Studies on Synthesis and Characterization of Aqueous Hybrid Silicone-Acrylic and Acrylic-Silicone Dispersions and Coatings. Part I
Abstract
:1. Introduction
- A process where monomer X is polymerized in aqueous dispersion of polymer Y or monomer Y is polymerized in aqueous dispersion of polymer X;
- A process where monomer X is added to aqueous dispersion of polymer Y or monomer Y is added to aqueous dispersion of polymer X and left for some time in order to achieve swelling of dispersion particles with the monomer, and only then is polymerization conducted;
- A process where a mixture of monomers X and Y is placed in the reactor before start of polymerization or is added dropwise during the polymerization. However, in this case formation of particles with hybrid structure would be possible only if the corresponding homopolymers are not compatible or either reactivities of monomers or their polymerization mechanisms differ significantly.
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis of Silicone Resin Dispersions and Hybrid Silicone-Acrylic and Acrylic-Silicone Dispersions
- Mixture designated as 1: D4—84.0%, MTES—9.5%, VTES—6.5%
- Mixture designated as 2: D4—88.0%, VTES—12%
2.3. Characterization of Dispersions
- Solids content, wt.%–percentage of sample mass remaining after drying for 1 h at 80 °C followed by 4 h at 125 °C. The measurements were conducted three times and the mean value was taken.
- pH—using standard indicator paper.
- Viscosity—using Bohlin Instruments CVO 100 rheometer (Cirencester, UK), cone-plate 60 mm diameter and 1° measuring device, shear rate 600 s−1.
- Coagulum content—after filtration of dispersion on 190 mesh net the solids remaining on the net were dried and weighed. Coagulum content (wt.%) was calculated from equation mc/md × 100% where mc was mass of dry coagulum remaining on the net and md was mass of dispersion.
- Acrylic and styrene monomers, ethanol and D4 content—by GC (HP 5890 series II apparatus FID detector, Hewlett Packard, Palo Alto, CA, USA)
- Mechanical stability—lack or occurrence of separation during rotation in Hettich Universal 32R centrifuge (Westphalian, The Netherlands) at 4000 r.p.m. for 90 min was considered as good stability.
- Average particle size (nm), particle size distribution and zeta potential (mV)—light-scattering method using Malvern Zeta Sizer apparatus.
- Dispersion particles appearance—transmission electron microscope (TEM) Hitachi 2700 (Tokyo, Japan), dispersions were diluted 1000× with water (1 part of dispersion per 1000 parts of water) for taking pictures. High Angle Annular Dark Field (HAADF) mode also called “Z-contrast” was applied for processing the images reproduced in this paper.
- Minimum film-forming temperature (MFFT)—according to ISO 2115 [34] using Coesfeld apparatus equipped with temperature gradient plate. Temperature range: –3–50 °C.
- Glass transition temperature (Tg) of dispersion solids—by differential scanning calorimetry (DSC) (TA Instruments Q2000 apparatus, New Castle, DE, USA), heat–cool–heat regime, 20 °C/min.
2.4. Characterization of Coatings
- Contact angle (water)—according to EN 828:2000, using KRUSS DSA 100E apparatus (KRÜSS GmbH, Hamburg, Germany). The measurements were conducted five times and the mean value was taken.
- Pendulum hardness (Koenig)—according to EN ISO 1522 [35]. The measurements were conducted seven times and the mean value was taken.
- Adhesion—according to EN ISO 2409 [36], the tests were repeated at least three times.
- Elasticity—according to EN ISO 1519 [37], the tests were repeated at least two times.
- Impact resistance (direct and reverse)—according to EN ISO 6272-1 [38], using Erichsen Variable Impact Tester Model 304 (Erichsen, Hemer, Germany). The measurements were conducted at least twice.
- Cupping—according to EN ISO 1520 [39], using Erichsen Cupping Tester (ERICHSEN GmbH & Co. KG, Hemer, Germany). The tests were repeated three times and the mean value was taken.
- Water resistance—glass Petri dishes of 50 mm diameter were filled with distilled water and placed upside-down on the coating, so the coating was covered with 7 mm thick layer of water. Assembles prepared this way were left for 72 h and appearance of coatings was examined for the bubbles size (S0—no bubbles, S2–S5—small to large size of bubbles) and density (0—no bubbles, 2–5 low to high density of bubbles) according to EN ISO 4628-2 [40]. Observation of changes of coating appearance after 6 days under water were also examined.
