Corrosion Resistance of Waterborne Epoxy Coatings by Incorporation of Dopamine Treated Mesoporous-TiO2 Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
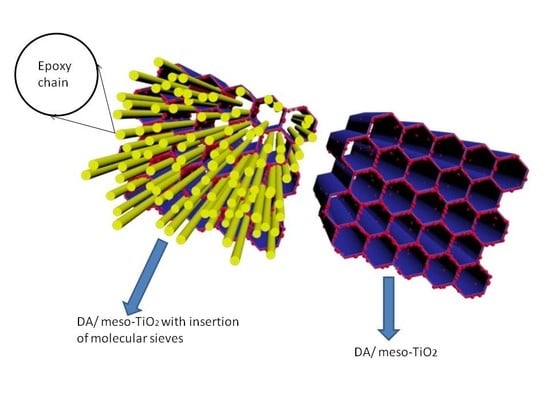
2.2. Preparation of DA/meso-TiO2
2.3. Preparation of the Anticorrosion Coating
2.4. Preparation of the Steel Substrate
2.5. Techniques and Analyses
3. Results and Discussion
3.1. FTIR Results
3.2. Nitrogen Adsorption–Desorption Results
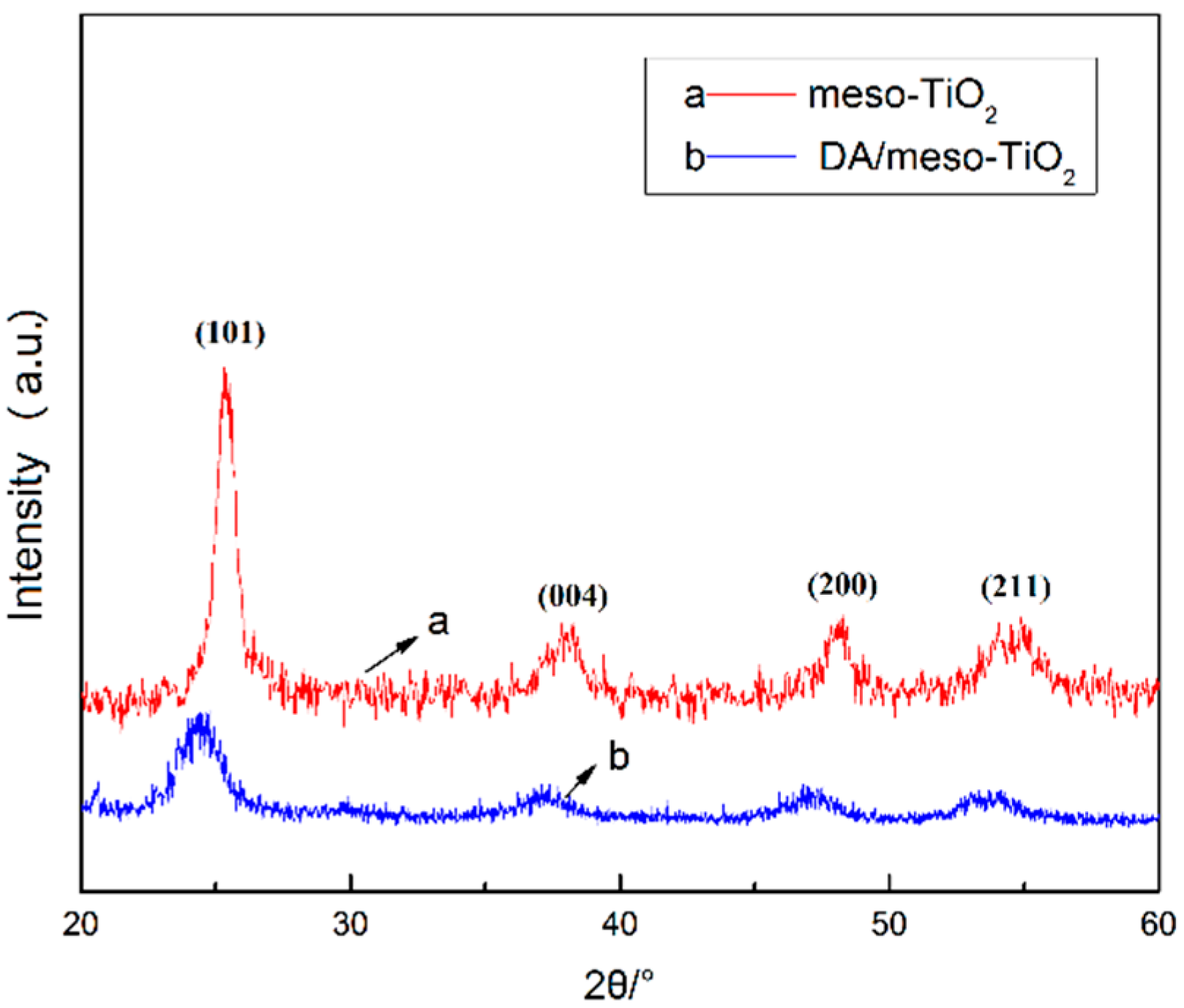
3.3. X-ray Diffraction Results
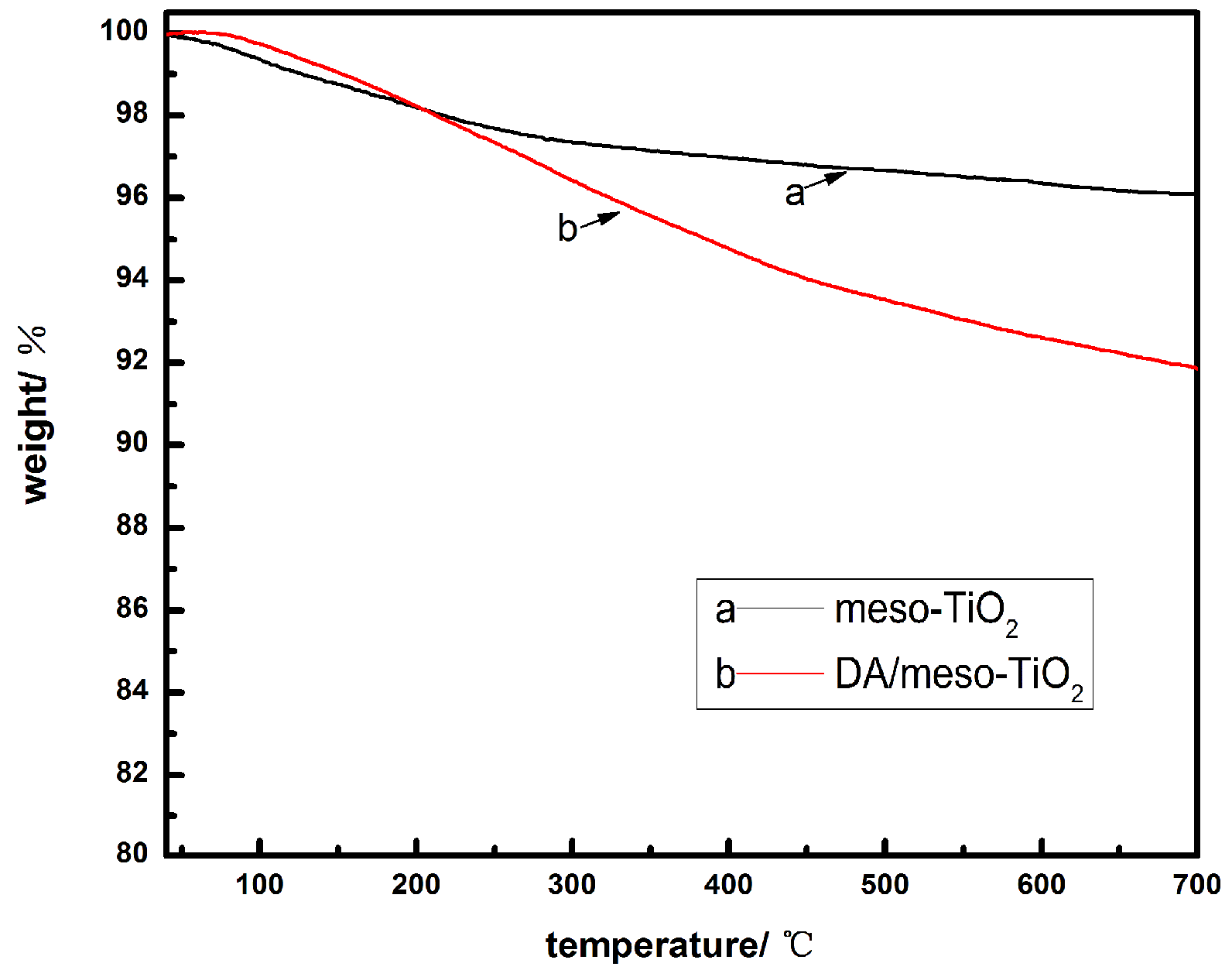
3.4. Thermo-Gravimetric Analyses (TGA)
3.5. Electrochemical Impedance Spectroscopy (EIS)
3.6. Salt Spray Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, N.; Wu, H.; Cheng, Q. Investigation on anticorrosion performance of polyaniline-mesoporous MCM-41 composites in new water-based epoxy coating. Mater. Corros. 2015, 65, 968–976. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vittoria, V. Development of epoxy mixtures for application in aeronautics and aerospace. RSC Adv. 2014, 4, 485–494. [Google Scholar] [CrossRef]
- Pourhashem, S.; Rashidi, A.; Vaezi, R. Excellent corrosion protection performance of epoxy composite coatings filled with amino-silane functionalized graphene oxide. Surf. Coat. Technol. 