Effect of Novel Micro-Arc Oxidation Implant Material on Preventing Peri-Implantitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Titanium Discs
2.2. Saliva Collection
2.3. Biofilm Development
2.4. SEM, AFM and EDX Observation
2.5. Crystal Violet Assay
2.6. MTT Assay
2.7. Live/Dead Staining
2.8. 16S rDNA Sequencing
2.9. Bioinformatics and Statistical Analysis
3. Results
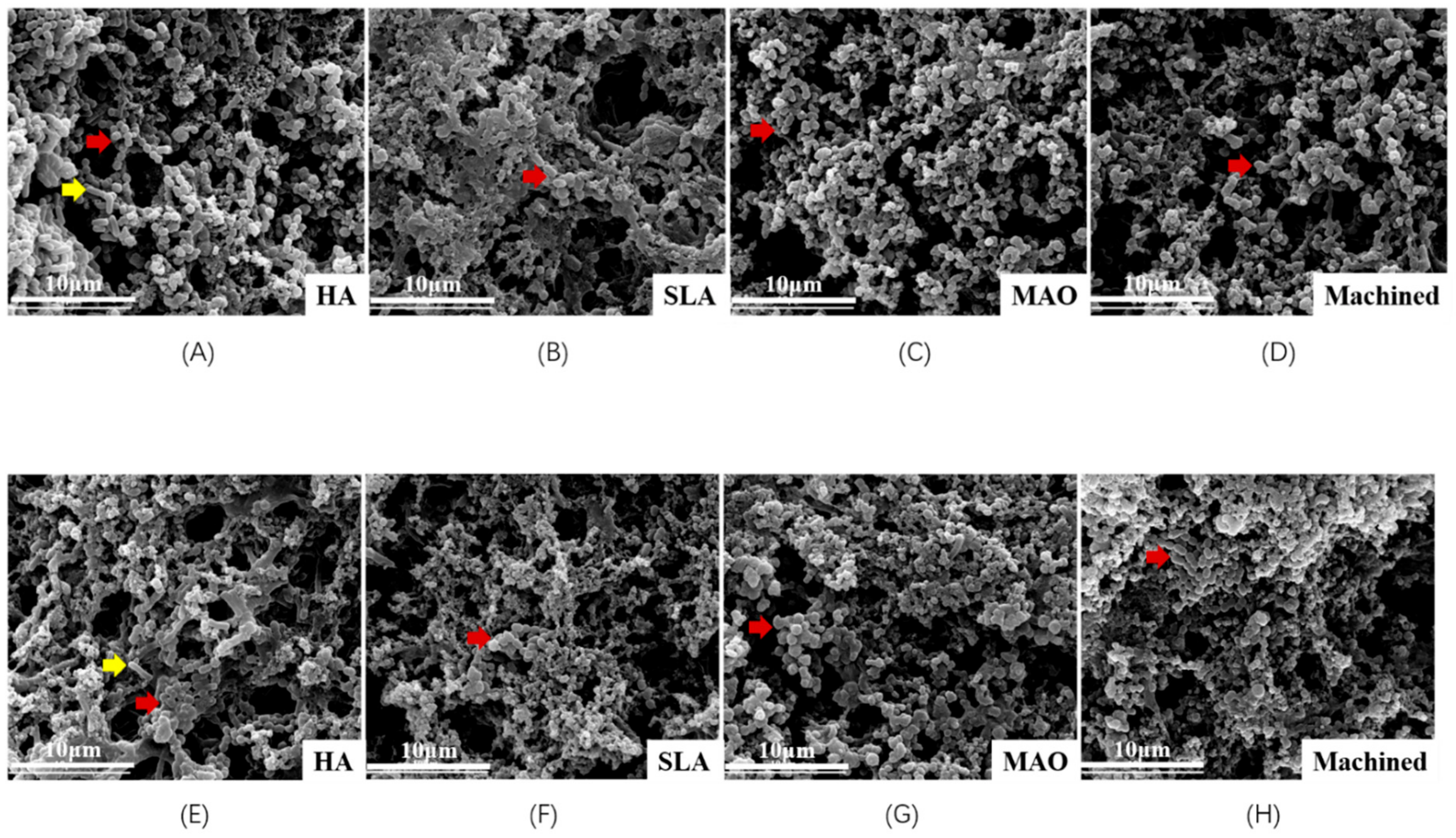
3.1. Scanning Electron Microscopy (SEM) Observation of the Surface of Titanium Discs
3.2. Scanning Electron Microscopy Observation of Biofilm on Titanium Discs
3.3. Metabolic Activity and Biomass Accumulation
3.4. Live/Dead Staining
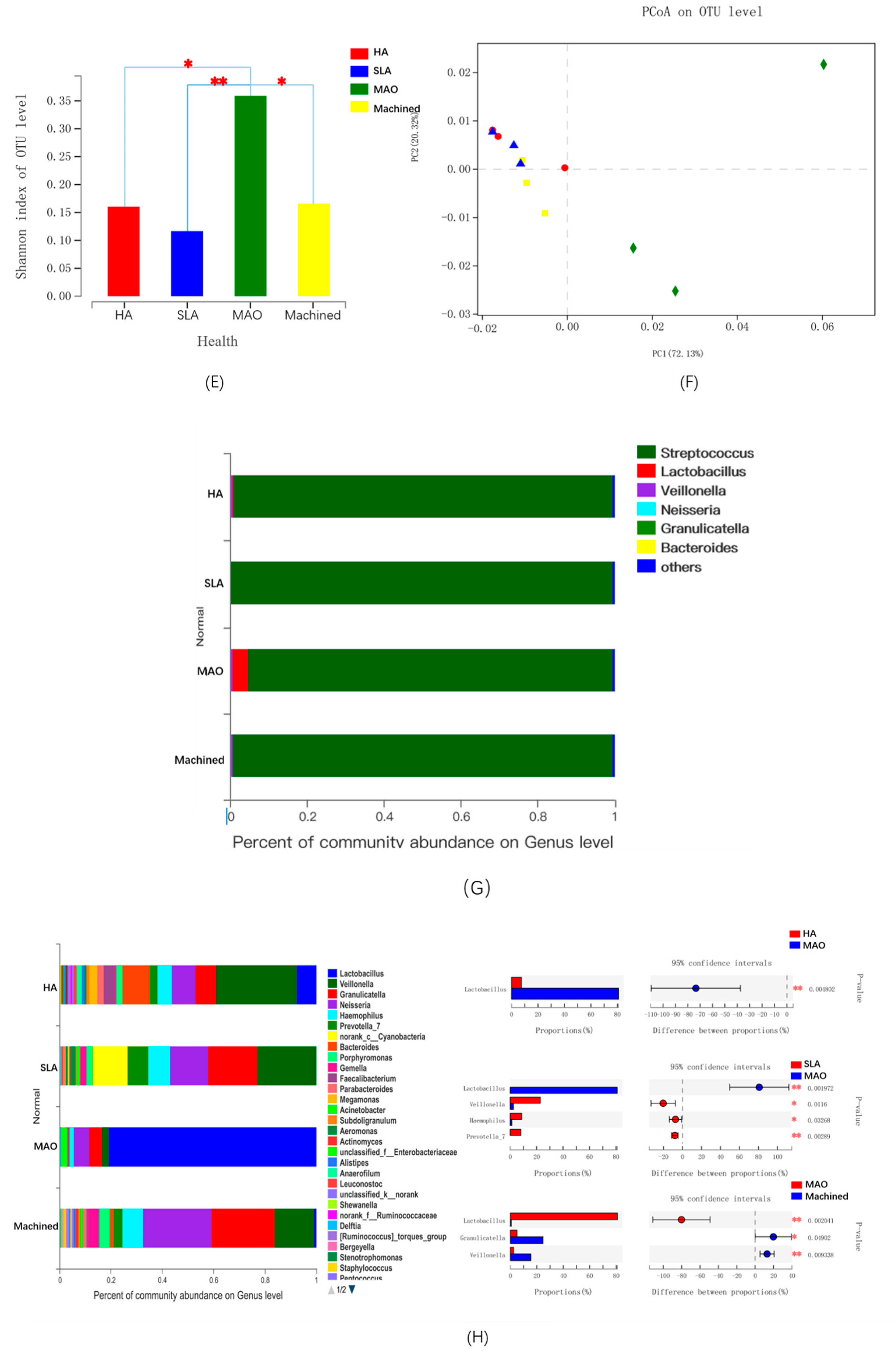
3.5. The Microbial Community of Saliva-Derived Biofilms
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robertson, K.; Shahbazian, T.; MacLeod, S. Treatment of peri-implantitis and the failing implant. Dent. Clin. N. Am. 2015, 59, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Mir-Orfila, P.; Figueiredo, R.; Valmaseda-Castellon, E.; Gay-Escoda, C. Prevalence of peri-implant diseases. A cross-sectional study based on a private practice environment. J. Clin. Periodontol. 2012, 39, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Mameno, T.; Wada, M.; Onodera, Y.; Fujita, D.; Sato, H.; Ikebe, K. Longitudinal study on risk indicators for peri-implantitis using survival-time analysis. J. Prosthodont. Res. 2018, 63, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Tu, Y.K.; Stolpe, M. Preventing and Treating Peri-Implantitis: A Cost-Effectiveness Analysis. J. Periodontol. 2015, 86, 1020–1029. [Google Scholar] [CrossRef]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Renvert, S.; Quirynen, M. Risk indicators for peri-implantitis. A narrative review. Clin. Oral Implant. Res. 2015, 26 (Suppl. 11), 15–44. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincon, M.V.; Gomez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Apatzidou, D.; Lappin, D.F.; Hamilton, G.; Papadopoulos, C.A.; Konstantinidis, A.; Riggio, M.P. Microbiome of peri-implantitis affected and healthy dental sites in patients with a history of chronic periodontitis. Arch. Oral Biol. 2017, 83, 145–152. [Google Scholar] [CrossRef]
- Belkacemi, S.; Mazel, A.; Tardivo, D.; Tavitian, P.; Stephan, G.; Bianca, G.; Terrer, E.; Drancourt, M.; Aboudharam, G. Peri-implantitis-associated methanogens: A preliminary report. Sci. Rep. 2018, 8, 9447. [Google Scholar] [CrossRef]
