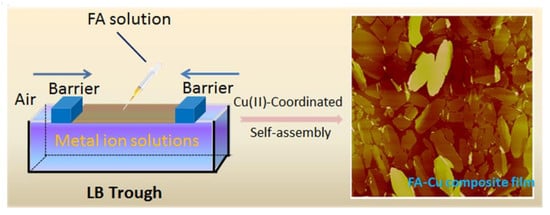
Self-Assembled Composite Langmuir Films via Fluorine-Containing Bola-Type Derivative with Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Langmuir Films
2.3. Characterization
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fuhrhop, J.H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2938. [Google Scholar] [CrossRef] [PubMed]
- Fuhrhop, J.H.; Matthieu, J. Routes to functional vesicle membranes without proteins. Angew. Chem. Int. Ed. 1984, 23, 100–113. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; Fritsch, D. Bolaamphiphiles form ultrathin, porous and unsymmetric monolayer lipid membranes. Acc. Chem. Soc. 1986, 19, 130–137. [Google Scholar] [CrossRef]
- Escamilla, G.H.; Newkome, G.R. Bolaamphiphiles: From golf balls to fibres. Angew. Chem. Int. Ed. 1994, 33, 1937–1940. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M. Induced chirality of supramolecular assemblies of some amphiphiles with beta-cyclodextrin through the interaction at the air/water interface. J. Colloid Interface Sci. 2007, 306, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, T.; Liu, M. Macroscopic chirality of supramolecular gels formed from achiral tris(ethyl cinnamate) benzene-1,3,5-tricarboxamides. Angew. Chem. Int. Ed. 2014, 53, 13424–13428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Q.; Liu, M. Fabrication of chiral Langmuir-Schaefer films from achiral TPPS and amphiphiles through the adsorption at the air/water interface. J. Phys. Chem. B 2003, 107, 2565–2569. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; David, H.H.; Mathieu, J.; Liman, U.; Winter, H.J.; Boekema, E. Bolaamphiphiles and monolayer lipid membranes. J. Am. Chem. Soc. 1986, 108, 1785–1791. [Google Scholar] [CrossRef]
- Iwaura, R.; Yoshida, K.; Masuda, M.; Yase, K.; Shimizu, T. Spontaneous fiber formation and hydrogelation of nucleotide bolaamphiphiles. Chem. Mater. 2002, 14, 3047–3053. [Google Scholar] [CrossRef]
- Iwaura, R.; Yoshida, K.; Masuda, M.; Mayumi, O.K.; Yoshida, M.; Shimizu, T. Oligonucleotide-templated self-assembly of nucleotide bolaamphiphiles: DNA-like nanofibers edged by a double-helical arrangement of A-T base pairs. Angew. Chem. Int. Ed. 2003, 42, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, S.; Liu, M. Configuration and photochemical reaction of a bolaamphiphilic diacid with a diazo resin in monolayers and Langmuir-Blodgett films. J. Colloid Interface Sci. 2003, 261, 417–422. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Moriya, A.; Kinoshita, M. Peculiar membrane morphologies of archaebacterial lipid models: 1,1′-polymethylenebis(2-alkyl-sn-glycero-3-phosphocholine). Biochim. Biophys. Acta 1989, 1003, 151–160. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sakamoto, Y.; Moriya, A.; Yamada, K.; Higuchi, T.; Kinoshita, M. Archaebacterial lipid models. Highly thermostable membranes from 1,1′-(1,32-dotriacontamethylene)-bis(2-phytanyl-sn-glycero- 3-phosphocholine). J. Am. Chem. Soc. 1990, 112, 3188–3191. [Google Scholar] [CrossRef]
- Cuvier, A.S.; Berton, J.; Stevens, C.V.; Fadda, G.C.; Babonneau, F.; Van Bogaert, I.N.A.; Soetaert, W.; Pehau-Arnaudet, G.; Baccile, N. pH-triggered formation of nanoribbons from yeast-derived glycolipid biosurfactants. Soft Matter 2014, 10, 3950–3959. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jia, K.K.; Wang, Y.C.; Shao, W.; Yao, C.H.; Peng, L.M.; Zhang, D.M.; Hu, X.Y.; Wang, L.Y. Dual-responsive bola-type supra-amphiphile constructed from water-soluble pillar[5]arene and naphthalimide-containing amphiphile for intracellular drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 4843–4850. [Google Scholar]