2017, 317, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.H.; Tang, Y.; Yu, F. Preparation of light color antistatic and anticorrosive waterborne epoxy coating for oil tanks. J. Coat. Technol. Res. 2008, 5, 259–269. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Wang, R. The role of functional groups on graphene oxide in epoxy nanocomposites. Polymer 2013, 54, 5821–5829. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S. Electrochemical behavior of nano-iron oxide modified alkyd based waterborne coatings. Mater. Chem. Phys. 2009, 117, 550–556. [Google Scholar] [CrossRef]
- Nematollahi, M.; Heidarian, M.; Peikari, M. Comparison between the effect of nanoglass flake and montmorillonite organoclay on corrosion performance of epoxy coating. Corros. Sci. 2010, 52, 1809–1817. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A. Investigating the effect of SiO2-graphene oxide hybrid as inorganic nanofiller on corrosion protection properties of epoxy coatings. Surf. Coat. Technol. 2017, 311, 282–294. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, K.; Wu, H. Effect of nano-sized mesoporous silica MCM-41 and MMT on corrosion properties of epoxy coating. Prog. Org. Coat. 2012, 75, 386–391. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Org. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Salami-Kalajahi, M.; Haddadi-Asl, V.; Rahimi-Razin, S. Investigating the effect of pristine and modified silica nanoparticles on the kinetics of methyl methacrylate polymerization. Chem. Eng. J. 2011, 174, 368–375. [Google Scholar] [CrossRef]
