Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
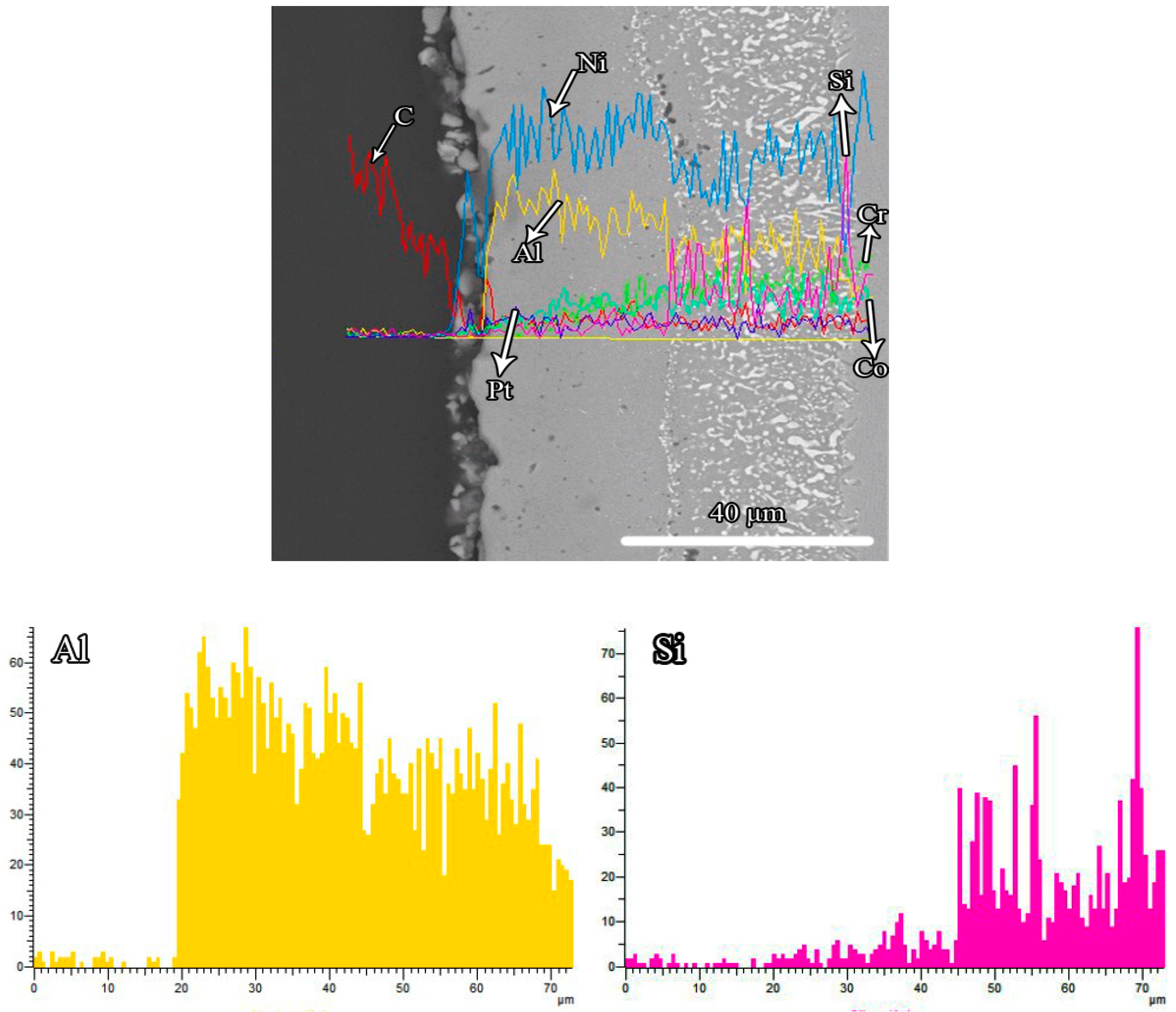
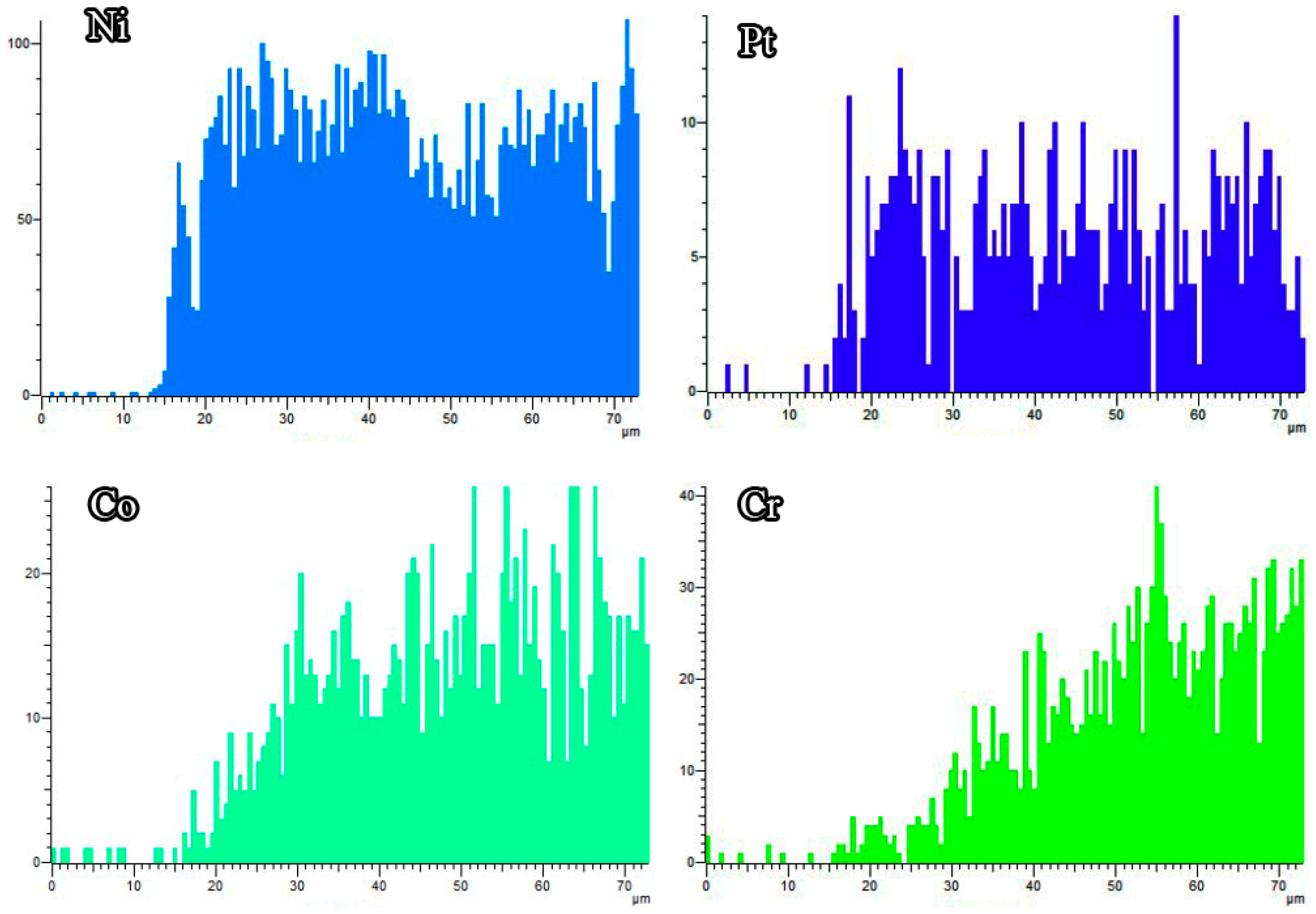
3.1. Microstructures of the Coatings
3.2. Oxidation Kinetic Curves
3.3. Oxidation Products
3.4. The Effect of Si on the Microstructure and Oxidation Resistance of the Pt Modified Aluminide Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, J.; Luan, Y.; Guo, H.; Peng, H.; Zhang, Y.; Zhang, T.; Gong, S. The role of Cr and Si in affecting high-temperature oxidation behaviour of minor Dy doped NiAl alloys. Corros. Sci. 2013, 77, 322–333. [Google Scholar] [CrossRef]
- Marino, K.A.; Carter, E.A. Ni and Al diffusion in Ni-rich NiAl and the effect of Pt additions. Intermetallics 2010, 18, 1470–1479. [Google Scholar] [CrossRef]
- Liu, R.D.; Jiang, S.M.; Yu, H.J.; Gong, J.; Sun, C. Preparation and hot corrosion behaviour of Pt modified AlSiY coating on a Ni-based superalloy. Corros. Sci. 2016, 104, 162–172. [Google Scholar] [CrossRef]
- Svensson, H.; Christensen, M.; Knutsson, P.; Wahnström, G.; Stiller, K. Influence of Pt on the metal–oxide interface during high temperature oxidation of NiAl bulk materials. Corros. Sci. 2009, 51, 539–546. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Bai, M.; Chandio, A.; Wu, R.; Xiao, P. Effect of platinum addition on oxidation behaviour of γ/γ′ nickel aluminide. Acta Mater. 2015, 86, 319–330. [Google Scholar] [CrossRef]
- Pomeroy, M.J. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, H.; Zhang, L.; Guo, H.; Gong, S. Microstructure and cyclic oxidation behaviour of low-Pt/Dy co-doped β-NiAl coatings on single crystal (SC) superalloy. Surf. Coat. Technol. 2016, 304, 108–116. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Yao, H.R.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Preparation and enhanced oxidation performance of a Hf-doped single-phase Pt-modified aluminide coating. Corros. Sci. 2016, 113, 17–25. [Google Scholar] [CrossRef]
