Dithienylpyrrole- and Tris[4-(2-thienyl)phenyl]amine-Containing Copolymers as Promising Anodic Layers in High-Contrast Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Electrochemical Preparation of Polymer Electrodes
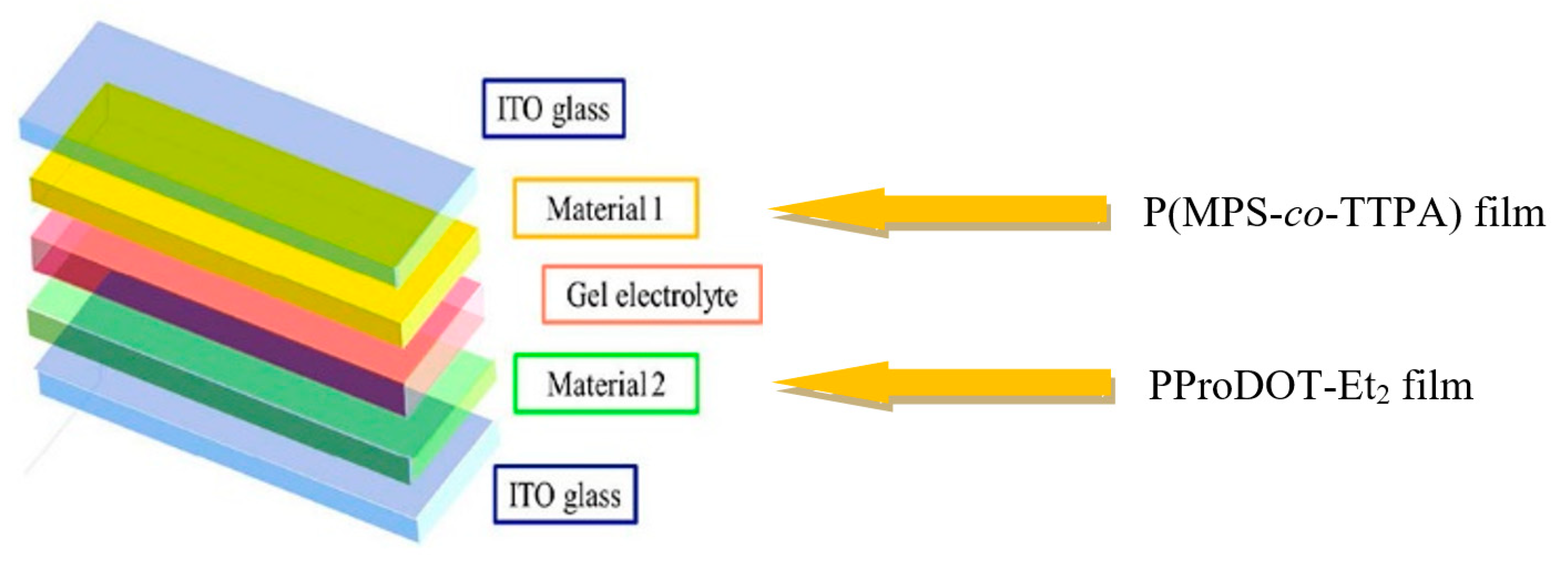
2.2. Fabrication of ECDs
2.3. Spectroelectrochemical Characterizations of Copolymer Films and ECDs
3. Results and Discussion
3.1. Preparation of Copolymer Films
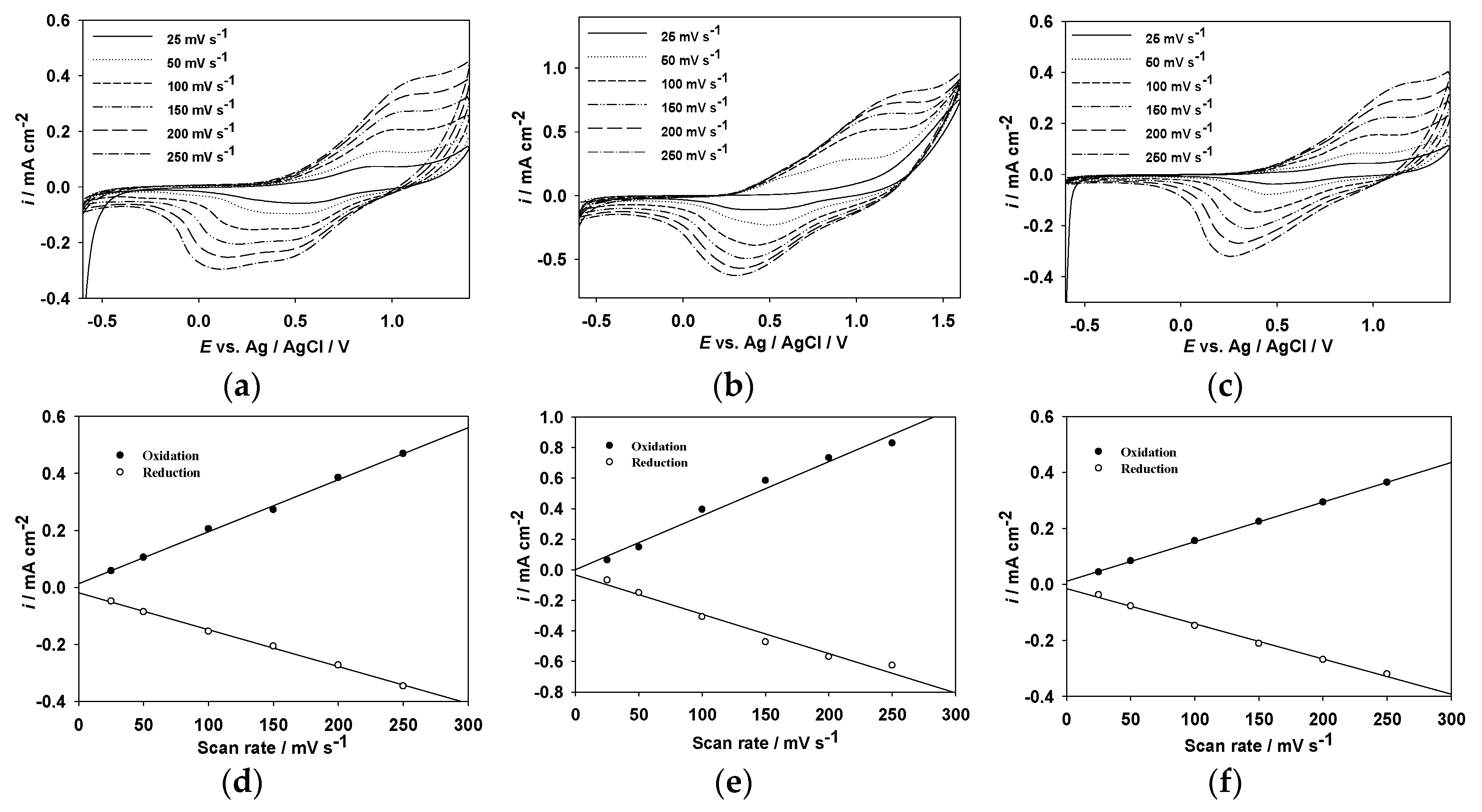
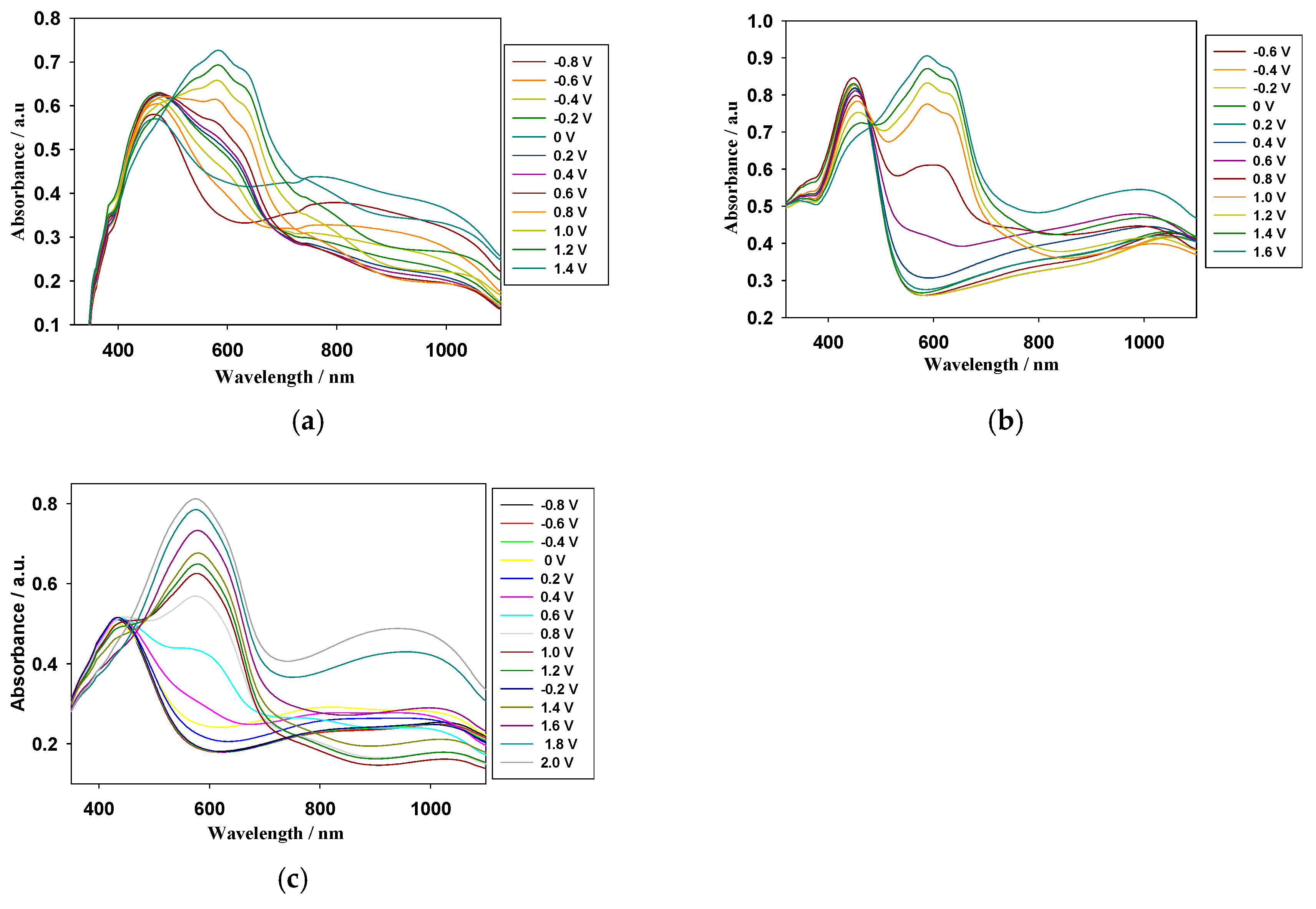
3.2. Spectroelectrochemical Studies of Copolymer Films
3.3. Spectroelectrochemistry of Dual Type ECDs
3.4. Optical Memory Effect of ECDs
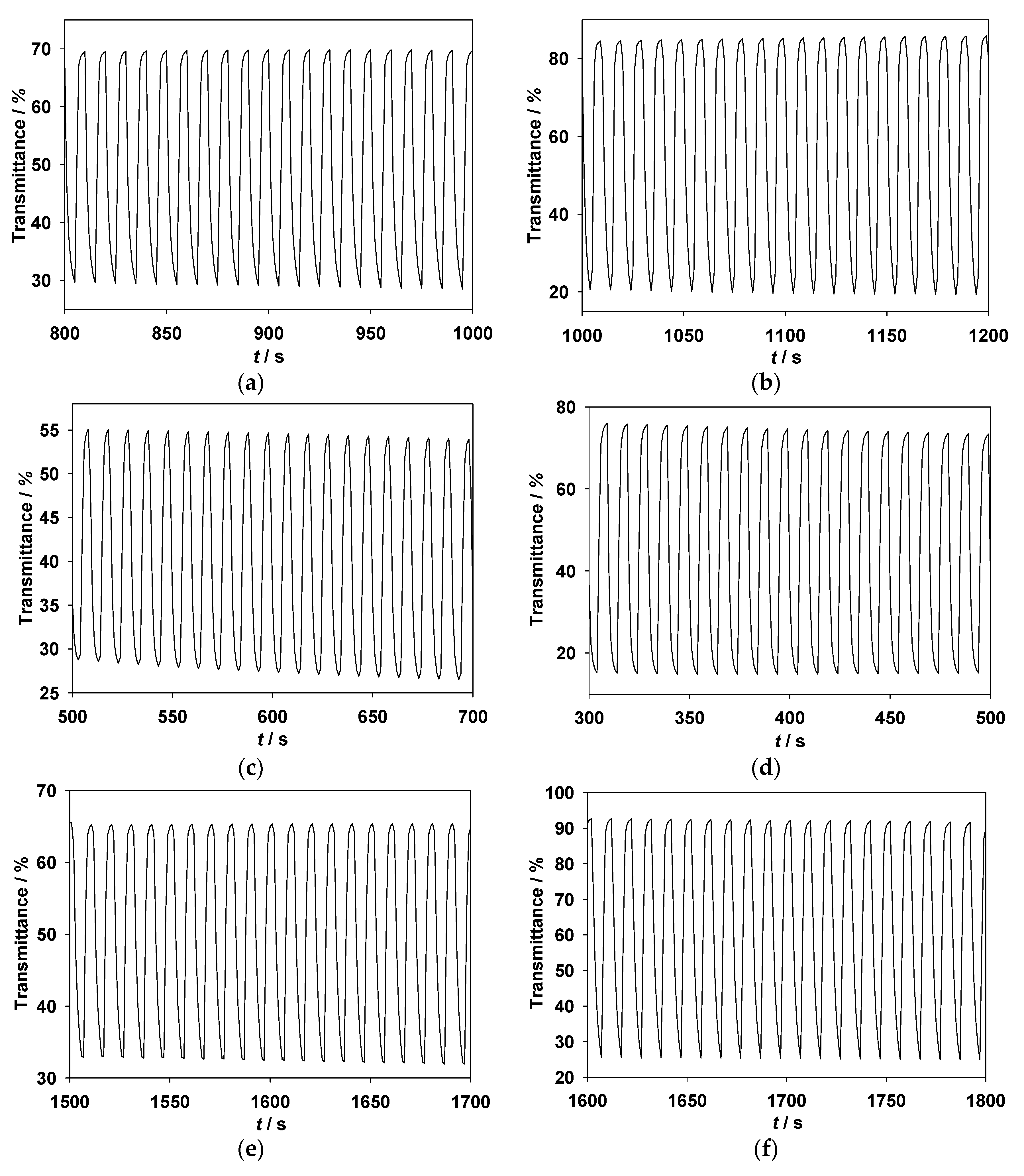
3.5. Redox Stability of ECDs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Beaujuge, P.M.; Reynolds, J.R. Color control in π–conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.G. Electrochromic materials. Chem. Soc. Rev. 1997, 26, 147–156. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lu, H.-Y. Electrosynthesis of aromatic poly(amide-amine) films from triphenylamine-based electroactive compounds for electrochromic applications. Polymers 2017, 9, 708. [Google Scholar] [CrossRef]
- Su, Y.-S.; Wu, T.-Y. Three carbazole-based polymers as potential anodically coloring materials for high-contrast electrochromic devices. Polymers 2017, 9, 284. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Dou, S.; Zhang, L.; Zhang, H.; Lv, H.; Wang, L.; Zhao, J.; Li, Y. A comprehensive study of electrochromic device with variable infrared emissivity based on polyaniline conducting polymer. Sol. Energy Mater. Sol. Cells 2017, 170, 120–126. