Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Sample Preparation
2.2. Methods
3. Results and Discussion
4. Conclusions
- The solution phase study indicated that addition of benzoimidazole to chloride solution decreased the corrosion of bare ST37 sample through development of a protective layer on the surface or stabilization of the corrosion products.
- Cathodic disbonding resistance of epoxy-polyamide coating was shown to be dependent on the inhibitor content.
- Inclusion of 0.75 wt.% corrosion inhibitor to the epoxy-polyamide coating formulation decreased the cathodic disbonded surface area effectively, which is consistent with the electrochemical data.
- The wet adhesion of the polymeric coating was significantly enhanced through the addition of 0.75 wt.% benzoimidazole.
Author Contributions
Funding
Conflicts of Interest
References
- Kamimura, T.; Kishikawa, H. Mechanism of cathodic disbonding of three-layer polyethylene-coated steel pipe. Corrosion 1998, 54, 979–987. [Google Scholar] [CrossRef]
- Leidheiser, H.; Wang, W.; Lgetoft, L. The mechanism for cathodic delamination of organic coating from a metal surface. J. Prog. Org. Coat. 1983, 11, 19–40. [Google Scholar] [CrossRef]
- Zhou, W.; Jeffers, T.E. Application temperature, cure, and film thickness affect cathodic disboniment of FBE coatings. Mater. Perform. 2006, 45, 24–28. [Google Scholar]
- Grundmeier, G.; Stratmann, M. Adhesion and de-adhesion mechanisms at polymer/metal interfaces: Mechanistic understanding based on in situ studies of buried interfaces. Annu. Rev. Mater. 2005, 35, 571–615. [Google Scholar] [CrossRef]
