis) in two habitats. Plant Mol. Biol. Rep. 2013, 31, 1131–1140. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Sun, C.D.; Zhang, L.L.; Xiao, D.; Xu, C.J.; Chen, K.S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. 2010, 126, 229–235. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci. 2004, 166, 293–302. [Google Scholar] [CrossRef]
- Hirai, M.; Ueno, I. Development of citrus fruits: Fruit development and enzymatic changes in juice vesicle tissue. Plant Cell Physiol. 1977, 18, 791–799. [Google Scholar]
- Yaman, O.; Bayoindirli, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, C.; Wan, C.; Chen, C.; Chen, M.; Chen, J. Quality and biochemical changes of navel orange fruits during storage as affected by cinnamaldehyde-chitosan coating. Sci. Hortic. 2018, 239, 80–86. [Google Scholar] [CrossRef]
- Petriccione, M.; Sanctis, F.D.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess. Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Deng, L.; Zeng, K.; Zhou, Y.; Huang, Y. Effects of postharvest oligochitosan treatment on anthracnose disease in citrus (Citrus sinensis L. Osbeck) fruit. Eur. Food Res. Technol. 2015, 240, 795–804. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Mares, M.; Paslaru, E.; Cristea, M.; Nica, V.; Simionescu, B.C. Imino-chitosan biopolymeric films. Obtaining, self-assembling, surface and antimicrobial properties. Carbohydr. Polym. 2015, 117, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Moraru, S.; Popescu, M.C.; Nicolescu, A.; Zgardan, C.; Simionescu, B.C.; Barboiu, M. Out-of-water constitutional self-organization of chitosan-cinnamaldehyde dynagels. Chem. Eur. J. 2014, 20, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; Mcclements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocolloids 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Ling, S.; Shen, D.; Yi, L.; Sun, X.; Wang, J.; Tao, L.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef]
- Kou, X.H.; Wang, S.; Zhang, Y.; Guo, R.Z.; Wu, M.S.; Chen, Q.; Xue, Z.H. Effects of chitosan and calcium chloride treatments on malic acid-metabolizing enzymes and the related gene expression in post-harvest pear cv. ‘Huang guan’. Sci. Hortic. 2014, 165, 252–259. [Google Scholar] [CrossRef]
- Peng, G.; Xie, X.L.; Jiang, Q.; Song, S.; Xu, C.J. Chlorophyll a/b binding protein plays a key role in natural and ethylene-induced degreening of Ponkan (Citrus reticulata Blanco). Sci. Hortic. 2013, 160, 37–43. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.K.; Zhu, H.Y.; Cao, M.J.; Zhang, J.J.; Wang, X.G.; Chen, Y.J. Relation between leaf mda content and protection system of low potassium tolerant maize inbred lines under different potassium concentration. J. Jilin Agric. Univ. 2010, 32, 5–8. [Google Scholar]
- Kou, X.H.; Guo, W.L.; Guo, R.Z.; Li, X.Y.; Xue, Z.H. Effects of chitosan, calcium chloride, and pullulan coating treatments on antioxidant activity in pear cv. “Huang guan” during storage. Food Bioprocess Technol. 2014, 7, 671–681. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Kan, C.; Chen, M.; Chen, C.; Chen, Y.; Fu, Y.; Wan, C.; Chen, J. Effects of Chitosan-Based Coatings Enriched with Cinnamaldehyde on Mandarin Fruit cv. Ponkan during Room-Temperature Storage. Coatings 2018, 8, 372. https://doi.org/10.3390/coatings8100372
Gao Y, Kan C, Chen M, Chen C, Chen Y, Fu Y, Wan C, Chen J. Effects of Chitosan-Based Coatings Enriched with Cinnamaldehyde on Mandarin Fruit cv. Ponkan during Room-Temperature Storage. Coatings. 2018; 8(10):372. https://doi.org/10.3390/coatings8100372
Chicago/Turabian StyleGao, Yang, Chaonan Kan, Ming Chen, Chuying Chen, Yuhuan Chen, Yongqi Fu, Chunpeng Wan, and Jinyin Chen. 2018. "Effects of Chitosan-Based Coatings Enriched with Cinnamaldehyde on Mandarin Fruit cv. Ponkan during Room-Temperature Storage" Coatings 8, no. 10: 372. https://doi.org/10.3390/coatings8100372
APA StyleGao, Y., Kan, C., Chen, M., Chen, C., Chen, Y., Fu, Y., Wan, C., & Chen, J. (2018). Effects of Chitosan-Based Coatings Enriched with Cinnamaldehyde on Mandarin Fruit cv. Ponkan during Room-Temperature Storage. Coatings, 8(10), 372. https://doi.org/10.3390/coatings8100372





