3.1. In-Flight Particle Diagnostics & Spray Parameter Selection
The process map resulting from the choice of spray parameters is illustrated in
Figure 2. It may be noticed that the HVOF-sprayed particles’ temperatures and velocities spread over a considerably wide range of values. On the other hand, the error bars (one standard deviation long) do not overlap. This shows that for each of the chosen HVOF deposition parameters, the particles absorb substantially different thermal and kinetic energies. This result was deliberately sought after in order to maximize the variability of HVOF coating microstructures and performances.
When compared to HVOF, the HVAF-sprayed particles experience significantly lower in-flight temperatures due to the flame cooling effect caused by the process’s relatively high air-flow rates. On average, their temperature is lower than the theoretical liquidus temperature of a bulk SS444 (1520 °C), which should substantially affect the high-temperature oxidation effects. It should be mentioned that the diagnostic tool used to characterize the HVAF-sprayed particles was the accuraspray system which determines temperatures and velocities as an ensemble (which is why the error bars are not reported) in a space volume of approximately 176 mm3. As for the HVOF-sprayed particles, the system used was the DPV-evolution, which can determine individual in-flight particle characteristics in a measurement volume inferior to 1 mm3.
The results in
Figure 2 show that the in-flight particle temperature and the substrate temperature after spraying are not strongly correlated. The effect seems to be more strongly linked to the combustion O/F ratio used for spraying. For example, coatings HVOF #1 and HVOF #8 were produced with fuel-rich flames, burning kerosene at higher rates when compared to HVOF #3 and HVOF #5.
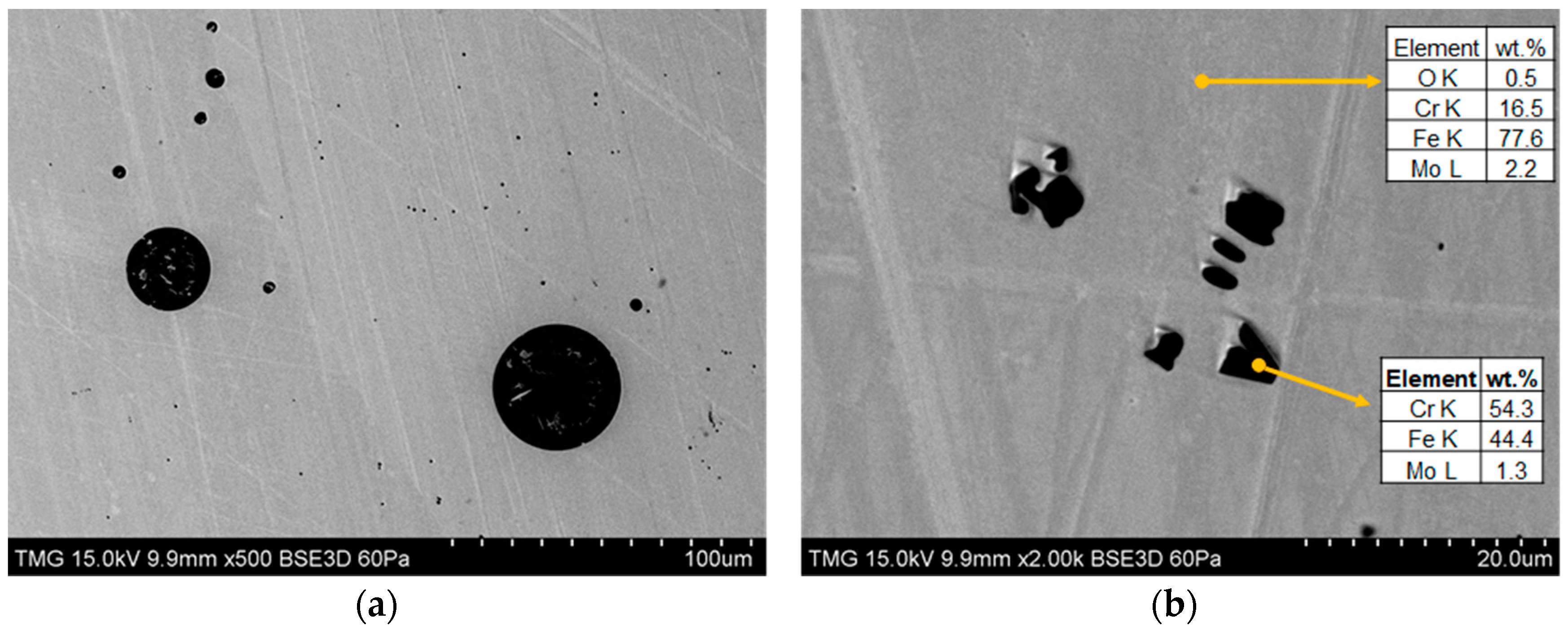
3.2. Microstructure and EDX Analysis
The powder feedstock displayed small dark precipitates which may also be found in the coating’s microstructures, as highlighted in
Figure 3 by dashed circles. Details on the chemical composition of the precipitates are discussed in the subsequent section.
A closer look at
Figure 3b reveals that the HVOF-sprayed SS444 coatings have three main microconstituents: unmolten splats that partially retain the feedstock morphology; a re-solidified zone comprising lamellar metallic and oxide splats that formed due to exposure to the high-temperature flames and; pores. These constituents are present in all the sprayed coatings.