- Nazarov, A.; Le Bozec, N.; Thierry, D. Assessment of steel corrosion and deadhesion of epoxy barrier paint by scanning Kelvin probe. Prog. Org. Coat. 2018, 114, 123–134. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. Steel surface pre-treated by an advance and eco-friendly cerium oxidenanofilm modified by graphene oxide nanosheets; electrochemical and adhesion measurements. J. Alloy. Compd. 2018, 747, 109–123. [Google Scholar] [CrossRef]
- Sorensen, P.; Dam-Johansen, K.; Weinell, C.; Kiil, S. Cathodic delamination: Quantification of ionic transport rates along coating–steel interfaces. Prog. Org. Coat. 2010, 68, 70–78. [Google Scholar] [CrossRef]
- Martinez, S.; Zulj, L.; Kapor, F. Disbonding of underwater-cured epoxy coating caused by cathodic protection current. Corros. Sci. 2009, 51, 2253–2258. [Google Scholar] [CrossRef]
- Fu, A.; Cheng, Y. Characterization of corrosion of X65 pipeline steel under disbonded coating by scanning Kelvin probe. Corros. Sci. 2009, 51, 914–920. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M. The effect of benzimidazole and zinc acetylacetonate mixture on cathodic disbonding of epoxy coated mild steel. Prog. Org. Coat. 2009, 66, 137–140. [Google Scholar] [CrossRef]
- Leidheiser, H. Coatings. In Corrosion Mechanisms; Mansfeld, F., Ed.; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Schweitzer, P.A. Corrosion Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Greenfield, D.; Scantlebury, D. The protective action of organic coatings on steel: A review. J. Cross. Sci. Eng. 2000, 3, 5. [Google Scholar]
- Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Naderi, R.; Attar, M. The role of zinc aluminum phosphate anticorrosive pigment in protective performance and cathodic disbondment of epoxy coating. Corros. Sci. 2010, 52, 1291–1296. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M. Cathodic disbondment of epoxy coating with zinc aluminum polyphosphate as a modified zinc phosphate anticorrosion pigment. Prog. Org. Coat. 2010, 69, 392–395. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M. Effect of zinc-free phosphate-based anticorrosion pigment on the cathodic disbondment of epoxy-polyamide coating. Prog. Org. Coat. 2014, 77, 830–835. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Tan, A.; Soutar, A. Hybrid sol–gel coatings for corrosion protection of Copper. Thin Solid Film 2008, 516, 5706–5709. [Google Scholar] [CrossRef]
- Yang, H. Plasma Treatment of Organic Inhibitors for Corrosion Protection of Aerospace Alloys. Master’s Thesis, University of Cincinnati, Cincinnat, OH, USA, 2003. [Google Scholar]
- Tadokoro, K.; Shoji, H.; Sakon, T.; Jitsuhara, I.; Yamasaki, M. Metallic Sheet Having rust-Preventive Organic Coating Thereon, Process for the Production Thereof and Treating Fluid Therefore. U.S. Patent 6,254,980, 3 July 2001. [Google Scholar]
- Cook, R.L. Releasable Corrosion Inhibitor Compositions. U.S. Patent 6,933,046, 23 August 2005. [Google Scholar]
- Brooman, E.W. Modifying organic coatings to provide corrosion resistance—Part III: Organic additives and conducting polymers. Met. Finish. 2002, 100, 104–110. [Google Scholar]
- Izadi, M.; Shahrabi, T.; Ramezanzadeh, B. Active corrosion protection performance of an epoxy coating applied on the mild steel modified with an eco-friendly sol-gel film impregnated with green corrosion inhibitor loaded nanocontainers. Appl. Surf. Sci. 2018, 440, 491–505. [Google Scholar] [CrossRef]
- Mahdavian, M.; Ashhary, S. Mercapto functional azole compounds as organic corrosion inhibitors in a polyester-melamine coating. Prog. Org. Coat. 2010, 68, 259–264. [Google Scholar] [CrossRef]
- Ebrahimi-Mehr, M.; Shahrabi, T.; Hosseini, M. Determination of suitable corrosion inhibitor formulation for a potable water supply. Anti-Corros. Methods Mater. 2004, 51, 399–405. [Google Scholar] [CrossRef]