- Water vapour permeability—according to ASTM F1249 [41]. TotalPerm 063 (Extra Solution) apparatus was used. Tests were conducted at 23 °C for 0.35 mm thick film. Fomblin perfluorinated grease from Solvay Solexis (Brussels, Belgium) was applied to seal the test vessels. The measurements were repeated at least twice.
- Moreover, coatings applied on PET film were examined for surface structure by X-ray photoelectron spectroscopy (XPS)—ULVAC/PHYSICAL ELECTRONICS PHI5000 VersaProbe apparatus (Physical Electronics, Inc., Chigasaki, Japan).
2.5. Characterization of Films
- Percentage swell, i.e., change of the mass caused by soaking in water or organic solvent—ca. 0.12 g samples of film were weighed and placed in 40 mL H2O or 40 mL toluene contained in closed glass cups and left for 20 h at 23 °C. Then the samples were taken out, delicately dried with filter paper and weighed. Percentage swell was calculated from the equation: % swell = m1-m0/m0 × 100%, where m0 = mass of the sample before test and m1 = mass of the sample after test. The tests were repeated three times.
- Mechanical properties (tensile strength and elongation at break)—using Instron 3345 testing machine (Instron, Norwood, MA, USA) according to EN-ISO 527-1 [42] at a pulling rate of 50 mm/min on dumbbell-shaped specimens. The measurements were conducted five times and the mean value was used taken.
3. Results and Discussion
3.1. Properties of Dispersions
3.1.1. Particle Size and Particle Size Distribution
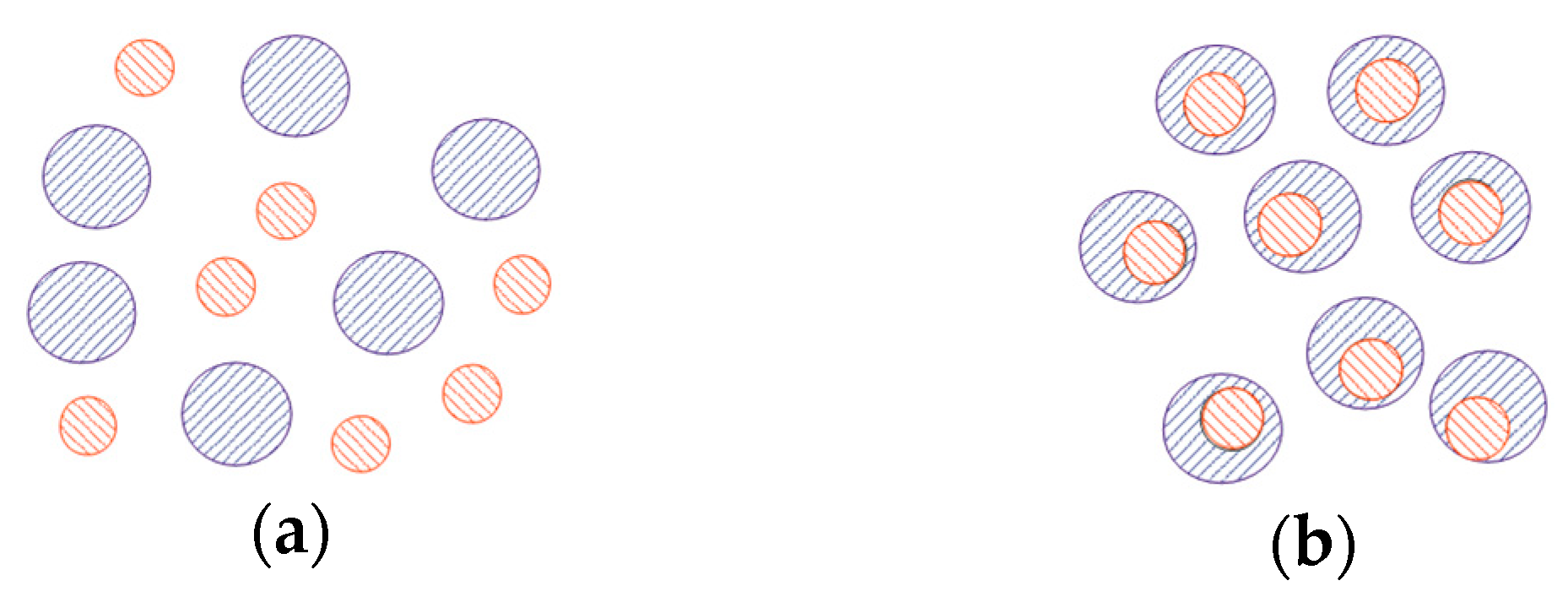
3.1.2. Particle Structure
3.1.3. Minimum Film-Forming Temperature (MFFT)
3.1.4. Glass Transition Temperature (Tg)
3.2. Properties of Coatings and Films
3.2.1. Surface Properties
3.2.2. Water Resistance
3.2.3. Swell in Water and in Toluene
3.2.4. Water Vapour Permeability
3.2.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez, R.; de Las Heras Alarcón, C.; Ekanayake, P.; McDonald, P.J.; Keddie, J.L.; Barandiaran, M.J.; Asua, J.M. Correlation of silicone incorporation into hybrid acrylic coatings with the resulting hydrophobic and thermal properties. Macromolecules 2008, 41, 8537–8546. [Google Scholar] [CrossRef]
