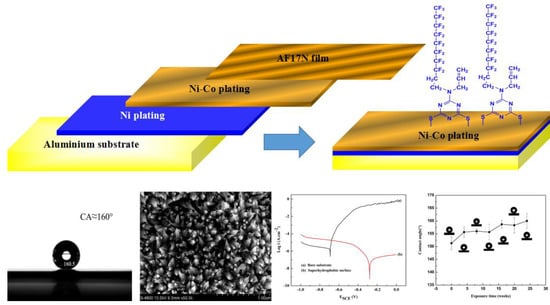
Fabrication of Superhydrophobic AA5052 Aluminum Alloy Surface with Improved Corrosion Resistance and Self Cleaning Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Superhydrophobic Coatings on AA5052 Surface
2.3. Characterization
3. Results
3.1. Surface Morphology and Chemical Compositions
3.2. Chemical Stability and Corrosion Resistance
3.3. Self-Cleaning Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loto, R.T.; Adeleke, A. Corrosion of aluminum alloy metal matrix composites in neutral chloride solutions. J. Fail. Anal. Prev. 2016, 16, 874–885. [Google Scholar] [CrossRef]
- Quazi, M.M.; Fazal, M.A.; Haseeb, A.S.M.A.; Yusof, F.; Masjuki, H.H.; Arslan, A. Laser-based surface modifications of aluminum and its alloys. Crit. Rev. Solid State Mater. Sci. 2016, 41, 106–131. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Gudić, S.; Vrsalović, L.; Kliškić, M.; Jerković, I.; Radonić, A.; Zekić, M. Corrosion inhibition of aa 5052 aluminium alloy in nacl solution by different types of honey. Int. J. Electrochem. Sci. 2016, 11, 998–1011. [Google Scholar]
- Uwiringiyimana, E.; O’Donnell, P.S.; Joseph, I.V.; Adams, F.V. The effect of corrosion inhibitors on stainless steels and aluminium alloys: A review. Afr. J. Pure Appl. Chem. 2016, 10, 23–32. [Google Scholar]
- Singh, B.P.; Jena, B.K.; Bhattacharjee, S.; Besra, L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surf. Coat. Technol. 2013, 232, 475–481. [Google Scholar] [CrossRef]
- Asmone, A.S.; Chew, M.Y.L. An investigation of superhydrophobic self-cleaning applications on external building façade systems in the tropics. J. Build. Eng. 2018, 17, 167–173. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced superhydrophobic coating on silicone rubber for longstanding anti-icing performance in severe conditions. Acs Appl. Mater. Interface 2017, 9, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Park, S.; Lim, H. Effects of morphology parameters on anti-icing performance in superhydrophobic surfaces. Appl. Surf. Sci. 2018, 435, 585–591. [Google Scholar] [CrossRef]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Chen, L.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated pvdf membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Wu, P.; Zhang, S.; Geng, B. Facile preparation of superhydrophobic and superoleophilic porous polymer membranes for oil/water separation from a polyarylester polydimethylsiloxane block copolymer. J. Mater. Sci. 2016, 51, 3211–3218. [Google Scholar] [CrossRef]
- Yagüe, J.L.; Segadó, P.; Auset, M.; Borrós, S. Textured superhydrophobic films on copper prepared using solvent-free methods exhibiting antifouling properties. Thin Solid Film 2017, 635, 32–36. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Coll. Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Li, S.; Han, Z.; Yu, S.; Ren, L. Fabrication of biomimetic super-hydrophobic surface on aluminum alloy. J. Mater. Sci. 2014, 49, 1624–1629. [Google Scholar] [CrossRef]
- Dey, S.; Chatterjee, S.; Singh, B.P.; Bhattacharjee, S.; Rout, T.K.; Sengupta, D.K.; Besra, L. Development of superhydrophobic corrosion resistance coating on mild steel by electrophoretic deposition. Surf. Coat. Technol. 2018, 72, 220–235. [Google Scholar] [CrossRef]
- Qian, H.C.; Li, H.Y.; Zhang, D.W. Research progress of superhydrophobic surface technologies in the field of corrosion protection. Surf. Technol. 2015, 3, 15–24. [Google Scholar]
- Hoshian, S.; Jokinen, V.; Somerkivi, V.; Lokanathan, A.R.; Franssila, S. Robust superhydrophobic silicon without a low surface-energy (hydrophobic) coating. ACS Appl. Mater. Interface 2015, 7, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, F.; Chen, H.; Chen, D. Superhydrophobic behavior achieved from hydrophilic surfaces. Appl. Phys. Lett. 2009, 95, 3063–3067. [Google Scholar] [CrossRef]