- Beiro, M.; Collazo, A.; Izquierdo, M.; Novoa, X.; Perez, C. Characterization of barrier properties of organic paints: The zinc phosphate effectiveness. Prog. Org. Coat. 2003, 46, 97–106. [Google Scholar] [CrossRef]
- Mousavifard, S.M.; MalekMohammadi Nouri, P.; Attar, M.M.; Ramezanzadeh, B. The effects of zinc aluminum phosphate (ZPA) and zinc aluminum polyphosphate (ZAPP) mixtures on corrosion inhibition performance of epoxy/polyamide coating. J. Ind. Eng. Chem. 2013, 19, 1031–1039. [Google Scholar]
- Mahdavian, M.; Naderi, R.; Peighambari, M.; Hamidpour, M.; Haddadi, S. Evaluation of cathodic disbondment of epoxy coating containing azole compounds. J. Ind. Eng. Chem. 2014, 21, 1167–1173. [Google Scholar] [CrossRef]
- Hang, T.; Duong, N.; Truc, T.; Hoang, T.; Thanh, D.; Daopiset, S.; Boonplean, A. Effects of hydrotalcite intercalated with corrosion inhibitor on cathodic disbonding of epoxy coatings. J. Coat. Technol. Res. 2015, 12, 375–383. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. The effects of addition of poly(vinyl) alcohol (PVA) as a greencorrosion inhibitor to the phosphate conversion coating on theanticorrosion and adhesion properties of the epoxy coating on thesteel substrate. Appl. Surf. Sci. 2015, 327, 174–181. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized grapheme oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Bentiss, F.; Jama, C.; Mernari, B.; El Attari, H.; El Kadi, L.; Lebrini, M.; Traisnel, M.; Lagrenee, M. Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4-triazole in normal hydrochloric acid medium. Corros. Sci. 2009, 51, 1628–1635. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedel, M.; Motte, C. Electrochemical investigation of high-performance silane sol-gel films containing clay nanoparticles. Prog. Org. Coat. 2010, 69, 158–166. [Google Scholar] [CrossRef]