- Kickelbick, G. Introduction to hybrid materials. In Hybrid Materials: Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–46. [Google Scholar]
- Castelvetro, V.; de Vita, C. Nanostructured hybrid materials from aqueous polymer dispersions. Adv. Colloid Interface Sci. 2004, 108–109, 167–185. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Koncka-Foland, A.; Skarżyński, J.; Legocka, I. Synthesis and characterization of aqueous hybrid polyurethane-urea-acrylic/styrene polymer dispersions. In Advances in Urethane Science and Technology, 1st ed.; Klempner, D., Frisch, K.C., Eds.; RAPRA Technology Ltd.: Shrewsbury, UK, 2001; pp. 261–334. [Google Scholar]
- Khanjani, J.; Pazokifard, S.; Zohuriaan-Mehr, M.J. Improving dirt pickup resistance in waterborne coatings using latex blends of acrylic/PDMS polymers. Prog. Org. Coat. 2017, 102, 151–166. [Google Scholar] [CrossRef]
- Peruzzo, P.J.; Anbinder, P.S.; Pardini, O.R.; Vega, J.; Costa, C.A.; Galembeck, F.; Amalvy, J.I. Waterborne polyurethane/acrylate: Comparison of hybrid and blend systems. Prog. Org. Coat. 2011, 72, 429–437. [Google Scholar] [CrossRef]
- Ma, J.Z.; Liu, Y.H.; Bao, Y.; Liu, J.L.; Zhang, J. Research advances in polymer emulsion based on “core-shell” structure design. Adv. Colloid Interface Sci. 2013, 197–198, 118–131. [Google Scholar] [CrossRef]
- Mittal, V. Advanced Polymer Nanoparticles: Synthesis and Surface Modification, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Guyot, A.; Landfester, K.; Schork, F.J.; Wang, C. Hybrid polymer latexes. Prog. Polym. Sci. 2007, 32, 1439–1461. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Ofat, I.; Trzaskowska, J. Silicone-containing aqueous polymer dispersions with hybrid particle structure. Adv. Colloid Interface Sci. 2015, 223, 1–39. [Google Scholar] [CrossRef]
- Holmes, D. Controlling the morphology of composite latex particles. Inquiry J. 2005, 4. Available online: https://scholars.unh.edu/inquiry_2005/4/ (accessed on 25 December 2018).