- Kang, Y.T.; Cai, Z.G.; Tang, X.Y.; Liu, K.; Wang, G.T.; Zhang, X. An Amylase-responsive bolaform supra-amphiphile. ACS Appl. Mater. Interfaces 2016, 8, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Van Bogaert, I.N.A.; Buyst, D.; Martins, J.C.; Roelants, S.L.K.W.; Soetaert, W.K. Synthesis of bolaform biosurfactants by an engineered Starmerella bombicola yeast. Biotechnol. Bioeng. 2016, 113, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Wang, C.X.; Abbott, N.L. Redox-triggered mixing and demixing of surfactants within assemblies formed in solution and at surfaces. J. Colloid Interface Sci. 2017, 502, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, M. Chiral molecular assemblies from a novel achiral amphiphilic 2-(heptadecyl) naphtha[2,3]imidazole through interfacial coordination. J. Am. Chem. Soc. 2003, 125, 5051–5056. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Huan, Y.; Li, Z.; He, G.; Liu, M. Self-assembled supramolecular nanotube yarn. Adv. Mater. 2013, 25, 5875–5879. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhu, X.; Liu, M. Self-assembly of racemic alanine derivatives: Unexpected chiral twist and enhanced capacity for the discrimination of chiral species. Angew. Chem. Int. Ed. 2013, 52, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhang, L.; Jiang, Y.; Liu, M. Role of achiral nucleobases in multicomponent chiral self-assembly: Purine-triggered helix and chirality transfer. Angew. Chem. Int. Ed. 2016, 55, 15062–15066. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, M.K.; Kim, D.H.; Hyeon, T. Designed assembly and integration of colloidal nanocrystals for device applications. Adv. Mater. 2016, 28, 1176–1207. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, Y.; Cervantes-Salguero, K.; Minami, K.; Kawamata, I.; Nakanishi, W.; Hill, J.P.; Murata, S.; Ariga, K. Supramolecular 1-D polymerization of DNA origami through a dynamic process at the 2-dimensionally confined air-water interface. Phys. Chem. Chem. Phys. 2016, 18, 12576–12581. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ma, L.; Choudhury, S.; Moganty, S.S.; Wei, S.; Archer, L.A. Fabricating multifunctional nanoparticle membranes by a fast layer-by-layer Langmuir-Blodgett process: Application in lithium-sulfur batteries. J. Mater. Chem. A 2016, 4, 14709–14719. [Google Scholar] [CrossRef]
- El Garah, M.; Dianat, A.; Cadeddu, A.; Gutierrez, R.; Cecchini, M.; Cook, T.R.; Ciesielski, A.; Stang, P.J.; Cuniberti, G.; Samori, P. Atomically precise prediction of 2D self-assembly of weakly bonded nanostructures: STM insight into concentration-dependent architectures. Small 2016, 12, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Lotito, V.; Zambelli, T. Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Colloid Interface Sci. 2017, 246, 217–274. [Google Scholar] [CrossRef] [PubMed]
- Toor, A.; Feng, T.; Russell, T.P. Self-assembly of nanomaterials at fluid interfaces. Eur. Phys. J. E 2016, 39, 57. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.J.; Fu, X.L.; Li, T.J.; Zhao, N.R.; Shi, X.T.; Cui, F.Z.; Du, C.; Wang, Y.J. Surface chemistry from wettability and charge for the control of mesenchymal stem cell fate through self-assembled monolayers. Colloid Surf. B 2016, 148, 549–556. [Google Scholar] [CrossRef] [PubMed]
- He, W.L.; Fang, F.; Ma, D.M.; Chen, M.; Qian, D.J.; Liu, M.H. Palladium-directed self-assembly of multi-titanium(IV)-porphyrin arrays on the substrate surface as sensitive ultrathin films for hydrogen peroxide sensing, photocurrent generation, and photochromism of viologen. Appl. Surf. Sci. 2018, 427, 1003–1010. [Google Scholar] [CrossRef]
- Hoffmann-Vogel, R. Imaging prototypical aromatic molecules on insulating surfaces: A review. Rep. Prog. Phys. 2018, 81, 016501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, F.; Ma, X.Y.; Qiang, X.; Li, Z.G.; Dong, C.; Zang, D.Y. Tadpole-shaped POSS-based copolymers and the aggregation behavior at air/water interface. Adv. Condens. Matter Phys. 2018, 3787843. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, F.; Jiao, T.; Xing, R.; Zhang, L.; Zhang, Q.; Peng, Q. Selective Cu(II) ion removal from wastewater via surface charged self-assembled polystyrene-Schiff base nanocomposites. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 545, 60–67. [Google Scholar] [CrossRef]
