Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bionanocomposites Production
2.3. Film Characterization
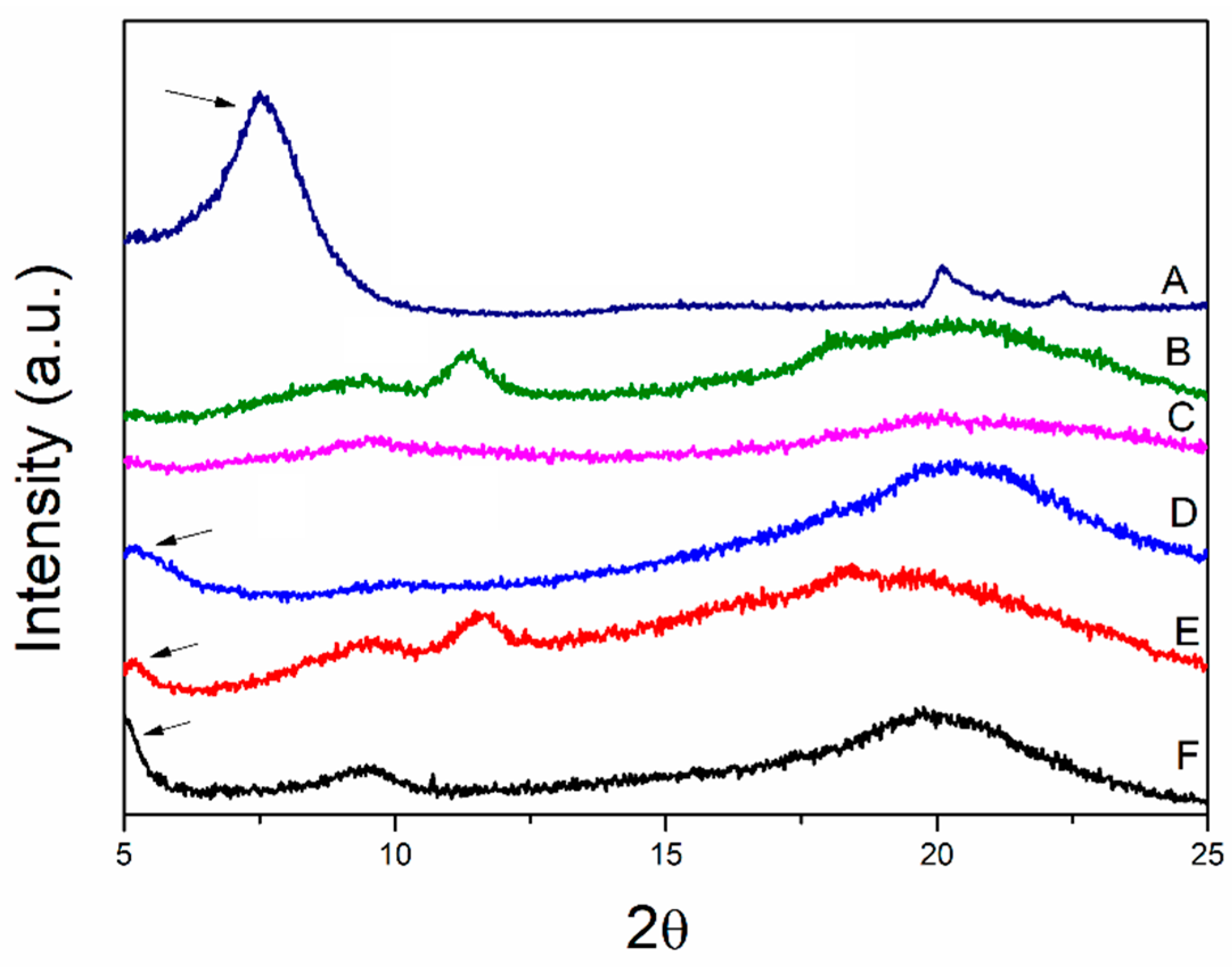
2.3.1. X-ray Diffraction (XRD)
2.3.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.3. Morphological Characterization: Scanning Electron Microscopy (SEM)
2.3.4. Thermal Properties
2.3.5. Thickness and Mechanical Properties
2.3.6. Optical Properties
2.3.7. Contact Angle (CA)
2.3.8. Solubility and Swelling Degree
2.3.9. Water Vapor Permeability (WVP)
2.3.10. Oxygen Permeability (OP)
2.4. Data Statistical Treatment
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.3. Morphological Characterization
3.4. Thermal Properties
3.5. Thickness and Mechanical Properties
3.6. Optical Properties
3.7. Solubility in Water, Swelling Degree and Contact Angle
3.8. Barrier Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. De Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Vilarinho, F.; Andrade, M.; Buonocore, G.G.; Stanzione, M.; Vaz, M.F.; Sanches Silva, A. Monitoring lipid oxidation in a processed meat product packaged with nanocomposite poly(lactic acid) film. Eur. Polym. J. 2018, 98, 362–367. [Google Scholar] [CrossRef]
- Fernando, A.L.; Duarte, M.P.; Vatsanidou, A.; Alexopoulou, E. Environmental aspects of fiber crops cultivation and use. Ind. Crops Prod. 2015, 68, 105–115. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer−Clay Nanocomposites Based on Chitosan Intercalated in Montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Hernández, M.; Chiralt, A.; González-Martínez, C. Antimicrobial activity of polysaccharide films containing essential oils. Food Control 2011, 22, 1302–1310. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Avérous, L. Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng. R Reports 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Alizadeh, V.; Barzegar, H.; Nasehi, B.; Samavati, V. Development of a chitosan-montmorillonite nanocomposite film containing Satureja hortensis essential oil. Iran. Food Sci. Technol. Res. J. 2018, 13, 131–143. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Influence of chitosan/clay functional bionanocomposite activated with rosemary essential oil on the shelf life of fresh silver carp. Int. J. Food Sci. Technol. 2014, 49, 811–818. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant Migration Studies in Chitosan Films Incorporated with Plant Extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S.; Du Plooy, W. Essential Oils and Other Plant Extracts as Food Preservatives. In Progress in Food Preservation; Wiley-Blackwell: Oxford, UK, 2012; pp. 539–579. [Google Scholar]