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, B.K.; Li, W.B.; Tseng, L.Y.; Wu, T.Y.; Tseng, C.G.; Chen, H.R.; Huang, Y.C. Effects of supporting electrolytes on spectroelectrochemical and electrochromic properties of polyaniline-poly(styrene sulfonic acid) and poly(ethylenedioxythiophene)-poly(styrene sulfonic acid)-based electrochromic device. J. Chin. Chem. Soc. 2014, 61, 563–570. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Vasilyeva, S.V.; Liu, D.Y.; Ellinger, S.; McCarley, T.D.; Reynolds, J.R. Structure-performance correlations in spray-processable green dioxythiophene-benzothiadiazole donor–acceptor polymer electrochromes. Chem. Mater. 2012, 24, 255–268. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.Y.; Huang, M.W. Electrochromic characterizations of copolymers based on 4,4′-bis(N-carbazolyl)-1,1′-biphenyl and indole-6-carboxylic acid and their applications in electrochromic devices. J. Taiwan Inst. Chem. Eng. 2016, 68, 481–488. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, S.; Liu, H.; Zhao, Y.; Wang, Z.; Xu, J. Effects on the electrochemical and electrochromic properties of 3 linked polythiophene derivative by the introduction of polyacrylate. Int. J. Electrochem. Sci. 2015, 10, 7720–7731. [Google Scholar]
- Baran, D.; Balan, A.; Celebi, S.; Esteban, B.M.; Neugebauer, H.; Sariciftci, N.S.; Toppare, L. Processable multipurpose conjugated polymer for electrochromic and photovoltaic applications. Chem. Mater. 2010, 22, 2978–2987. [Google Scholar] [CrossRef]
