Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen and Plasma Electrolytic Oxidation (PEO) Treatment
2.2. Microstructural and Phase Composition
2.3. Corrosion Test
2.3.1. Electrochemical Experiments
2.3.2. Long-Term Immersion Tests
2.4. Cytotoxicity and Cell Morphology Examination
3. Results and Discussion
3.1. Phase Composition
3.2. Surface and Cross-Section Microstructure
3.3. Corrosion Studies
3.3.1. Electrochemical Measurements
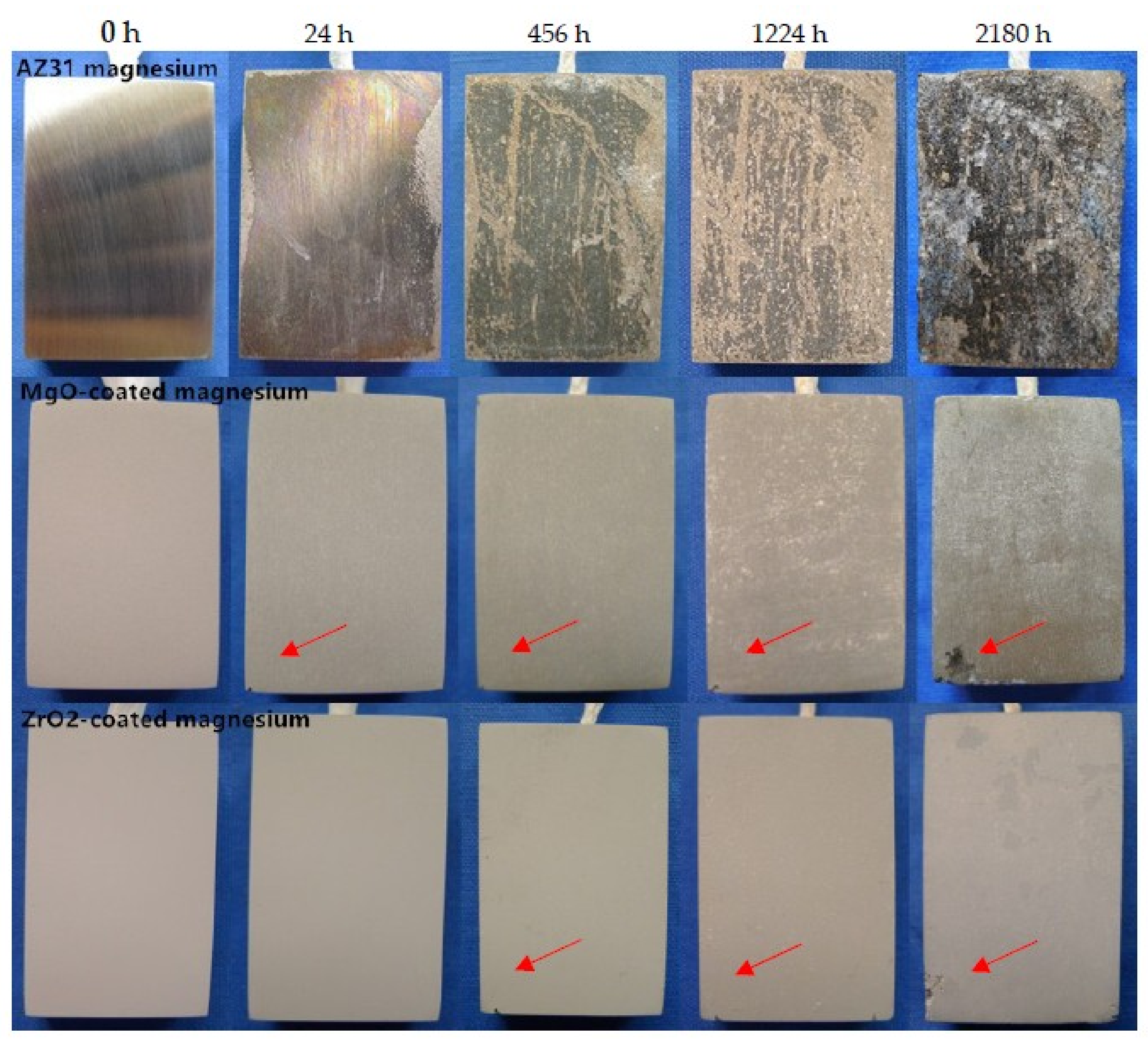
3.3.2. Long-Term Immersion Test
3.3.3. Hydrogen Evolution Tests
3.4. Cytotoxicity Test
4. Conclusions
- The coating formed in the basic phosphate electrolyte was porous and mainly composed of MgO and MgF2, while the coating formed in fluorozirconate electrolyte was dense and mainly consisted of t-ZrO2 and m-ZrO2.