- Pascoal, A.; Quirantes-Piné, R.; Fernando, A.L.; Alexopoulou, E.; Segura-Carretero, A. Phenolic composition and antioxidant activity of kenaf leaves. Ind. Crops Prod. 2015, 78, 116–123. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crops Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytopathogenic fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C.; Nerín, C. Trends in microbial control techniques for poultry products. Crit. Rev. Food Sci. Nutr. 2016, 58, 1–19. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: II. Application in bio-based plastics for active packaging. Carbohydr. Polym. 2013, 96, 586–592. [Google Scholar] [CrossRef]
- Higueras, L.; López-carballo, G.; Cerisuelo, J.P.; Gavara, R. Preparation and characterization of chitosan/HP-β-cyclodextrins composites with high sorption capacity for carvacrol. Carbohydr. Polym. 2012, 97, 262–268. [Google Scholar] [CrossRef]
- ASTM—America Society Standard Testing and Materials. Standard Test Method for Tensile Properties of Thin Plastic Sheeting—D882-12; ASTM: West Conshohocken, PA, USA, 2012; p. 12. [Google Scholar]
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-I.; Zhao, Y. Incorporation of a high concentration of mineral or vitamin into chitosan-based films. J. Agric. Food Chem. 2004, 52, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Alves, V.D.; Costa, N.; Coelhoso, I.M. Barrier properties of biodegradable composite films based on kappa-carrageenan/pectin blends and mica flakes. Carbohydr. Polym. 2010, 79, 269–276. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Dias, M.V.; Machado Azevedo, V.; Borges, S.V.; Soares, N.D.F.F.; de Barros Fernandes, R.V.; Marques, J.J.; Medeiros, É.A.A. Development of chitosan/montmorillonite nanocomposites with encapsulated α-tocopherol. Food Chem. 2014, 165, 323–329. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Ávila, A.; Bierbrauer, K.; Pucci, G.; López-González, M.; Strumia, M. Study of optimization of the synthesis and properties of biocomposite films based on grafted chitosan. J. Food Eng. 2012, 109, 752–761. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Yuan, M.L.; Fan, J.; Zhao, T.R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass transition temperature of chitosan and miscibility of chitosan/poly(N-vinyl pyrrolidone) blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Characterization of chitosan composites with various clays. Int. J. Biol. Macromol. 2014, 65, 534–541. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jingjid, S.; Damrongsakkul, S.; Tiptipakorn, S.; Takeichi, T. Biodegradability and property characterizations of Methyl Cellulose: Effect of nanocompositing and chemical crosslinking. Carbohydr. Polym. 2008, 72, 444–455. [Google Scholar] [CrossRef]