- Nikpour, S.; Naderi, R.; Mahdavian, M. Fabrication of silane coating with improved protection performance using Mentha longifolia extract. J. Taiwan Inst. Chem. Eng. 2018, 88, 261–276. [Google Scholar] [CrossRef]
- Bahrani, A.; Naderi, R.; Mahdavian, M. Chemical modification of talc with corrosion inhibitors to enhance the corrosion protective properties of epoxy-ester coating. Prog. Org. Coat. 2018, 120, 110–122. [Google Scholar] [CrossRef]
- Dias, S.; Lamaka, S.; Nogueira, C.; Diamantino, T.; Ferreira, M. Sol-gel coatings modified with zeolite fillers for active corrosion protection of AA2024. Corros. Sci. 2012, 62, 153–162. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Poznyak, S.; Rodrigues, L.; Raps, D.; Hack, T.; Dick, L.; Nunes, T.; Ferreira, M. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Naderi, R.; Fedel, M.; Urios, T.; Poelman, M.; Olivier, M.; Deflorian, F. Optimization of silane sol-gel coatings for the protection of aluminium components of heat exchangers. Surf. Interface Anal. 2013, 45, 1457–1466. [Google Scholar] [CrossRef]
- Ismail, I.; Harun, M. Cathodic disbonding of industrial chlorinated rubber-based primer used in rubber/metal composites: An electrochemical impedance spectroscopy analysis. Rubber Chem. Technol. 2016, 89, 712–723. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Li, Y.; Zheng, G.; Tu, X. Electrochemical impedance spectroscopy study for cathodic disbonding test technology on three layer polyethylene anticorrosive coating under full immersion and alternating dry–wet environments. Int. J. Electrochem. Sci. 2016, 11, 10884–10894. [Google Scholar] [CrossRef]
- Hirayama, R.; Haruyama, S. Electrochemical impedance for degraded coated steel having pores. Corrosion 1991, 47, 952–957. [Google Scholar] [CrossRef]