- The corrosion resistance of the ZrO2 coating was superior to that of the MgO coating in the polarization tests after 0.5 h of exposure to the corrosive environment. The long-term EIS tests and the immersion tests revealed the extent of corrosion deterioration of the PEO coatings on the AZ31 Mg alloy exposed to the SBF solution. The deterioration process of both coatings started from a slow corrosion damage of PEO coating and then progressed, finally, to localized corrosion damage of the magnesium alloy substrate. The results indicated that the ZrO2 coating provides a much more stable corrosion-resistant layer than the MgO coating at different immersion times.
- In-vitro cytotoxicity tests indicated that the ZrO2-coated AZ31 did not induce a cytotoxic reaction to L-929 cells and promoted cell growth during the first 7 days.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of metal orthopaedic implants. JBJS 1998, 88, 268–282. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, Y.; Sun, J.; Yang, L.; Guo, C.; Liang, J.; Cao, B. Preparation and characterization of aminated hydroxyethyl cellulose-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Metals 2017, 7, 214. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhong, M.; Zheng, C.; Cao, L.; Wang, D.; Wang, L.; Liang, J.; Cao, B. Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 281, 82–88. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, S.; Wang, L.; Yang, Y.; Liang, J.; Cao, B. Preparation of hydroxyapatite/tannic acid coating to enhance the corrosion resistance and cytocompatibility of AZ31 magnesium alloys. Coatings 2017, 7, 105. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Erinc, M.; Sillekens, W.H.; Mannens, R.G.T.M.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant materials. In Proceedings of the Magnesium Technology 2009, San Francisco, CA, USA, 15–19 February 2009. [Google Scholar]
- Liu, C.; Liang, J.; Zhou, J.; Wang, L.; Li, Q. Effect of laser surface melting on microstructure and corrosion characteristics of AM60B magnesium alloy. Appl. Surf. Sci. 2015, 343, 133–140. [Google Scholar] [CrossRef]
- Liu, C.; Liang, J.; Zhou, J.; Li, Q.; Peng, Z.; Wang, L. Characterization and corrosion behavior of plasma electrolytic oxidation coated AZ91-T6 magnesium alloy. Surf. Coat. Technol. 2016, 304, 179–187. [Google Scholar] [CrossRef]
- Liu, C.; Liang, J.; Zhou, J.; Li, Q.; Peng, Z.; Wang, L. Corrosion behavior of plasma electrolytic oxidation coated AZ91 Mg alloy: Influence of laser surface melting pretreatment. RSC Adv. 2016, 6, 70343–70351. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Microstructure and corrosion behavior of plasma electrolytic oxidation coated magnesium alloy pre-treated by laser surface melting. Surf. Coat. Technol. 2012, 206, 3109–3115. [Google Scholar] [CrossRef]
- Kumar, N.; Subasri, R. Corrosion protection and self-healing by sol-gel process: A review of recent patent literature. Recent Pat. Corros. Sci. 2012, 2, 148–163. [Google Scholar] [CrossRef]
- Liu, C.; Liang, J.; Zhou, J.; Li, Q.; Wang, L. Characterization of AZ31 magnesium alloy by duplex process combining laser surface melting and plasma electrolytic oxidation. Appl. Surf. Sci. 2016, 382, 47–55. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Apelfeld, A.; Krit, B.; Ludin, V.; Morozova, N.; Vladimirov, B.; Wu, R.Z. The characterization of plasma electrolytic oxidation coatings on AZ41 magnesium alloy. Surf. Coat. Technol. 2017, 322, 127–133. [Google Scholar] [CrossRef]