- Schmatz, B.; Ponder, J.F., Jr.; Reynolds, J.R. Multifunctional triphenylamine polymers synthesized via direct (hetero) arylation polymerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 147–153. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, W.-K.; Liou, G.-S. Synthesis and electrochromism of highly organosoluble polyamides and polyimides with bulky trityl-substituted triphenylamine units. Polymers 2017, 9, 511. [Google Scholar] [CrossRef]
- Su, Y.S.; Chang, J.C.; Wu, T.Y. Applications of three dithienylpyrroles-based electrochromic polymers in high-contrast electrochromic devices. Polymers 2017, 9, 114. [Google Scholar] [CrossRef]
- Koyuncu, F.B.; Sefer, E.; Koyuncu, S.; Ozdemir, E. A new low band gap electrochromic polymer containing 2,5-bis-dithienyl-1H-pyrrole and 2,1,3-benzoselenadiazole moiety with high contrast ratio. Polymer 2011, 52, 5772–5779. [Google Scholar] [CrossRef]
- Soganci, T.; Soyleyici, S.; Soyleyici, H.C.; Ak, M. High contrast electrochromic polymer and copolymer materials based on amide-substituted poly(dithienyl pyrrole). J. Electrochem. Soc. 2017, 164, H11–H20. [Google Scholar] [CrossRef]
- Guven, N.; Camurlu, P. Optoelectronic properties of poly(2,5-dithienylpyrrole)s with fluorophore groups. J. Electrochem. Soc. 2015, 162, H867–H876. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, J.; Fu, Y.; Cui, C.; Zhang, X. Electrosynthesis and characterization of a multielectrochromic copolymer of tris[4-(2-thienyl)phenyl]amine with 3,4-ethylenedioxythiophene. J. Electrochem. Soc. 2013, 160, G6–G13. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, Y.S. Electrochemical synthesis and characterization of 1,4-benzodioxan-based electrochromic polymer and its application in electrochromic devices. J. Electrochem. Soc. 2015, 162, G103–G112. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, J.; Cui, C.; Fu, Y.; Zhang, X. Star-shaped conjugated systems derived from thienyl-derivatized poly(triphenylamine)s as active materials for electrochromic devices. J. Electroanal. Chem. 2012, 677, 24–30. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratio and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Hao, L.; Lin, K.F.; Sun, I.W. Thermophysical properties of a room temperature ionic liquid (1-methyl-3-pentyl-imidazolium hexafluorophosphate) with poly(ethylene glycol). J. Taiwan Inst. Chem. Eng. 2011, 42, 914–921. [Google Scholar] [CrossRef]
- Wu, T.Y.; Liao, J.W.; Chen, C.Y. Electrochemical synthesis, characterization and electrochromic properties of indan and 1,3-benzodioxole-based poly(2,5-dithienylpyrrole) derivatives. Electrochim. Acta 2014, 150, 245–262. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Chang, J.-K.; Lin, Y.-C.; Wu, T.-Y.; Lee, P.-Y.; Ho, T.-H. Poly(tris(4-carbazoyl-9-ylphenyl)amine)/three poly(3,4-ethylenedioxythiophene) derivatives in complementary high-contrast electrochromic devices. Polymers 2017, 9, 543. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Lee, P.-Y. Electrosynthesis of copolymers based on 1,3,5-tris(N-carbazolyl)benzene and 2,2′-bithiophene and their applications in electrochromic devices. Polymers 2017, 9, 518. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, Y.-C. Facile synthesis of electroactive and electrochromic triptycene poly(ether-imide)s containing triarylamine units via oxidative electro-coupling. Polymers 2017, 9, 497. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Wu, T.-L.; Lin, Y.-C.; Chang, J.-K.; Chen, H.-R.; Wu, T.