- Beigzadeh Ghelejlu, S.; Esmaiili, M.; Almasi, H. Characterization of chitosan-nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016, 86, 613–621. [Google Scholar] [CrossRef]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan–cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Zhang, P.; Ye, L.; Wu, L.; He, S. Physical Characterization and Pork Packaging Application of Chitosan Films Incorporated with Combined Essential Oils of Cinnamon and Ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Sarantópoulos, C.G.L.; Oliveira, L.M.; Padula, M.; Coltro, L.; Aalves, R.M.V.; Garcia, E.C. Embalagens Plásticas Flexíveis: Principais Polímeros e Avaliação de Propriedades; CETEA/ITAL: Campinas, Brazil, 2002; p. 267. [Google Scholar]
- Russo, T.; D’Amora, U.; Gloria, A.; Tunesi, M.; Sandri, M.; Rodilossi, S.; Albani, D.; Forloni, G.; Giordano, C.; Cigada, A.; et al. Systematic analysis of injectable materials and 3D rapid prototyped magnetic scaffolds: From CNS applications to soft and hard tissue repair/regeneration. Procedia Eng. 2013, 59, 233–239. [Google Scholar] [CrossRef]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A.E. Antimicrobial, mechanical and barrier properties of triticale protein films incorporated with oregano essential oil. Food Biosci. 2013, 1, 2–9. [Google Scholar] [CrossRef]
- Zeid, A.; Karabagias, I.K.; Nassif, M.; Kontominas, M.G. Preparation and evaluation of antioxidant packaging films made of polylactic acid containing thyme, rosemary, and oregano essential oils. J. Food Process. Preserv. 2019, 1–11. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan-caffeic acid-genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahan, F.J. Development of active packaging containing natural antioxidants. Procedia Food Sci. 2011, 1, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Soares, F.; Pires, A.C.S.; Camilloto, G.P.; Santiago-Silva, P.; Espitia, P.J.P.; Silva, W.A. Recent patents on active packaging for food application. Recent Pat. Food. Nutr. Agric. 2009, 1, 171–178. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Atarés, L.; Pérez-Masiá, R.; Chiralt, A. The role of some antioxidants in the HPMC film properties and lipid protection in coated toasted almonds. J. Food Eng. 2011, 104, 649–656. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.D.; Bai, J. Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781138198937. [Google Scholar]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite. Int. J. Biol. Macromol. 2017, 109, 1219–1231. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Alonso, J.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Modifications induced by the addition of a nanoclay in the functional and active properties of an EVOH film containing carvacrol for food packaging. J. Memb. Sci. 2012, 423, 247–256. [Google Scholar] [CrossRef]



| Film | Tg (°C) | Δ1 | Δ2 | Δ3 | Δ4 | Residue (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Td (°C) | ΔM (%) | Td (°C) | ΔM (%) | Td (°C) | ΔM (%) | Td (°C) | ΔM (%) | |||
| Ch | 206.7 ± 3.7 ns** | 70.9 ± 2.5 | 6.5 ± 0.0 | 170.1 ± 1.5 | 12.6 ± 0.8 | 283.6 ± 0.7 | 24.3 ± 0.6 | − | − | 23.6 ± 0.4 |
| Ch + MMT | 188.9 ± 6.7 ns | 63.6 ± 2.4 | 6.4 ± 0.8 | 173.2 ± 0.8 | 14.0 ± 0.8 | 283.5 ± 0.5 | 23.2 ± 0.2 | − | − | 25.8 ± 0.1 |
| Ch + 0.5% GEO | 202.2 ± 2.7 ns | 65.6 ± 2.4 | 6.1 ± 0.2 | 174.1 ± 2.1 | 13.7 ± 0.1 | 284.0 ± 1.0 | 20.4 ± 0.4 | 407.4 ± 5.6 | 26.6 ± 0.6 | 18.0 ± 0.1 |
| Ch+MMT + 0.5% GEO | 202.8 ± 5.4 ns | 64.0 ± 0.0 | 6.2 ± 0.3 | 168.4 ± 3.2 | 11.5 ± 0.6 | 284.0 ± 0.9 | 20.4 ± 0.0 | 407.8 ± 0.4 | 26.9 ± 0.4 | 20.2 ± 0.4 |
| Ch + 1% GEO | 194.4 ± 7.1 ns | 63.2 ± 1.5 | 4.9 ± 0.2 | 167.4 ± 1.3 | 11.1 ± 2.7 | 284.1 ± 2.5 | 19.9 ± 1.7 | 397.1 ± 6.2 | 27.1 ± 5.7 | 16.9 ± 0.8 |
| Ch + MMT + 1% GEO | 196.2 ± 3.4 ns | 64.8 ± 1.9 | 4.7 ± 0.1 | 172.6 ± 3.7 | 16.1 ± 0.3 | 290.8 ± 1.1 | 25.2 ± 0.4 | 409.5 ± 2.6 | 26.9 ± 0.8 | 17.7 ± 0.3 |
| Ch + 2% GEO | 195.5 ± 4.8 ns | 59.6 ± 6.3 | 4.1 ± 0.2 | 135.5 ± 3.2 | 13.1 ± 1.3 | 284.8 ± 0.7 | 33.0 ± 0.5 | 407.1 ± 3.2 | 25.0 ± 0.6 | 14.2 ± 1.0 |
| Ch + MMT + 2% GEO | 194.3 ± 6.8 ns | 63.7 ± 0.9 | 5.0 ± 0.2 | 183.4 ± 2.5 | 16.6 ± 0.6 | 287.7 ± 0.5 | 23.2 ± 0.9 | 408.7 ± 3.1 | 25.9 ± 0.6 | 18.0 ± 0.4 |
| GEO (%) | Thickness (µm) | Tensile Strength (MPa) | EAB (%) | Elastic Modulus (GPa) | ||||
| 0% MMT | 2.5% MMT | 0% MMT | 2.5% MMT | 0% MMT | 2.5% MMT | 0% MMT | 2.5% MMT | |
| 0 | 41.7 ± 2.9 Da* | 39.5 ± 2.0 Ca | 46.7 ± 1.7 Ab | 66.6 ± 3.1 Aa | 17.9 ± 1.2 Bb | 33.5 ± 2.5 Aa | 2.05 ± 0.19 Aa | 1.86 ± 0.14 Aa |
| 0.5 | 55.5 ± 1.3 Ca | 52.5 ± 0.6 Bb | 46.3 ± 5.1 Aa | 42.0 ± 4.2 Ba | 23.8 ± 5.3 ABa | 22.3 ± 2.9 Ba | 1.66 ± .012 Aa | 1.30 ± 0.30 Ba |
| 1.0 | 68.2 ± 3.1 Ba | 67.4 ± 1.1 Aa | 27.2 ± 5.8 Ba | 34.6 ± 1.0 BCa | 36.0 ± 9.4 Aa | 33.1 ± 2.3 Aa | 0.30 ± 0.09 Cb | 0.88 ± 0.13 BCa |
| 2.0 | 81.3 ± 4.5 Aa | 68.6 ± 0.9 Ab | 32.1 ± 3.9 Ba | 30.3 ± 0.7 Ca | 33.6 ± 6.6 ABa | 35.8 ± 1.3 Aa | 0.71 ± 0.15 Ba | 0.47 ± 0.05 Ca |