- Winzor, C.L.; Sundberg, D.C. Conversion dependent morphology predictions for composite emulsion polymers: 1. Synthetic lattices. Polymer 1992, 33, 3797–3809. [Google Scholar] [CrossRef]
- Chen, Y.C.; Dimonie, V.; El-Aasser, M.S. Interfacial phenomena controlling particle morphology of composite latexes. J. Appl. Polym. Sci. 1991, 42, 1049–1063. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Russel, G.T.; Gilbert, R.G. Modelling secondary particle formation in emulsion polymerisation: Application to making core–shell morphologies. Polymer 2002, 43, 4557–4570. [Google Scholar] [CrossRef]
- Sundberg, D.S.; Durant, Y.G. Latex particle morphology, fundamental aspects: A review. Polym. React. Eng. 2003, 11, 379–432. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- He, W.D.; Cao, C.T.; Pan, C.Y. Formation mechanism of silicone rubber particles with core-shell structure by seeded emulsion polymerization. J. Appl. Polym. Sci. 1996, 61, 383–388. [Google Scholar] [CrossRef]
- He, W.D.; Pan, C.Y. Influence of reaction between second monomer and vinyl group of seed polysiloxane on seeded emulsion polymerization. J. Appl. Polym. Sci. 2001, 80, 2752–2758. [Google Scholar] [CrossRef]
- Lin, M.; Chu, F.; Gujot, A.; Putaux, J.L.; Bourgeat-Lami, E. Silicone-polyacrylate composite latex particles. Particles formation and film properties. Polymer 2005, 46, 1331–1337. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, L.X.; Sun, J.W.; Kan, C.Y. Fabrication and characterization of polysiloxane/polyacrylate composite latexes with balanced water vapor permeability and mechanical properties: Effect of silane coupling agent. J. Coat. Technol. Res. 2018, 15, 165–173. [Google Scholar] [CrossRef]
- Kan, C.Y.; Kong, X.Z.; Yuan, Q.; Liu, D.S. Morphological prediction and its application to the synthesis of polyacrylate/polysiloxane core/shell latex particles. J. Appl. Polym. Sci. 2001, 80, 2251–2258. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Tissot, I.; Lefevbre, F. Synthesis and characterization of SiOH-functionalized polymer latexes using methacryloxy propyl trimethoxysilane in emulsion polymerization. Macromolecules 2002, 35, 6185–6191. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Rościszewski, P.; Rokicki, G.; Kołdoński, G.; Skarżyński, J.; Koncka-Foland, A. Aqueous dispersions of siloxane-acrylic/styrene copolymers for use in coatings–preliminary investigations. Surf. Coat. Int. Part B 2001, 84, 301–307. [Google Scholar] [CrossRef]
- Kan, C.Y.; Zhu, X.L.; Yuan, Q.; Kong, X.Z. Graft emulsion copolymerization of acrylates and siloxane. Polym. Adv. Technol. 1997, 8, 631–633. [Google Scholar] [CrossRef]
- Li, W.; Shen, W.; Yao, W.; Tang, J.; Xu, J.; Jin, L.; Zhang, J.; Xu, Z. A novel acrylate-PDMS composite latex with controlled phase compatibility prepared by emulsion polymerization. J. Coat. Technol. Res. 2017, 14, 1259–1269. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.; Hao, L.; Zhang, D.; Zhang, M. Synthesis and characterization of self-crosslinking fluorinated polyacrylate soap-free lattices with core-shell structure. Appl. Surf. Sci. 2013, 268, 373–380. [Google Scholar] [CrossRef]
- Hao, G.; Zhu, L.; Yang, W.; Chen, Y. Investigation on the film surface and bulk properties of fluorine and silicon contained polyacrylate. Prog. Org. Coat. 2015, 85, 8–14. [Google Scholar] [CrossRef]
- Li, J.; Zhong, S.; Chen, Z.; Yan, X.; Li, W.; Yi, L. Fabrication and properties of polysilsesquioxane-based trilayer core-shell structure latex coatings with fluorinated polyacrylate and silica nanocomposite as the shell layer. J. Coat. Technol. Res. 2018, 15, 1077–1078. [Google Scholar] [CrossRef]
- Xu, W.; Hao, L.; An, Q.; Wang, X. Synthesis of fluorinated polyacrylate/polysilsesquioxane composite soap-free emulsion with partial trilayer core-shell structure and its hydrophobicity. J. Polym. Res. 2015, 22, 20. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Ofat, I.; Legocka, I.; Trzaskowska, J. Silicone-acrylic hybrid aqueous dispersions of core–shell particle structure and corresponding silicone-acrylic nanopowders designed for modification of powder coatings and plastics. Part I–Effect of silicone resin composition on properties of dispersions and corresponding nanopowders. Prog. Org. Coat. 2014, 77, 568–578. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Ofat, I.; Trzaskowska, J.; Kuczynska, H. Silicone-acrylic hybrid aqueous dispersions of core–shell particle structure and corresponding silicone-acrylic nanopowders designed for modification of powder coatings and plastics. Part II: Effect of modification with silicone-acrylic nanopowders and of composition of silicone resin contained in those nanopowders on properties of epoxy-polyester and polyester powder coatings. Prog. Org. Coat. 2015, 78, 419–428. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Kozakiewicz, J.; Ofat, I.; Trzaskowska, J.; Spirkova, M. Silicone-acrylic hybrid aqueous dispersions of core–shell particle structure and corresponding silicone-acrylic nanopowders designed for modification of powder coatings and plastics. Part III: Effect of modification with selected silicone-acrylic nanopowders on properties of polyurethane powder coatings. Prog. Org. Coat. 2015, 78, 429–436. [Google Scholar] [CrossRef]
- ISO 2115 Polymer Dispersions–Determination of White Point Temperature and Minimum Film-Forming Temperature; International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 1552 Paints and Varnishes–Pendulum Damping Test; International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 2409 Paints and Varnishes–Cross-Cut Test; International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 1519 Paints and Varnishes–Bend Test (Cylindrical Mandrel); International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 6272-1 Paints and Varnishes–Rapid-Deformation (Impact Resistance) Tests–Part 1: Falling-Weight Test, Large-Area Indenter; International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 1520 Paints and Varnishes–Cupping Test; International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 4628-2 Paints and Varnishes–Evaluation of Degradation of Coatings–Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance–Part 2: Assessment of Degree of Blistering; International Organization for Standardization: Geneva, Switzerland, 2016.