-Y. Copolymers based on 1,3-bis(carbazol-9-yl)benzene and three 3,4-ethylenedioxythiophene derivatives as potential anodically coloring copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 368. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W. Synthesis and characterization of organic dyes containing various donors and acceptors. Int. J. Mol. Sci. 2010, 11, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.H.; Wu, T.Y.; Wang, H.P.; Sun, I.W.; Su, S.G.; Lin, Y.C.; Chang, C.W. An efficient metal free sensitizer for dye-sensitized solar cells. Mater. Lett. 2011, 65, 583–586. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Chung, H.-H. Applications of tris(4-(thiophen-2-yl)phenyl)amine- and dithienylpyrrole-based conjugated copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, H.P.; Wu, T.Y.; Sun, I.W. Optical and electrochromic characterizations of four 2,5-dithienylpyrrole-based conducting polymer films. Electrochim. Acta 2014, 119, 225–235. [Google Scholar] [CrossRef]
- Guven, N.; Camurlu, P. Electrosyntheses of anthracene clicked poly(thienylpyrrole)s and investigation of their electrochromic properties. Polymer 2015, 73, 122–130. [Google Scholar] [CrossRef]
- Soganci, T.; Soyleyici, H.C.; Ak, M.; Cetisli, H. An amide substituted dithienylpyrrole based copolymer: Its electrochromic properties. J. Electrochem. Soc. 2016, 163, H59–H66. [Google Scholar] [CrossRef]
- Tarkuc, S.; Sahmetlioglu, E.; Tanyeli, C.; Akhmedov, I.M.; Toppare, L. Electrochromic properties of poly (1-(phenyl)-2,5-di(2-thienyl)-1H-pyrrole-co-3,4-ethylenedioxy thiophene) and its application in electrochromic devices. Opt. Mater. 2008, 30, 1489–1494. [Google Scholar] [CrossRef]