- Song, Y.; Murakami, H.; Zhou, C. Cyclic Corrosion Behavior of Pt/Ru-Modified Bond Coatings Exposed to NaCl Plus Water Vapor at 1050 °C. J. Mater. Sci. Technol. 2010, 26, 217–222. [Google Scholar] [CrossRef]
- Song, Y.; Murakami, H.; Zhou, C. Cyclic-Oxidation Behavior of Multilayered Pt/Ru-Modified Aluminide Coating. J. Mater. Sci. Technol. 2011, 27, 280–288. [Google Scholar] [CrossRef]
- Hong, S.J.; Hwang, G.H.; Han, W.K.; Lee, K.S.; Kang, S.G. Effect of zirconium addition on cyclic oxidation behavior of platinum-modified aluminide coating on nickel-based superalloy. Intermetallics 2010, 18, 864–870. [Google Scholar] [CrossRef]
- Firouzi, A.; Shirvani, K. The structure and high temperature corrosion performance of medium-thickness aluminide coatings on nickel-based superalloy GTD-111. Corros. Sci. 2010, 52, 3579–3585. [Google Scholar] [CrossRef]
- Shirvani, K.; Mastali, S.; Rashidghamat, A.; Abdollahpour, H. The effect of silicon on thermal shock performance of aluminide-thermal barrier coatings. Corros. Sci. 2013, 75, 142–147. [Google Scholar] [CrossRef]
- Taniguchi, S.; Uesaki, K.; Zhu, Y.C.; Matsumoto, Y.; Shibata, T. Influence of implantation of Al, Si, Cr or Mo ions on the oxidation behaviour of TiAl under thermal cycle conditions. Mater. Sci. Eng. A 1999, 266, 267–275. [Google Scholar] [CrossRef]
- Clemens, D.; Vosberg, V.; Hobbs, L.W.; Breuer, U.; Quadakkers, W.J.; Nickel, H. TEM and SNMS studies of protective alumina scales on NiCrAlY-alloys. Fresenius J. Anal. Chem. 1996, 355, 703–706. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, Z.; Wang, W.; Zhou, C. Microstructure and hot corrosion behavior of Co–Si modified aluminide coating on nickel based superalloys. Corros. Sci. 2015, 100, 466–473. [Google Scholar] [CrossRef]