- Jorcin, J.; Aragon, E.; Merlatti, C.; Pebere, N. Delaminated areas beneath organic coating: A local electrochemical impedance approach. Corros. Sci. 2006, 48, 1779–1790. [Google Scholar] [CrossRef] [Green Version]

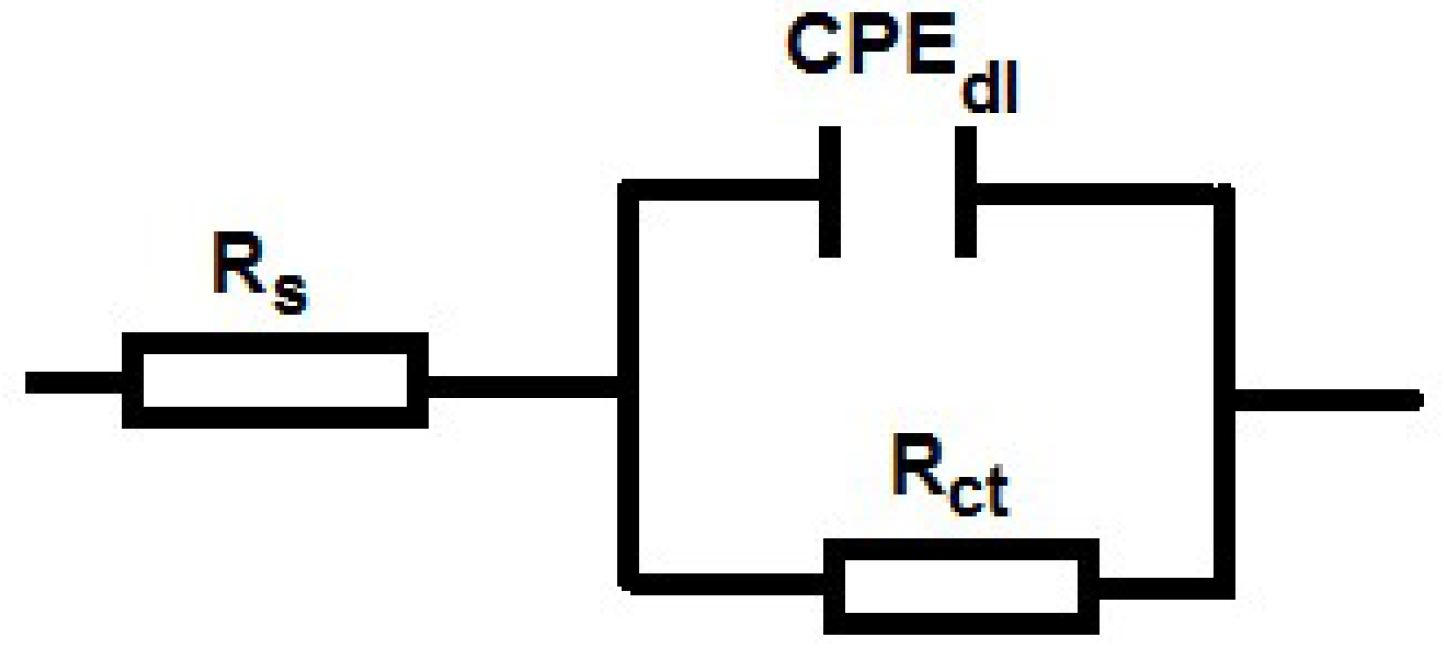
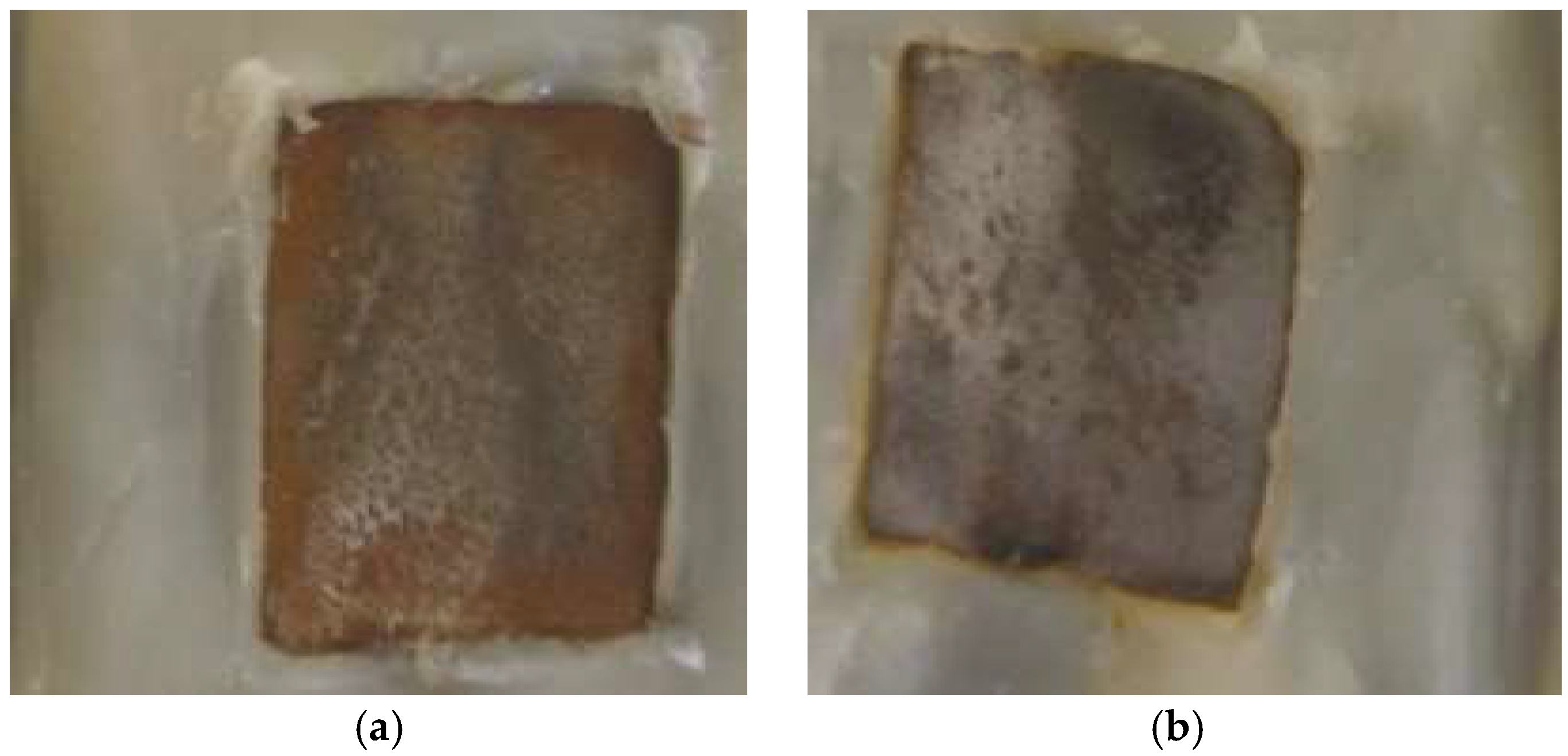



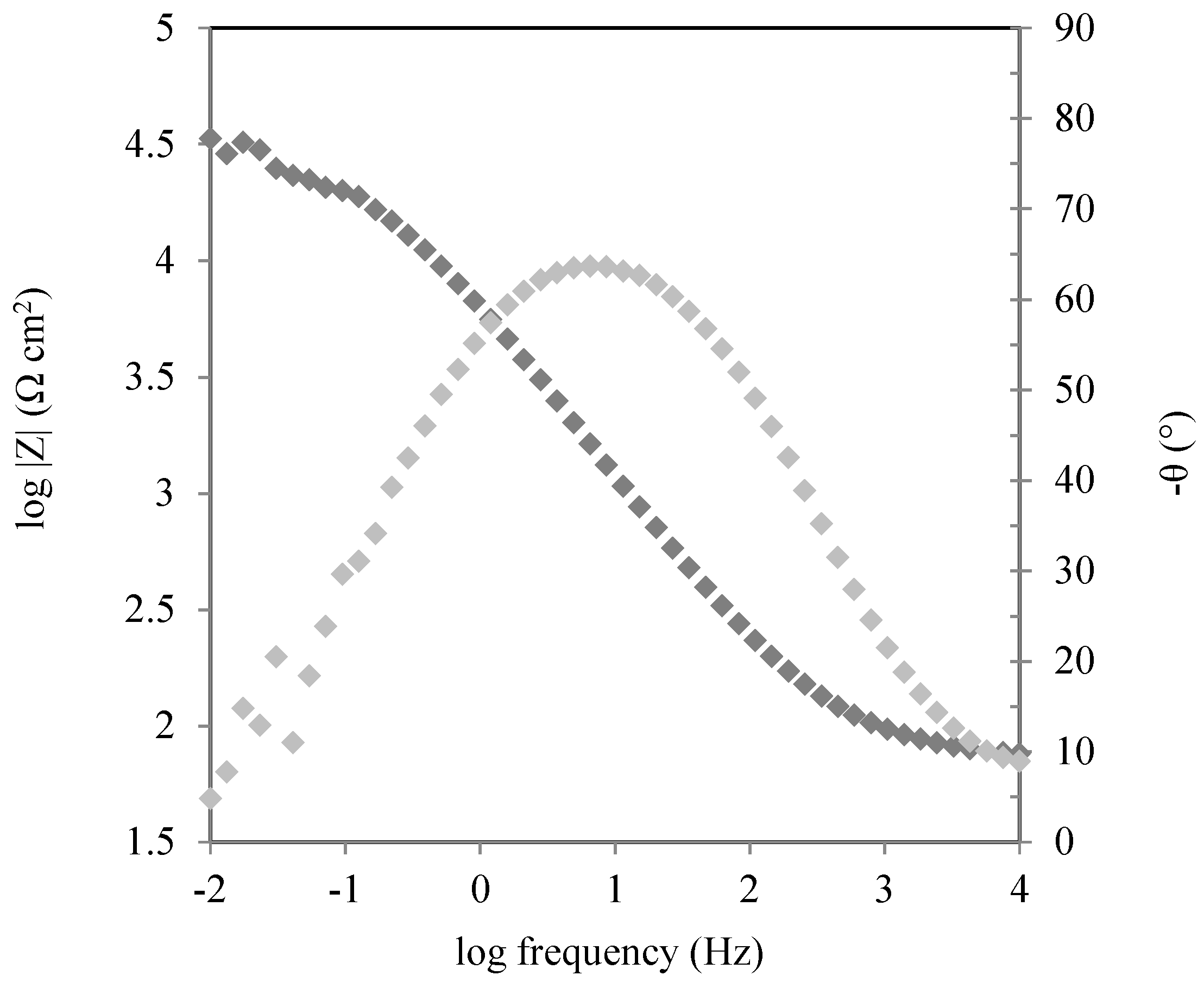
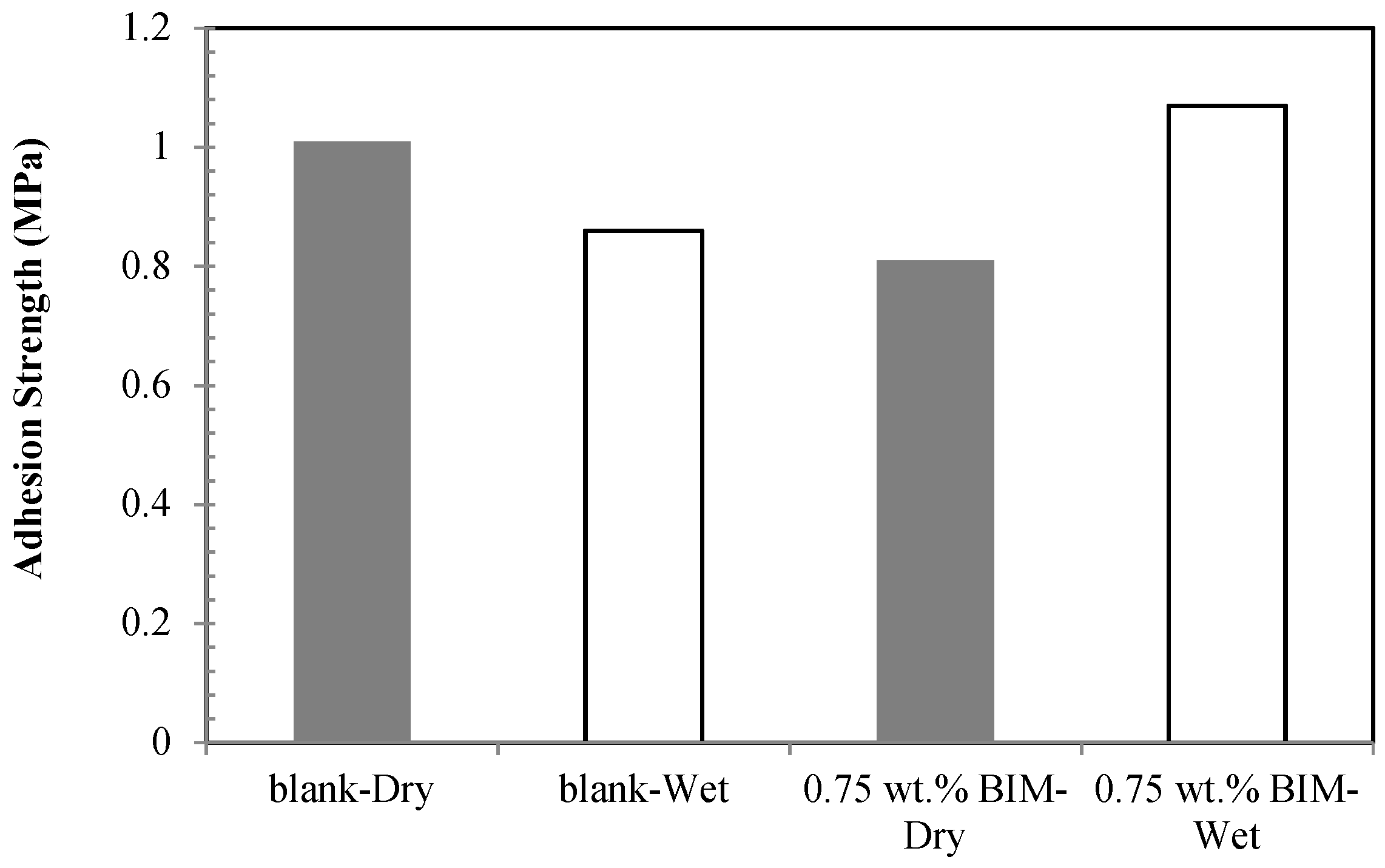

| BIM Concentration (mM) | Immersion Time (h) | Rct (Ω·cm2) | Cdl (µF·cm−2) |
|---|---|---|---|
| 0 | 5 | 1292 | 752 |
| 24 | 912 | 529 | |
| 1 | 5 | 2001 | 469 |
| 24 | 2256 | 404 | |
| Standard deviation | Rc | 1.7%–9.3% | |
| Cdl | 3.8%–13.8% | ||
| BIM Concentration (wt.%) | Immersion Time (h) | R (kΩ·cm2) | C (µF·cm−2) |
|---|---|---|---|
| 0 | 12 | 15.7 | 39.6 |
| 24 | 14.8 | 39.4 | |
| 0.5 | 12 | 17.5 | 31.1 |
| 24 | 12.5 | 43.4 | |
| 0.75 | 12 | 27.8 | 36.2 |
| 24 | 23.6 | 22.9 | |
| 1 | 12 | 15.9 | 17.2 |
| 24 | 14.9 | 46.4 | |
| Standard deviation | R | 1.3%–10.1% | |
| C | 3%–14.4% | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavian, S.; Naderi, R.; Asadi, N. Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating. Coatings 2018, 8, 471. https://doi.org/10.3390/coatings8120471
Nabavian S, Naderi R, Asadi N. Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating. Coatings. 2018; 8(12):471. https://doi.org/10.3390/coatings8120471
Chicago/Turabian StyleNabavian, Saghar, Reza Naderi, and Najmeh Asadi. 2018. "Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating" Coatings 8, no. 12: 471. https://doi.org/10.3390/coatings8120471
APA StyleNabavian, S., Naderi, R., & Asadi, N. (2018). Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating. Coatings, 8(12), 471. https://doi.org/10.3390/coatings8120471