- Bingol, B.E.; Tekin, B.; Carbas, B.B. An investigation on electrochromic properties of new copolymers based on dithienylpyrrole and propylenedioxythiophene. J. Electroanal. Chem. 2017, 806, 107–115. [Google Scholar] [CrossRef]
- Varis, S.; Ak, M.; Akhmedov, I.M.; Tanyeli, C.; Toppare, L. A novel multielectrochromic copolymer based on 1-(4-nitrophenyl)-2,5-di(2-thienyl)-1H-pyrrole and EDOT. J. Electroanal. Chem. 2007, 603, 8–14. [Google Scholar] [CrossRef]
- Camurlu, P.; Gűltekin, C. A comprehensive study on utilization of N-substituted poly(2,5-dithienylpyrrole) derivatives in electrochromic devices. Sol. Energy Mater. Sol. Cells 2012, 107, 142–147. [Google Scholar] [CrossRef]
- Camurlu, P.; Tarkuc, S.; Sahmetlioğlu, E.; Akhmedov, I.M.; Tanyeli, C.; Toppare, L. Multichromic conducting copolymer of 1-benzyl-2,5-di(thiophen-2-yl)-1H-pyrrole with EDOT. Sol. Energy Mater. Sol. Cells 2008, 92, 154–159. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]








| Polymer Films | Onset Potentials/V | Oxidation Potentials/V | Reduction Potentials/V |
|---|---|---|---|
| P(MPS-co-TTPA) | 0.72 | 1.12 | 0.34 |
| P(MPO-co-TTPA) | 0.70 | 1.21 | 0.21 |
| P(ANIL-co-TTPA) | 0.78 | 1.19 | 0.22 |
| Polymer Films | 0 V | 0.6 V | 1.2 V | 1.6 V |
|---|---|---|---|---|
| P(MPS-co-TTPA) |  |  |  |  |
| P(MPO-co-TTPA) |  |  |  |  |
| P(ANIL-co-TTPA) |  |  |  |  |
| Polymer Films in [EPI+][TFSI–] | λmax/nm | Cycle No. | ∆T/% | τc/s | τb/s |
|---|---|---|---|---|---|
| T90% | T90% | ||||
| P(MPS-co-TTPA) | 570 | 1 | 39.47 | 2.05 | 1.85 |
| 50 | 40.82 | 2.16 | 1.88 | ||
| 100 | 39.35 | 2.21 | 2.02 | ||
| 964 | 1 | 67.04 | 2.06 | 1.90 | |
| 50 | 66.68 | 2.16 | 1.82 | ||
| 100 | 67.18 | 2.21 | 1.84 | ||