- White, L.; Koo, Y.; Neralla, S.; Sankar, J.; Yun, Y. Enhanced mechanical properties and increased corrosion resistance of a biodegradable magnesium alloy by plasma electrolytic oxidation (PEO). Mater. Sci. Eng. B 2016, 208, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Comparison of electrochemical corrosion behavior of MgO and ZrO2, coatings on AM50 magnesium alloy formed by plasma electrolytic oxidation. Corros. Sci. 2009, 51, 2483–2492. [Google Scholar] [CrossRef]
- Liu, F.; Shan, D.; Song, Y.; Han, E.H.; Ke, W. Corrosion behavior of the composite ceramic coating containing zirconium oxides on AM30 magnesium alloy by plasma electrolytic oxidation. Corros. Sci. 2011, 53, 3845–3852. [Google Scholar] [CrossRef]
- Mu, W.; Han, Y. Characterization and properties of the MgF2/ZrO2, composite coatings on magnesium prepared by micro-arc oxidation. Surf. Coat. Technol. 2008, 202, 4278–4284. [Google Scholar] [CrossRef]
- Hong, C.; Jianmin, H. Study on preparing of ZrO2 ceramic coating and bone biological activity of magnesium alloys for medicine. Rare Met. Mater. Eng. 2014, 43, 150–153. [Google Scholar]
- Li, J.; Zhang, X.; He, X.; Hang, R.; Huang, X.; Tang, B. Preparation, biocompatibility and wear resistance of microstructured Zr and ZrO2, alloyed layers on 316L stainless steel. Mater. Lett. 2017, 203, 24–27. [Google Scholar] [CrossRef]
- Wang, G.; Meng, F.; Ding, C.; Chu, P.K.; Liu, X. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010, 6, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Mosgau, S.; Schliephake, H.; Radespiel-Tröger, M.; Neukam, F.W. Osseointegration of endodontic endosseous cones: Zirconium oxide vs titanium. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 89, 91–98. [Google Scholar] [CrossRef]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.P.; Kübler, N.R.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Liu, H.; Li, W.; Zhu, L. Effect of zirconia sol in electrolyte on the characteristics of microarc oxidation coating on AZ91D magnesium. Mater. Lett. 2011, 65, 413–415. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Q.; Wei, B.; Yu, B.; He, J.; Li, D. Study on the microstructure and corrosion resistance of ZrO2-containing ceramic coatings formed on magnesium alloy by plasma electrolytic oxidation. J. Alloy. Compd. 2009, 474, 551–556. [Google Scholar] [CrossRef]
- Mu, W.; Yong, H. Study on micro-arc oxidized coatings on magnesium in three different electrolytes. Rare Met. Mater. Eng. 2010, 39, 1129–1134. [Google Scholar]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Xiong, Y.; Hu, Q.; Song, R.G. A study on microstructure and corrosion resistance of ZrO2-containing PEO coatings formed on AZ31 Mg alloy in phosphate-based electrolyte. Appl. Surf. Sci. 2015, 357, 1463–1471. [Google Scholar] [CrossRef]