- Stokman, M.A.; van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K.; Raghoebar, G.M. Bacterial colonization of the peri-implant sulcus in dentate patients: A prospective observational study. Clin. Oral Investig. 2017, 21, 717–724. [Google Scholar] [CrossRef]
- Flichy-Fernandez, A.J.; Ata-Ali, J.; Alegre-Domingo, T.; Candel-Marti, E.; Ata-Ali, F.; Palacio, J.R.; Penarrocha-Diago, M. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: A double-blind randomized controlled trial. J. Periodontal Res. 2015, 50, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Sajedinejad, N.; Paknejad, M.; Houshmand, B.; Sharafi, H.; Jelodar, R.; Shahbani Zahiri, H.; Noghabi, K.A. Lactobacillus salivarius NK02: A Potent Probiotic for Clinical Application in Mouthwash. Probiotics Antimicrob. Proteins 2018, 10, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Masaki, C.; Tsuka, S.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. The effects of Lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. J. Prosthodont. Res. 2018, 62, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.O.; Freitas, A.R.; Pinheiro, M.L.L.; do Nascimento, C.; Watanabe, E.; Albuquerque, R.F. Oral Biofilm Formation on Different Materials for Dental Implants. J. Vis. Exp. 2018, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.W.-A.-A.M.; Faust, J.; Bachle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm formation and composition on different implant materials in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 101–109. [Google Scholar] [CrossRef]
- Bermejo, P.; Sanchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Biofilm formation on dental implants with different surface micro-topography: An in vitro study. Clin. Oral Implant. Res. 2019, 30, 725–734. [Google Scholar] [CrossRef]
- Zhao, B.; van der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef]
- Scarano, A.P.M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef]
- Namgoong, H.; Kim, M.D.; Ku, Y.; Rhyu, I.C.; Lee, Y.M.; Seol, Y.J.; Gu, H.J.; Susin, C.; Wikesjo, U.M.; Koo, K.T. Bone reconstruction after surgical treatment of experimental peri-implantitis defects at a sandblasted/acid-etched hydroxyapatite-coated implant: An experimental study in the dog. J. Clin. Periodontol. 2015, 42, 960–966. [Google Scholar] [CrossRef]