| P(MPO-co-TTPA) | 592 | 1 | 25.74 | 2.02 | 1.90 |
| 50 | 26.09 | 2.04 | 1.95 | ||
| 100 | 25.57 | 2.12 | 2.01 | ||
| 914 | 1 | 60.69 | 1.99 | 1.93 | |
| 50 | 58.06 | 2.01 | 1.96 | ||
| 100 | 56.23 | 1.97 | 1.91 | ||
| P(ANIL-co-TTPA) | 548 | 1 | 32.43 | 2.19 | 1.87 |
| 50 | 31.45 | 2.15 | 1.97 | ||
| 100 | 32.37 | 2.14 | 1.82 | ||
| 960 | 1 | 66.38 | 2.17 | 1.96 | |
| 50 | 66.27 | 2.18 | 1.87 | ||
| 100 | 67.08 | 2.11 | 1.85 |
| Polymer Films | Electrolyte | λ/nm | ΔTmax/% | ∆ODmax/% | ηmax/cm2 C−1 | Ref. |
|---|---|---|---|---|---|---|
| P(TPVB-co-EDOT) | 0.1 M TBP6/DCM | 555 | 44 | – | – | [32] |
| P(TPVB-co-EDOT) | 0.1 M TBP6/DCM | 1000 | 75 | – | – | [32] |
| P(SNS-PN-co-EDOT) | 0.1 M NaClO4/LiClO4/ACN | 480 | 8 | – | – | [33] |
| P(SNS-PN-co-ProDOT) | 0.1 M LiClO4/ACN | 850 | 42 | – | 256.0 | [34] |
| P(MPS-co-TTPA) | [EPI+][TFSI–] | 964 | 67.2 | 66.5 | 260.3 | This work |
| P(MPO-co-TTPA) | [EPI+][TFSI–] | 914 | 60.7 | 69.7 | 176.6 | This work |
| P(ANIL-co-TTPA) | [EPI+][TFSI–] | 960 | 67.1 | 55.9 | 230.8 | This work |
| ECDs | 0 V | 0.6 V | 1.0 V | 1.4 V |
|---|---|---|---|---|
| P(MPS-co-TTPA)/PProDOT-Et2 |  |  |  |  |
| P(MPO-co-TTPA)/PProDOT-Et2 |  |  |  |  |
| P(ANIL-co-TTPA)/PProDOT-Et2 |  |  |  |  |
| ECDs | λmax/nm | Cycle No. | ΔT/% | τc/s | τb/s |
|---|---|---|---|---|---|
| T95% | T95% | ||||
| P(MPS-co-TTPA)/PProDOT-Et2 | 576 | 1 | 49.21 | 1.01 | 0.93 |
| 50 | 49.53 | 1.01 | 0.96 | ||
| 100 | 48.89 | 1.14 | 0.97 | ||
| P(MPO-co-TTPA)/PProDOT-Et2 | 580 | 1 | 55.12 | 0.98 | 0.99 |
| 50 | 54.12 | 1.01 | 0.99 | ||
| 100 | 53.60 | 1.05 | 1.13 | ||
| P(ANIL-co-TTPA)/PProDOT-Et2 | 582 | 1 | 46.34 | 1.17 | 1.34 |
| 50 | 45.76 | 0.98 | 1.25 | ||