- ASTM F1249 Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor; ASTM International: West Conshohocken, PA, USA, 2013.
- ISO 527-1 Plastics–Determination of Tensile Properties–Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2012.
- Liu, Y. Silicone Dispersions, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mequanint, K.; Sanderson, R. Self-assembling of metal coatings from phosphate and siloxane-modified polyurethane dispersions: An analysis of the coating interface. J. Appl. Polym. Sci. 2003, 88, 893–899. [Google Scholar] [CrossRef]
- Ofat, I.; Kozakiewicz, J. Modification of epoxy-polyester and polyester powder coatings with silicone-acrylic nanopowders–effect on surface properties of coatings. Polimery 2014, 59, 643–649. [Google Scholar] [CrossRef]














| Designation of Dispersions | pH | Solids Content % | Coagulum Content % | Viscosity at 23 °C mPa·s | Average Particle Size nm | Particle Size Distribution nm | Polydispersity | Zeta Potential mV | Minimum Film-Forming Temperature (MFFT) °C | Tg (Disp. Solids) °C |
|---|---|---|---|---|---|---|---|---|---|---|
| SIL 1 | 6.3 | 19.3 | 0.00 | 2 | 121.7 | 104–157 | 0.121 | −50.9 | N.A. | −117.33 |
| SIL 2 | 6.2 | 18.5 | 0.00 | 3 | 128.6 | 116–154 | 0.086 | −49.5 | N.A. | −119.46 |
| ACR A | 6.2 | 51.1 | <0.1 | 94 | 105.7 | 74–119 | 0.112 | −51.0 | +11.4 | +17.17 |
| ACR B | 6.2 | 51.5 | <0.1 | 98 | 112.2 | 108–135 | 0.066 | −56.0 | +32.7 | +32.36 |
| SIL-ACR 1-A | 5.8 | 42.2 | 0.06 | 20 | 143.8 | 69–160 | 0.074 | −57.7 | +7.3 | −126.66 +17.66 |
| ACR-SIL A-1 | 6.3 | 43.2 | 0.38 | 140 | 111.7 | 71–131 | 0.096 | −55.2 | −0.5 | −130.54 +14.58 |
| SIL-ACR 1-B | 6.3 | 42.3 | 0.11 | 24 | 140.3 | 69–190 | 0.072 | −59.5 | 26.2 | −129.09 +30.87 |
| ACR-SIL B-1 | 6.3 | 42.0 | 0.21 | 61 | 114.4 | 98–133 | 0.071 | −55.0 | 16.0 | −126.33 +27.98 |
| SIL-ACR 2-A | 6.1 | 41.7 | 0.04 | 20 | 151.2 | 107–214 | 0.064 | −59.2 | −1.0 1 | −132.94 +17.29 |
| ACR-SIL A-2 | 6.3 | 41.6 | 0.13 | 55 | 109.3 | 98–122 | 0.085 | −47.2 | −0.4 | +16.13 |
| SIL-ACR 2-B | 6.3 | 41.4 | 0.05 | 16 | 149.9 | 125–156 | 0.057 | −55.7 | +26.0 1 | −131.46 +32.56 |
| ACR-SIL B-2 | 6.3 | 42.2 | 0.24 | 56 | 115.9 | 104–130 | 0.069 | −53.6 | +14.8 | +20.04 |
| Designation of Dispersions | Contact Angle (H2O) (°) | Water Vapour Permeability g/m2/24h | Water Resistance after 72 h | Impact Resistance (direct) J | Impact Resistance (reverse) J | Cupping mm | Elasticity (Rod Diameter 2 mm) | Hardness (Koenig) | Adhesion to Glass | Swell in H2O % | Swell in Toluene % | Tensile Strength MPa | Elongation at Break % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIL 1 | 111 | N.A. | 18 | 202 | N.A. | ||||||||