- Derkach, K.V.; Romanova, I.V.; Shpakov, A.O. Functional interaction between the dopamine and melanocortin systems of the brain. Neurosci. Behav. Physiol. 2018, 48, 213–219. [Google Scholar] [CrossRef]
- Hu, Y.R.; Guo, C.; Wang, F. Improvement of microalgae harvesting by magnetic nanocomposites coated with polyethylenimine. Chem. Eng. J. 2014, 242, 341–347. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Mititelu-Mija, A.; Vincent, L. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim. Acta 2006, 447, 167–177. [Google Scholar] [CrossRef]
- Wang, N.; Fu, W.; Zhang, J. Corrosion performance of waterborne epoxy coatings containing polyethylenimine treated mesoporous-TiO2 nanoparticles on mild steel. Prog. Org. Coat. 2015, 89, 114–122. [Google Scholar] [CrossRef]
- Wang, N.; Fang, Q.; Zhang, J. Incorporation of nano-sized mesoporous MCM-41 material used as fillers in natural rubber composite. Mater. Sci. Eng. A 2011, 528, 3321–3325. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G. Preparation of mesoporous titanium dioxide anode by a film- and pore-forming agent for the dye-sensitized solar cell. Mater. Res. Bull. 2016, 76, 140–146. [Google Scholar] [CrossRef]
- ASTM B117-03 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2003.