- Luo, X.; Ma, K.; Jiao, T.; Xing, R.; Zhang, L.; Zhou, J.; Li, B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Cai, J.F. Silver(I) ion induced reverse U-shape monolayers of poly(methylenebis(benzimidazoles)) at the air/water interface. Langmuir 2000, 16, 2899–2901. [Google Scholar] [CrossRef]
- Lu, Q.; Luo, Y.; Li, L.; Liu, M. Self-assembled supramolecular architecture of a bolaamphiphilic diacid on the subphases containing Ag(I) and Eu(III) metal ions. Langmuir 2003, 19, 285–291. [Google Scholar] [CrossRef]
- Jiao, T.; Liu, M. Supramolecular nano-architectures and two-dimensional/three-dimensional aggregation of a bolaamphiphilic diacid at the air/water interface. Thin Solid Films 2005, 479, 269–276. [Google Scholar] [CrossRef]
- Duan, P.; Qin, L.; Liu, M. Langmuir-Blodgett films and chiroptical switch of an azobenzene-containing dendron regulated by the in situ host-guest reaction at the air/water interface. Langmuir 2011, 27, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, H.; Yang, D.; Zhang, L.; Jiang, L.; Liu, M. Two-dimensional alignment of self-assembled organic nanotubes through Langmuir–Blodgett technique. Langmuir 2016, 32, 13065–13072. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhuo, C.; Hu, C.; Liu, M. Evolution of nanoflowers and nanospheres of zinc bisporphyrinate tweezers at the air/water interface. Langmuir 2017, 33, 3694–3701. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, L.; Liu, M. A supramolecular chiroptical switch exclusively from an achiral amphiphile. Adv. Mater. 2006, 18, 177–180. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Jiang, S.G.; Wang, X.S.; Zhang, B.W.; Liu, M.H. Self-assembled spiral nanoarchitecture and supramolecular chirality in Langmuir-Blodgett films of an achiral amphiphilic barbituric acid. J. Am. Chem. Soc. 2004, 126, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Meng, Y.; Zhang, L.; Liu, M. Self-assembled single-walled metal-helical nanotube (M-HN): Creation of efficient supramolecular catalysts for asymmetric reaction. J. Am. Chem. Soc. 2016, 138, 15629–15635. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Jiao, T.; Li, R.; Chen, Y.; Guo, W.; Zhang, L.; Zhou, J.; Zhang, Q.; Peng, Q. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Jin, Q.; Zhang, L.; Liu, M. A chiroptical logic circuit based on self-assembled soft materials containing amphiphilic spiropyran. Adv. Mater. 2016, 28, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Jiao, T.; Xing, R.; Yang, Z.; Fan, J.; Liu, J.; Li, B.; Peng, Q. Preparation and enhanced structural integrity of electrospun poly(ε-caprolactone)-based fibers by freezing amorphous chains through thiol-ene click reaction. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 538, 7–13. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Duan, P.F.; Li, Y.G.; Jiang, J.; Wang, T.Y.; Liu, M.H. Towards a universal organogelator: A general mixing approach to fabricate various organic compounds into organogels. Sci. China Chem. 2011, 54, 1051–1063. [Google Scholar] [CrossRef]
- Lu, X.; Tao, L.; Song, D.; Li, Y.; Gao, F. Bimetallic Pd@Au nanorods based ultrasensitive acetylcholinesterase biosensor for determination of organophosphate pesticides. Sens. Actuators B Chem. 2018, 255, 2575–2581. [Google Scholar] [CrossRef]