| GEO (%) | Water Solubility (%) | Swelling Degree (%) | Contact Angle (Degrees) | |||||
| 0% MMT | 2.5% MMT | 0% MMT | 2.5% MMT | 0% MMT | 2.5% MMT | |||
| 0 | 23.1 ± 1.6 Aa | 20.4 ± 0.6 Bb | 132.3 ± 10.0 Ab* | 192.2 ± 13.3 Aa | 70.9 ± 4.0 Ab | 90.6 ± 5.5 Aa | ||
| 0.5 | 20.3 ± 0.4 Ab | 21.9 ± 0.6 Ba | 124.6 ± 3.5 Aa | 35.1 ± 20.7 Cb | 70.3 ± 7.5 Aa | 68.6 ± 1.6 Ba | ||
| 1.0 | 16.4 ± 0.4 Bb | 18.7 ± 0.2 Ba | 97.8 ± 18.2 Ba | 97.0 ± 13.4 Ba | 66.6 ± 5.5 Aa | 70.9 ± 3.2 Ba | ||
| 2.0 | 19.6 ± 2.1 ABa | 23.5 ± 2.4 Aa | 87.0 ± 2.5 Ba | 54.7 ± 20.5 BCa | 57.2 ± 1.9 Ba | 63.8 ± 7.6 Ba | ||
| Film | WVP (10−11 mol/m·s·Pa) | OP (10−16 mol/m·s·Pa) | Cromaticity | Hue * | Opacity |
|---|---|---|---|---|---|
| Ch | 1.40 ± 0.09 C* | 0.184 ± 0.052 DE | 3.1 ± 0.1 D | 129.0 ± 1.0 A | 1.1 ± 0.2 C |
| Ch + MMT | 1.75 ± 0.10 BC | 0.098 ± 0.008 F | 4.2 ± 0.4 C | 118.8 ± 1.8 B | 1.7 ± 0.3 C |
| Ch + 0.5% GEO | 1.93 ± 0.36 ABC | 0.182 ± 0.008 DE | 5.8 ± 0.3 B | 112.3 ± 0.6 C | 2.6 ± 0.1 B |
| Ch + MMT + 0.5% GEO | 1.94 ± 0.27 ABC | 0.171 ± 0.001 E | 6.8 ± 0.6 B | 109.8 ± 1.4 CD | 3.1 ± 0.7 B |
| Ch + 1% GEO | 1.95 ± 0.21 ABC | 0.255 ± 0.010 BC | 7.0 ± 0.8 B | 110.2 ± 1.3 CD | 4.1 ± 0.1 AB |
| Ch + MMT + 1% GEO | 2.12 ± 0.08 AB | 0.246 ± 0.013 CD | 8.0 ± 0.2 AB | 107.9 ± 0.1 DE | 5.0 ± 0.2 AB |
| Ch + 2% GEO | 1.94 ± 0.10 ABC | 0.325 ± 0.037 A | 9.6 ± 1.9 A | 107.1 ± 1.8 DE | 4.2 ± 1.4 AB |
| Ch + MMT+ 2% GEO | 2.41 ± 0.16 A | 0.285 ± 0.015 AB | 9.7 ± 1.3 A | 105.1 ± 0.7 E | 6.2 ± 1.8 A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings 2019, 9, 700. https://doi.org/10.3390/coatings9110700
Souza VGL, Pires JRA, Rodrigues C, Rodrigues PF, Lopes A, Silva RJ, Caldeira J, Duarte MP, Fernandes FB, Coelhoso IM, et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings. 2019; 9(11):700. https://doi.org/10.3390/coatings9110700
Chicago/Turabian StyleSouza, Victor Gomes Lauriano, João Ricardo Afonso Pires, Carolina Rodrigues, Patricia Freitas Rodrigues, Andréia Lopes, Rui Jorge Silva, Jorge Caldeira, Maria Paula Duarte, Francisco Braz Fernandes, Isabel Maria Coelhoso, and et al. 2019. "Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil" Coatings 9, no. 11: 700. https://doi.org/10.3390/coatings9110700
APA StyleSouza, V. G. L., Pires, J. R. A., Rodrigues, C., Rodrigues, P. F., Lopes, A., Silva, R. J., Caldeira, J., Duarte, M. P., Fernandes, F. B., Coelhoso, I. M., & Fernando, A. L. (2019). Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings, 9(11), 700. https://doi.org/10.3390/coatings9110700