- Liu, F.; Shan, D.; Song, Y.; Han, E.H. Effect of additives on the properties of plasma electrolytic oxidation coatings formed on AM50 magnesium alloy in electrolytes containing K2ZrF6. Surf. Coat. Technol. 2011, 206, 455–463. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Wang, H.; Peng, J. The influence of additives on the stability behavior of electrolyte, discharges and PEO films characteristics. J. Alloy. Compd. 2010, 493, 445–452. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Corrosion mechanism of plasma electrolytic oxidation coated magnesium alloy with laser surface melting pretreatment. J. Electrochem. Soc. 2014, 161, C20–C24. [Google Scholar] [CrossRef]
- Duan, H.; Du, K.; Yan, C.; Wang, F. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim. Acta 2006, 51, 2898–2908. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—a framework for improved alloy performance. Adv. Eng. Mater. 2010, 5, 837–858. [Google Scholar] [CrossRef]
- Yan, Y.; Han, Y. Structure and bioactivity of micro-arc oxidized zirconia films. Surf. Coat. Technol. 2007, 201, 5692–5695. [Google Scholar] [CrossRef]











| Group | Electrolyte Concentration (g·L−1) | Current Frequency (Hz) | Duty Ratio (%) | Process Time (min) | pH |
|---|---|---|---|---|---|
| Control group | (NaPO3)6 = 3, KF = 8 | 1000 | 40 | 10 | 8.0 |
| Experimental group | K2ZrF6 = 3, Na2HPO4 = 1 | 1000 | 40 | 10 | 5.6 |
| Samples | Ecorr (V vs. AgCl) | Icorr (A·cm−2) |
|---|---|---|
| AZ31 | −1.4689 ± 0.015 | (1.8895 ± 0.2866) × 10−6 |
| MgO/AZ31 | −1.4174 ± 0.011 | (3.5754 ± 0.4781) × 10−7 * |
| ZrO2/AZ31 | −1.3911 ± 0.012 | (1.5072 ± 0.2991) × 10−7 * |
| Time (h) | R1 (Ω·cm2) | CPE1-T | CPE1-P | R2 (Ω·cm2) | CPE2-T | CPE2-P |
|---|---|---|---|---|---|---|
| 0.5 | 3.8 × 104 | 9.24 × 10−7 | 0.66 | 1.0 × 105 | 1.56 × 10−6 | 0.72 |
| 2 | 2.5 × 104 | 1.84 × 10−6 | 0.58 | 7.1 × 104 | 1.50 × 10−6 | 0.88 |
| 5 | 5.7 × 102 | 3.82 × 10−6 | 0.68 | 6.1 × 104 | 3.82 × 10−6 | 0.65 |
| 20 | 6.1 × 103 | 1.99 × 10−7 | 0.68 | 1.9 × 105 | 1.79 × 10−6 | 0.65 |
| 112 | 4.2 × 103 | 3.77 × 10−7 | 0.68 | 3.0 × 105 | 1.37 × 10−6 | 0.66 |
| 160 | 4.8 × 103 | 3.14 × 10−7 | 0.67 | 2.8 × 105 | 1.35 × 10−6 | 0.73 |
| Time (h) | R1 (Ω·cm2) | CPE1-T | CPE1-P | R2 (Ω·cm2) | CPE2-T | CPE2-P |
|---|---|---|---|---|---|---|
| 0.5 | 5.2 × 103 | 4.09 × 10−7 | 0.68 | 1.3 × 105 | 1.00 × 10−6 | 0.67 |
| 2 | 2.5 × 103 | 1.16 × 10−6 | 0.67 | 3.5 × 105 | 1.42 × 10−7 | 1.06 |
| 5 | 2.8 × 103 | 6.72 × 10−7 | 0.63 | 6.5 × 104 | 8.37 × 10−7 | 0.89 |
| 20 | 2.3 × 104 | 3.47 × 10−7 | 0.67 | 3.5 × 105 | 4.68 × 10−7 | 0.95 |
| 112 | 1.8 × 104 | 2.88 × 10−7 | 0.67 | 2.1 × 105 | 5.32 × 10−7 | 0.97 |
| 160 | 1.2 × 104 | 1.96 × 10−7 | 0.70 | 1.5 × 105 | 1.33 × 10−7 | 0.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fu, L.; Nai, Z.; Liang, J.; Cao, B. Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation. Coatings 2018, 8, 441. https://doi.org/10.3390/coatings8120441
Wang S, Fu L, Nai Z, Liang J, Cao B. Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation. Coatings. 2018; 8(12):441. https://doi.org/10.3390/coatings8120441
Chicago/Turabian StyleWang, Shimeng, Lingxia Fu, Zhenggang Nai, Jun Liang, and Baocheng Cao. 2018. "Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation" Coatings 8, no. 12: 441. https://doi.org/10.3390/coatings8120441
APA StyleWang, S., Fu, L., Nai, Z., Liang, J., & Cao, B. (2018). Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation. Coatings, 8(12), 441. https://doi.org/10.3390/coatings8120441