- Song, J.; Xing, R.; Jiao, T.; Peng, Q.; Yuan, C.; Möhwald, H.; Yan, X. Crystalline dipeptide nanobelts based on solid-solid phase transformation self-assembly and their polarization imaging of cells. ACS Appl. Mater. Interfaces 2018, 10, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem. Int. Ed. 2017, 56, 12174–12178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.P.; Repo, E.; Meng, Y.; Wang, X.T.; Yin, D.L.; Sillanpaa, M. An EDTA-beta-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.S.; Bakr, A.S.A.; El Naggar, A.M.A.; Sultan, E.S.A. Water decontamination via the removal of Pb (II) using a new generation of highly energetic surface nano-material: Co+2Mo+6 LDH. J. Colloid Interface Sci. 2016, 461, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Catala, L.; Mallah, T. Nanoparticles of Prussian blue analogs and related coordination polymers: From information storage to biomedical applications. Coord. Chem. Rev. 2017, 346, 32–61. [Google Scholar] [CrossRef]
- Galanti, A.; Kotova, O.; Blasco, S.; Johnson, C.J.; Peacock, R.D.; Mills, S.; Boland, J.J.; Albrecht, M.; Gunnlaugsson, T. Exploring the Effect of Ligand Structural Isomerism in Langmuir-Blodgett Films of Chiral Luminescent Eu-III Self-Assemblies. Chem. Eur. J. 2016, 22, 9709–9723. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.V.; Meshkov, I.N.; Enakieva, Y.Y.; Zvyagina, A.I.; Ezhov, A.A.; Mikhaylov, A.A.; Gorbunova, Y.G.; Chernyshev, V.V.; Kalinina, M.A.; Arslanov, V.V. Effect of metalation-demetalation reactions on the assembly and properties of 2D supramolecular arrays of tetrapyridylporphyrin and its Zn(II)-complex. Surf. Sci. 2017, 660, 39–46. [Google Scholar] [CrossRef]
- Das, K.; Kundu, S. Subphase pH induced monolayer to multilayer collapse of fatty acid Salt Langmuir monolayer at lower surface pressure. Colloid Surf. A-Physicochem. Eng. Asp. 2016, 492, 54–61. [Google Scholar] [CrossRef]
- He, W.L.; Chen, J.L.; Chen, M.; Qian, D.J. Interfacial self-assembly, characterization, electrochemical, and photo-catalytic properties of porphyrin-ruthenium complex/polyoxomelate triad hybrid multilayers. Colloid Surf. A 2016, 509, 1–10. [Google Scholar] [CrossRef]
- Li, S.Y.; Du, L.; Wei, Z.M.; Wang, W.X. Aqueous-phase aerosols on the air-water interface: Response of fatty acid Langmuir monolayers to atmospheric inorganic ions. Sci. Total Environ. 2017, 580, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Todosijevic, M.N.; Brezesinski, G.; Savic, S.D.; Neubert, R.H.H. Sucrose esters as biocompatible surfactants for penetration enhancement: An insight into the mechanism of penetration enhancement studied using stratum corneum model lipids and Langmuir monolayers. Eur. J. Pharm. Sci. 2017, 99, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.M.; Champagne, A.M.; Williams, J.B.; Allen, H.C. Interfacial properties of avian stratum corneum monolayers investigated by Brewster angle microscopy and vibrational sum frequency generation. Chem. Phys. Lipids 2017, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, N.; Sun, S.; Zhao, Q.; Jiao, T.; Zhou, J.; Xing, R.; Gao, F.; Zhang, L.; Peng, Q. Self-Assembled Composite Langmuir Films via Fluorine-Containing Bola-Type Derivative with Metal Ions. Coatings 2018, 8, 141. https://doi.org/10.3390/coatings8040141
Qu N, Sun S, Zhao Q, Jiao T, Zhou J, Xing R, Gao F, Zhang L, Peng Q. Self-Assembled Composite Langmuir Films via Fluorine-Containing Bola-Type Derivative with Metal Ions. Coatings. 2018; 8(4):141. https://doi.org/10.3390/coatings8040141
Chicago/Turabian StyleQu, Nianrui, Shuxin Sun, Qianran Zhao, Tifeng Jiao, Jingxin Zhou, Ruirui Xing, Faming Gao, Lexin Zhang, and Qiuming Peng. 2018. "Self-Assembled Composite Langmuir Films via Fluorine-Containing Bola-Type Derivative with Metal Ions" Coatings 8, no. 4: 141. https://doi.org/10.3390/coatings8040141
APA StyleQu, N., Sun, S., Zhao, Q., Jiao, T., Zhou, J., Xing, R., Gao, F., Zhang, L., & Peng, Q. (2018). Self-Assembled Composite Langmuir Films via Fluorine-Containing Bola-Type Derivative with Metal Ions. Coatings, 8(4), 141. https://doi.org/10.3390/coatings8040141