- Forooshani, H.M.; Aliofkhazraei, M.; Rouhaghdam, A.S. Superhydrophobic aluminum surfaces by mechanical/chemical combined method and its corrosion behavior. J. Taiwan Inst. Chem. Eng. 2017, 72, 220–235. [Google Scholar] [CrossRef]
- Xiong, J.; Sarkar, D.K.; Chen, X.G. Superhydrophobic honeycomb-like cobalt stearate thin films on aluminum with excellent anti-corrosion properties. Appl. Surf. Sci. 2017, 407, 361–370. [Google Scholar] [CrossRef]
- Karthik, N.; Yong, R.L.; Sethuraman, M.G. Fabrication of corrosion resistant mussel-yarn like superhydrophobic composite coating on aluminum surface. J. Taiwan Inst. Chem. Eng. 2017, 77, 302–310. [Google Scholar] [CrossRef]
- Kang, Z.; Ye, Q.; Sang, J.; Li, Y. Fabrication of super-hydrophobic surface on copper surface by polymer plating. J. Mater. Process. Technol. 2009, 209, 4543–4547. [Google Scholar] [CrossRef]
- Fang, W.; Wang, Y.; Li, Y.; Qian, W. Fabrication of triazinedithiol functional polymeric nanofilm by potentiostatic polymerization on aluminum surface. Appl. Surf. Sci. 2011, 257, 2423–2427. [Google Scholar]
- Zhao, Q.; Tang, T.; Dang, P.; Zhang, Z.; Wang, F. Preparation and analysis of complex barrier layer of heterocyclic and long-chain organosilane on copper alloy surface. Metals 2016, 6, 162. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, T.; Dang, P.; Zhang, Z.; Wang, F. The corrosion inhibition effect of triazinedithiol inhibitors for aluminum alloy in a 1 m hcl solution. Metals 2017, 7, 44. [Google Scholar] [CrossRef]
- Mori, K.; Hirahara, H.; Oishi, Y.; Kumagai, N. Effect of triazine dithiols on the polymer plating of magnesium alloys. Mater. Sci. Forum 2000, 350, 223–234. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Tan, C.; Zhou, J.; Li, L. Highly anticorrosion, self-cleaning superhydrophobic Ni–Co surface fabricated on AZ91D magnesium alloy. Surf. Coat. Technol. 2014, 251, 7–14. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lee, Y.; Ju, K.Y.; Lee, J.K. Stable biomimetic superhydrophobic surfaces fabricated by polymer replication method from hierarchically structured surfaces of al templates. Langmuir 2010, 26, 14103–14110. [Google Scholar] [CrossRef] [PubMed]










| Compositions | Concentration (g L−1) | Conditions |
|---|---|---|
| NiCl2·6H2O | 113 | Current densities: 3 mA cm−2 |
| CoCl2·6H2O | 8 | pH: 3.6 |
| C2H8N2·2HCl | 100 | Temperature: 50 °C |
| H3BO3 | 15 | Time: 360 s |
| Sample | Ecorr (V) | Icorr (A cm−2) | Corrision Rate (mm a−1) | PE (%) |
|---|---|---|---|---|
| Bare aluminum alloy | −0.69 | 1.03 × 10−2 | 1.12 × 10−1 | − |
| Superhydrophobic | −0.28 | 6.76 × 10−5 | 7.39 × 10−4 | 99.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Tang, T.; Wang, F. Fabrication of Superhydrophobic AA5052 Aluminum Alloy Surface with Improved Corrosion Resistance and Self Cleaning Property. Coatings 2018, 8, 390. https://doi.org/10.3390/coatings8110390
Zhao Q, Tang T, Wang F. Fabrication of Superhydrophobic AA5052 Aluminum Alloy Surface with Improved Corrosion Resistance and Self Cleaning Property. Coatings. 2018; 8(11):390. https://doi.org/10.3390/coatings8110390
Chicago/Turabian StyleZhao, Qian, Tiantian Tang, and Fang Wang. 2018. "Fabrication of Superhydrophobic AA5052 Aluminum Alloy Surface with Improved Corrosion Resistance and Self Cleaning Property" Coatings 8, no. 11: 390. https://doi.org/10.3390/coatings8110390
APA StyleZhao, Q., Tang, T., & Wang, F. (2018). Fabrication of Superhydrophobic AA5052 Aluminum Alloy Surface with Improved Corrosion Resistance and Self Cleaning Property. Coatings, 8(11), 390. https://doi.org/10.3390/coatings8110390