- Azarmehr, S.A.; Shirvani, K.; Schütze, M.; Galetz, M. Microstructural evolution of silicon-platinum modified aluminide coatings on superalloy GTD-111. Surf. Coat. Technol. 2017, 321, 455–463. [Google Scholar] [CrossRef]
- Fan, Q.X.; Peng, X.; Yu, H.J.; Jiang, S.M.; Gong, J.; Sun, C. The isothermal and cyclic oxidation behaviour of two Co modified aluminide coatings at high temperature. Corros. Sci. 2014, 84, 42–53. [Google Scholar] [CrossRef]
- Liu, R.D.; Jiang, S.M.; Guo, C.Q.; Gong, J.; Sun, C. The alumina scale growth and interdiffusion behaviour of Pt modified AlSiY coating during cyclic oxidation. Corros. Sci. 2017, 120, 121–129. [Google Scholar] [CrossRef]
- Hong, S.J.; Hwang, G.H.; Han, W.K.; Kang, S.G. The effect of Pt contents on the surface morphologies of Pt-modified aluminide coating. Surf. Coat. Technol. 2009, 203, 3066–3071. [Google Scholar] [CrossRef]
- Hong, S.J.; Hwang, G.H.; Han, W.K.; Kang, S.G. Cyclic oxidation of Pt/Pd-modified aluminide coating on a nickel-based superalloy at 1150 °C. Intermetallics 2009, 17, 381–386. [Google Scholar] [CrossRef]
- Fan, Q.X.; Jiang, S.M.; Wu, D.L.; Gong, J.; Sun, C. Preparation and hot corrosion behaviour of two Co modified NiAl coatings on a Ni-based superalloy. Corros. Sci. 2013, 76, 373–381. [Google Scholar] [CrossRef]
- Shirvani, K.; Saremi, M.; Yamaoto, Y. The approaches to thin film preparation and TEM observations on slurry Si-modified aluminide coatings. Mater. Corros. 2006, 57, 182–184. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Codeposition of Al and Si on nickel base superalloys by pack cementation process. Mater. Sci. Eng. A 2003, 356, 136–144. [Google Scholar] [CrossRef]