| 100 | 44.91 | 1.17 | 1.32 |
| ECDs | λ/nm | ∆Tmax/% | ∆ODmax/% | η/cm2 C–1 | Ref. |
|---|---|---|---|---|---|
| P(NTP-co-EDOT)/PEDOT | 650 | 23 | – | – | [35] |
| P(SNS-An-Fc-co-EDOT)/PEDOT | 601 | 22 | – | 484 | [36] |
| P(SNBS-co-EDOT)/PEDOT | 485 | 15 | – | – | [37] |
| P(PTP-co-EDOT)/PEDOT | 545 | 15 | – | – | [33] |
| P(Cz4-co-CIn1)/PProDOT-Me2 | 575 | 32 | 24.6 | 372.7 | [38] |
| P(MPS-co-TTPA)/PProDOT-Et2 | 576 | 49.53 | 60.94 | 691.2 | This work |
| P(MPO-co-TTPA)/PProDOT-Et2 | 580 | 55.12 | 67.58 | 766.5 | This work |
| P(ANIL-co-TTPA)/PProDOT-Et2 | 582 | 46.34 | 45.03 | 625.4 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-Y.; Su, Y.-S.; Chang, J.-C. Dithienylpyrrole- and Tris[4-(2-thienyl)phenyl]amine-Containing Copolymers as Promising Anodic Layers in High-Contrast Electrochromic Devices. Coatings 2018, 8, 164. https://doi.org/10.3390/coatings8050164
Wu T-Y, Su Y-S, Chang J-C. Dithienylpyrrole- and Tris[4-(2-thienyl)phenyl]amine-Containing Copolymers as Promising Anodic Layers in High-Contrast Electrochromic Devices. Coatings. 2018; 8(5):164. https://doi.org/10.3390/coatings8050164
Chicago/Turabian StyleWu, Tzi-Yi, Yuh-Shan Su, and Jui-Cheng Chang. 2018. "Dithienylpyrrole- and Tris[4-(2-thienyl)phenyl]amine-Containing Copolymers as Promising Anodic Layers in High-Contrast Electrochromic Devices" Coatings 8, no. 5: 164. https://doi.org/10.3390/coatings8050164
APA StyleWu, T.-Y., Su, Y.-S., & Chang, J.-C. (2018). Dithienylpyrrole- and Tris[4-(2-thienyl)phenyl]amine-Containing Copolymers as Promising Anodic Layers in High-Contrast Electrochromic Devices. Coatings, 8(5), 164. https://doi.org/10.3390/coatings8050164