| SIL 2 | 104 | N.A. | 10 | 387 | N.A. | ||||||||
| ACR A | 30 | 28.1 | >5(S5) | 2.0 | 19.6 | 10.7 | passed | 0.082 | 5 | 14 | 1156 | 4.2 | 1000 |
| ACR B | 35 | 15.6 | 5(S2) Medium whitening | 2.0 | 0 | 10.7 | failed | 0.458 | 5 | 15 | 1547 | 12.0 | 340 |
| SIL-ACR 1-A | 83 | 56.5 | 5(S2) Medium whitening | 9.8 | 19.6 | 11.6 | passed | 0.040 | 3 | 26 | 591 | 2.1 | 773 |
| ACR-SIL A-1 | 95 | 45 | 0(S0) Light whitening | 15.7 | 19.6 | 11.0 | passed | 0.022 | 2 | 21 | 1050 | 0.8 | 1851 |
| SIL-ACR 1-B | 81 | 64.5 | 5(S2) Light whitening | 0 | 0 | 10.9 | passed | 0.085 | 5 | 11 | 561 | 4.3 | 11 |
| ACR-SIL B-1 | 92 | 34.6 | 0(S0) Medium whitening | 5.9 | 19.6 | 10.9 | passed | 0.050 | 5 | 26 | 905 | 3.1 | 1015 |
| SIL-ACR 2-A | 77 | N.A. | 20 | 605 | |||||||||
| ACR-SIL A-2 | 92 | 39.4 | 0(S0) Medium whitening | 13.7 | 19.6 | 10.5 | passed | 0.034 | 5 | 16 | 1112 | 0.9 | 1516 |
| SIL-ACR 2-B | 85 | N.A. | 9 | 665 | N.A. | ||||||||
| ACR-SIL B-2 | 82 | 28.0 | 0(S0) Medium whitening | 3.9 | 19.6 | 11.0 | passed | 0.058 | 5 | 31 | 991 | 3.2 | 947 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakiewicz, J.; Trzaskowska, J.; Domanowski, W.; Kieplin, A.; Ofat-Kawalec, I.; Przybylski, J.; Woźniak, M.; Witwicki, D.; Sylwestrzak, K. Studies on Synthesis and Characterization of Aqueous Hybrid Silicone-Acrylic and Acrylic-Silicone Dispersions and Coatings. Part I. Coatings 2019, 9, 25. https://doi.org/10.3390/coatings9010025
Kozakiewicz J, Trzaskowska J, Domanowski W, Kieplin A, Ofat-Kawalec I, Przybylski J, Woźniak M, Witwicki D, Sylwestrzak K. Studies on Synthesis and Characterization of Aqueous Hybrid Silicone-Acrylic and Acrylic-Silicone Dispersions and Coatings. Part I. Coatings. 2019; 9(1):25. https://doi.org/10.3390/coatings9010025
Chicago/Turabian StyleKozakiewicz, Janusz, Joanna Trzaskowska, Wojciech Domanowski, Anna Kieplin, Izabela Ofat-Kawalec, Jarosław Przybylski, Monika Woźniak, Dariusz Witwicki, and Krystyna Sylwestrzak. 2019. "Studies on Synthesis and Characterization of Aqueous Hybrid Silicone-Acrylic and Acrylic-Silicone Dispersions and Coatings. Part I" Coatings 9, no. 1: 25. https://doi.org/10.3390/coatings9010025
APA StyleKozakiewicz, J., Trzaskowska, J., Domanowski, W., Kieplin, A., Ofat-Kawalec, I., Przybylski, J., Woźniak, M., Witwicki, D., & Sylwestrzak, K. (2019). Studies on Synthesis and Characterization of Aqueous Hybrid Silicone-Acrylic and Acrylic-Silicone Dispersions and Coatings. Part I. Coatings, 9(1), 25. https://doi.org/10.3390/coatings9010025