- Madi, M.; Zakaria, O.; Ichinose, S.; Kasugai, S. Effect of Induced Periimplantitis on Dental Implants With and Without Ultrathin Hydroxyapatite Coating. Implant. Dent. 2016, 25, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Conserva, E.; Generali, L.; Bandieri, A.; Cavani, F.; Borghi, F.; Consolo, U. Plaque accumulation on titanium disks with different surface treatments: An in vivo investigation. Odontology 2018, 106, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Biao, M.; Chen, Y.; Xie, M.; Yang, B. Regulating the osteogenic function of rhBMP 2 by different titanium surface properties. J. Biomed. Mater. Res. A 2016, 104, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, B. Bioactive Titanium Surfaces with the Effect of Inhibiting Biofilm Formation. J. Bionic Eng. 2014, 11, 589–599. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacteria and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, E2013. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Zhou, X.; Wu, P.; Li, M.; Feng, M.; Peng, X.; Ren, B.; Cheng, L. Effects of different substrates/growth media on microbial community of saliva-derived biofilm. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Zhou, C.; Weir, M.D.; Zhang, K.; Deng, D.; Cheng, L.; Xu, H.H. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dent. Mater. 2013, 29, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, S.; Peng, X.; Hu, Y.; Ren, B.; Li, M.; Hao, L.; Feng, M.; Cheng, L.; Zhou, X. Effects of water and microbial-based aging on the performance of three dental restorative materials. J. Mech. Behav. Biomed. Mater. 2018, 80, 42–50. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef]
- Ge, Y.; Ren, B.; Zhou, X.; Xu, H.H.K.; Wang, S.; Li, M.; Weir, M.D.; Feng, M.; Cheng, L. Novel Dental Adhesive with Biofilm-Regulating and Remineralization Capabilities. Materials 2017, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.H.; Li, M.; Li, C.Q.; Kou, G.J.; Zuo, X.L.; Li, Y.Q. Human colorectal mucosal microbiota correlates with its host niche physiology revealed by endomicroscopy. Sci. Rep. 2016, 6, 21952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G. QIIME allows analysis of highthroughput community sequencing data. Na. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Chai, B.; Farris, R.J.; Wang, Q.; Kulam, S.A.; McGarrell, D.M.; Garrity, G.M.; Tiedje, J.M. The Ribosomal Database Project (RDP-II): Sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 2005, 33, 294–296. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Matos, A.O.; Ricomini-Filho, A.P.; Beline, T.; Ogawa, E.S.; Costa-Oliveira, B.E.; de Almeida, A.B.; Nociti Junior, F.H.; Rangel, E.C.; da Cruz, N.C.; Sukotjo, C.; et al. Three-species biofilm model onto plasma-treated titanium implant surface. Coll. Surf. B Biointerfaces 2017, 152, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An In Vitro Model for Candida albicans(-)Streptococcus gordonii Biofilms on Titanium Surfaces. J. Fungi 2018, 4, 66. [Google Scholar] [CrossRef]
- Gokmenoglu, C.; Kara, N.B.; Belduz, M.; Kamburoglu, A.; Tosun, I.; Sadik, E.; Kara, C. Evaluation of Candida albicans biofilm formation on various parts of implant material surfaces. Niger. J. Clin. Pract. 2018, 21, 33–37. [Google Scholar] [CrossRef]
- Caglar, E.; Karqul, B.; Tanboga, I. Bacteriotherapy and probiotics’ role on oral health. Oral Dis. 2005, 11, 131–137. [Google Scholar] [CrossRef]
- Pena, M.; Barallat, L.; Vilarrasa, J.; Vicario, M.; Violant, D.; Nart, J. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: A triple-blind randomized clinical trial. Clin. Oral Investig. 2019, 23, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Alqahtani, M.; Shafqat, S.S.; Akram, Z.; Al-Kheraif, A.A.; Javed, F. Efficacy of mechanical debridement with adjunctive probiotic therapy in the treatment of peri-implant mucositis in cigarette-smokers and never-smokers. Clin. Implant. Dent. Relat. Res. 2019, 21, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Koõll-Klais, P.; Mandar, R.; Leibur, E.; Marcotte, H.; Hammarstrom, L.; Mikelsaar, M. Oral lactobacilli in chronic periodontitis and periodontal health: Species composition and antimicrobial activity. Oral Microbiol. Immunol. 2005, 20, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hammerle, C.H.F.; Jung, R.E.; Teles, F.R.F. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef]
- Kroger, A.; Hulsmann, C.; Fickl, S.; Spinell, T.; Huttig, F.; Kaufmann, F.; Heimbach, A.; Hoffmann, P.; Enkling, N.; Renvert, S.; et al. The severity of human peri-implantitis lesions correlates with the level of submucosal microbial dysbiosis. J. Clin. Periodontol. 2018, 45, 1498–1509. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Cortelli, J.R.; Romeiro, R.L.; Costa, F.O.; Aquino, D.R.; Orzechowski, P.R.; Araujo, V.C.; Duarte, P.M. Frequency of periodontal pathogens in equivalent peri-implant and periodontal clinical statuses. Arch. Oral Biol. 2013, 58, 67–74. [Google Scholar] [CrossRef]