- Wang, N.; Zhang, Y.; Chen, J. Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog. Org. Coat. 2017, 109, 126–134. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Mahdavian, M.; Karimi, E. Evaluation of corrosion protection properties of epoxy coating containing sol-gel surface modified nano-zirconia on mild steel. RSC Adv. 2015, 5, 28769–28777. [Google Scholar] [CrossRef]
- Ko, Y.I.; Kim, B.S.; Bae, J.S. Silicone-coated elastomeric polylactide nanofiber filaments: Mechanical properties and shape memory behavior. RSC Adv. 2013, 3, 20091–20098. [Google Scholar] [CrossRef]
- Francis, B.; Thomas, S.; Jose, J. Hydroxyl terminated poly(ether ether ketone) with pendent methyl group toughened epoxy resin: Miscibility, morphology and mechanical properties. Polymer 2005, 46, 12372–12385. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Arabindoo, B. Enhanced photocatalytic degradation of 4-chlorophenol by Zr4+ doped nano TiO2. J. Mol. Catal. A Chem. 2007, 266, 158–165. [Google Scholar] [CrossRef]
- Irala, D.R.; Fontana, L.C.; Sagás, J.C. The effects of plasma nitriding pretreatment in steel substrates on the photocatalytic activity of TiO2 films. Surf. Coat. Technol. 2014, 240, 154–159. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q. Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Shinde, V.P. Study of water transport characteristics of poly(o-ethylaniline) coatings: Corrosion mechanism. Ionics 2018, 24, 605–615. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, T.; Gopal, M. EIS studies of a corrosion inhibitor behavior under multiphase flow conditions. Corros. Sci. 2000, 42, 979–990. [Google Scholar] [CrossRef]
- Yang, X.K.; Li, Q.; Zhang, S.Y. Electrochemical corrosion behaviors and protective properties of Ni–Co–TiO2 composite coating prepared on sintered NdFeB magnet. J. Solid State Electrochem. 2010, 14, 1601–1608. [Google Scholar] [CrossRef]
- Scully, J.R. Electrochemical impedance of organic-coated steel: Correlation of impedance parameters with long-term coating deterioration. J. Electrochem. Soc. 1989, 136, 979–990. [Google Scholar] [CrossRef]
- Liu, B.; Du, P.; Zhao, C.; Wang, P. Synthesis of l-histidine-attached graphene nanomaterials and their application for steel protection. Appl. Nano Mater. 2018, 1, 1385–1395. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, P.; Wu, J. Enhancement of anticorrosion protection via inhibitor-loaded ZnAlCe-LDH nanocontainers embedded in sol-gel coatings. J. Coat. Technol. Res. 2018, 15, 303–313. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Eduok, U.; Suleiman, R.; Gittens, J. Anticorrosion/antifouling properties of bacterial spore-loaded sol-gel type coating for mild steel in saline marine condition: A case of thermophilic strain of Bacillus licheniformis. RSC Adv. 2015, 5, 93818–93830. [Google Scholar] [CrossRef]









| Sample | Waterborne Epoxy Resin (g) | Pigment (g) | Curing Agent (g) | Water (g) |
|---|---|---|---|---|
| Neat epoxy | 20 | – | 10 | 10 |
| Meso-TiO2 (1.0 wt %) | 20 | 0.2 | 10 | 10 |
| DA/meso-TiO2 (0.5 wt %) | 20 | 0.1 | 10 | 10 |
| DA/meso-TiO2 (0.7 wt %) | 20 | 0.14 | 10 | 10 |
| DA/meso-TiO2 (1.0 wt %) | 20 | 0.2 | 10 | 10 |
| DA/meso-TiO2 (2.0 wt %) | 20 | 0.4 | 10 | 10 |
| Samples | Average Pore-Size (nm) | BET (m2·g−1) | Pore Volume (mL·g−1) |
|---|---|---|---|
| meso-TiO2 | 5.8 | 94.43 | 0.253 |
| DA/meso-TiO2 | 4.7 | 9.84 | 0.074 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Diao, X.; Zhang, J.; Kang, P. Corrosion Resistance of Waterborne Epoxy Coatings by Incorporation of Dopamine Treated Mesoporous-TiO2 Particles. Coatings 2018, 8, 209. https://doi.org/10.3390/coatings8060209
Wang N, Diao X, Zhang J, Kang P. Corrosion Resistance of Waterborne Epoxy Coatings by Incorporation of Dopamine Treated Mesoporous-TiO2 Particles. Coatings. 2018; 8(6):209. https://doi.org/10.3390/coatings8060209
Chicago/Turabian StyleWang, Na, Xinlin Diao, Jing Zhang, and Ping Kang. 2018. "Corrosion Resistance of Waterborne Epoxy Coatings by Incorporation of Dopamine Treated Mesoporous-TiO2 Particles" Coatings 8, no. 6: 209. https://doi.org/10.3390/coatings8060209
APA StyleWang, N., Diao, X., Zhang, J., & Kang, P. (2018). Corrosion Resistance of Waterborne Epoxy Coatings by Incorporation of Dopamine Treated Mesoporous-TiO2 Particles. Coatings, 8(6), 209. https://doi.org/10.3390/coatings8060209