- Fu, C.; Kong, W.K.; Cao, G.H. Microstructure and oxidation behavior of Al+Si co-deposited coatings on nickel-based superalloys. Surf. Coat. Technol. 2014, 258, 347–352. [Google Scholar] [CrossRef]
- Dai, P.; Wu, Q.; Ma, Y.; Li, S.; Gong, S. The effect of silicon on the oxidation behavior of NiAlHf coating system. Appl. Surf. Sci. 2013, 271, 311–316. [Google Scholar] [CrossRef]
- Hamadi, S.; Bacos, M.P.; Poulain, M.; Seyeux, A.; Maurice, V.; Marcus, P. Oxidation resistance of a Zr-doped NiAl coating thermochemically deposited on a nickel-based superalloy. Surf. Coat. Technol. 2009, 204, 756–760. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Zhao, C.; Hao, W.; Wang, X.; Xiao, P. The oxidation performance for Zr-doped nickel aluminide coating by composite electrodepositing and pack cementation. Corros. Sci. 2017, 123, 103–115. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, D.; Xu, H.; Han, Y. Thermal barrier coatings with two layer bond coat on intermetallic compound Ni3Al based alloy. Intermetallics 2005, 13, 295–299. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Yu, H.; Wang, T.; Liu, Y. Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy. Coatings 2018, 8, 264. https://doi.org/10.3390/coatings8080264
Fan Q, Yu H, Wang T, Liu Y. Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy. Coatings. 2018; 8(8):264. https://doi.org/10.3390/coatings8080264
Chicago/Turabian StyleFan, Qixiang, Haojun Yu, Tiegang Wang, and Yanmei Liu. 2018. "Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy" Coatings 8, no. 8: 264. https://doi.org/10.3390/coatings8080264
APA StyleFan, Q., Yu, H., Wang, T., & Liu, Y. (2018). Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy. Coatings, 8(8), 264. https://doi.org/10.3390/coatings8080264