- Belstrøm, D.; Fiehn, E.; Nielsen, C.H.; Kirkby, N.; Twetman, S.; Klepac-Ceraj, V.; Paster, B.J.; Holmstrup, P. Differences in bacterial saliva profile between periodontitis patients and a control cohort. J. Clin. Periodontol. 2014, 41, 104–112. [Google Scholar] [CrossRef]
- Du, Q.; Wei, D.; Wang, Y.; Cheng, S.; Liu, S.; Zhou, Y.; Jia, D. The effect of applied voltages on the structure, apatite-inducing ability and antibacterial ability of micro arc oxidation coating formed on titanium surface. Bioact. Mater. 2018, 3, 426–433. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, Y.M.; Biao, M.N.; Zhang, X.D.; Yang, B.C. Bio-functionalization of biomedical metals. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1057–1070. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, K.; Wu, C.; Lei, X.; Ding, J.; Shi, X.; Liu, C. Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium. Materials 2017, 10, 1446. [Google Scholar] [CrossRef]
- Yuan, X.; Tan, F.; Xu, H.; Zhang, S.; Qu, F.; Liu, J. Effects of different electrolytes for micro-arc oxidation on the bond strength between titanium and porcelain. J. Prosthodont. Res. 2017, 61, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, B. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; MÜLler, P.; Attin, T.; Wieland, M.; Hofer, D.; Guggenheim, B. Polyspecies biofilm formation on implant surfaces with different surface characteristics. J. Appl. Oral Sci. 2013, 21, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Melo, F.; do Nascimento, C.; Souza, D.O.; de Albuquerque, R.F. Identification of oral bacteria on titanium implant surfaces by 16S rDNA sequencing. Clin. Oral Implant. Res. 2017, 28, 697–703. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhou, W.; Zhou, X.; Hu, Y.; Xiang, P.; Li, B.; Yang, B.; Peng, X.; Ren, B.; Li, M.; et al. Effect of Novel Micro-Arc Oxidation Implant Material on Preventing Peri-Implantitis. Coatings 2019, 9, 691. https://doi.org/10.3390/coatings9110691
Huang X, Zhou W, Zhou X, Hu Y, Xiang P, Li B, Yang B, Peng X, Ren B, Li M, et al. Effect of Novel Micro-Arc Oxidation Implant Material on Preventing Peri-Implantitis. Coatings. 2019; 9(11):691. https://doi.org/10.3390/coatings9110691
Chicago/Turabian StyleHuang, Xiaoyu, Wen Zhou, Xuedong Zhou, Yao Hu, Pengfei Xiang, Bolei Li, Bangcheng Yang, Xian Peng, Biao Ren, Mingyun Li, and et al. 2019. "Effect of Novel Micro-Arc Oxidation Implant Material on Preventing Peri-Implantitis" Coatings 9, no. 11: 691. https://doi.org/10.3390/coatings9110691