Table 3 summarizes the fraction of each constituent as measured by image analysis carried out on the coating cross-sections illustrated in
Figure 4. The coatings produced at a higher O/F ratio (HVOF #3 and #5) have a higher fraction of unmolten particles. Conversely, the coatings produced with a lower O/F Ratio (HVOF #1 and #8) are mostly composed of re-solidified splats. As will be discussed later, this effect is attributed to different particle trajectories.
The porosity in coating HVOF #5 was significantly higher than the others, which probably derives from an undesirable combination of high in-flight particle temperatures and low velocities. The effect could also have been caused by splat pull-out during sectioning and/or polishing procedures. Another important remark concerns the coating HVOF #8 which, despite being formed by particles that experienced lowest in-flight temperature, also exhibited oxide clusters characterized by a darker grey color.
It is evident from
Figure 4 that the coating HVAF #1 was formed mainly by unmolten splats. This may be explained by the larger particle sizes used and the particularly low in-flight particle temperatures used for its production. On the other hand, the HVAF #4 coating shared the same aspects of the HVOF coatings. This may be explained by the fact that the feedstock used to produce coating HVAF #4 was the same used to produce HVOF coatings and that the particle velocities prior to impact were relatively slow as compared with those that generated coating HVAF #1.
Based on thickness measurements, higher deposition efficiencies (DE) are achieved when HVOF-spraying with lower O/F ratios. According to [
22], increasing the in-flight particle temperature also increases their ductility, which increases the process deposition efficiency until the material’s melting point is reached, leading to the process’s highest attainable DE. Coatings HVOF #1 and #8 contain a higher fraction of particles that experienced complete melting prior to deposition, hence their higher DE. The same rationale may explain why the DE of HVOF coatings was higher than that of HVAF. However, due to the use of a different, self-regulated powder feeding system, the flow rate for depositing coating HVAF #4 was significantly lower than the rest, which explains why this coating had the lowest thickness.
The EDX analysis main results are summarized in
Table 4. The higher oxygen content in the resolidified zones, when compared to the unmolten splats, is probably linked to the influence of oxides more present in those regions (
Figure 3b). The general composition results point-out to a higher oxygen uptake in coating HVOF #8, which can be linked to the presence of the large oxide clusters (
Figure 4). Additionally, the HVAF coatings have an overall lower oxygen content, especially in the case of HVAF #1.
It is useful to compare the coating’s chemical composition with that of the wrought SS444, which under the same analysis conditions, exhibits an oxygen content of 0.5 wt.%. In terms of chromium content, the main element responsible for the protective passivation of stainless steels, most coatings display weight percentage values slightly inferior to that of the expected nominal range of 18–20% according to the general EDX analysis. In contrast, the local chromium content of the unmolten splats falls within the required nominal range. This apparent reduction of Cr wt.% may be a result of the interaction volume between the electron beam and the coating, comprising pores and oxides that have less Cr in their composition. On the other hand, the results also mean that Cr distribution on the coating surfaces is very heterogeneous. For example, the EDX analysis performed on precipitates present in both the feedstock and coatings revealed an enrichment in chromium and silicon, which was also reported in [
18]. These factors may play an important role in the coating’s electrochemical behavior.
The arc melting process led to significant densification of the sintered pack of particles. However, pores and different types precipitates were observed (
Figure 5). Due to their spherical geometry, it is believed that the pores were originated from gases entrapped in the melting pool. The EDX analysis performed on the precipitates revealed a Cr weight fraction of 54%. As compared with melt atomization, the cooling rates after arc melting are relatively slow. Therefore, one may argue that such precipitates are formed by coalescence of the small precipitates found in the feedstock.
The oxygen content of the arc melted steel was of 0.5 wt.%, considerably lower than what was measured for the coatings. Two factors may explain such findings. Firstly, the arc melted feedstock did not undergo the oxidation associated with the thermal spray process. Secondly, a slag formed and floated on top of the melt pool during the process. It is believed that the slag may have absorbed part of the oxygen entrapped in the powder during atomization. Upon solidification and polishing, the slag was completely removed from the feedstock.
3.3. Potentiodynamic Polarization
For the sake of clarity, only a limited number of polarization curves are shown in
Figure 6. When comparing the polarization curves of the (HVOF #1—1 h) coating to that of the wrought SS444, one will notice a significantly lower corrosion potential as well as a two-order of magnitude higher corrosion current. The measured
and
values for the SS444 coatings after 1 hour of immersion are in good agreement with other stainless steel coatings produced by HVOF [
5,
6], as well as with a vacuum-sintered 434L powder metallurgy (PM) stainless steel [
23]. When comparing the polarization behaviors of the arc melted feedstock and the wrought stainless steel, one may notice a much closer behavior when compared to that of the coatings. Nevertheless, the breakdown at
implies some impairment of the corrosion resistance of the arc melted disc, which may be caused by the pores and Cr-rich precipitates shown in
Figure 5.
At potentials significantly far from the corrosion potential , the sprayed coatings behavior distinguishes from that of the wrought materials by the absence of a passivation plateau. With increasing immersion time, changes are observed in the shapes of both anodic and cathodic branches of the curves, suggesting a modification in the kinetics of the corrosion reactions.
When compared to HVOF, the use of HVAF spraying does not lead to significant changes in the electrochemical response of the stainless steel coatings. Their corrosion potential was approximately 100 mV higher than that of HVOF coatings throughout the tests.
The polarization behavior of the sprayed SS444 coatings was similar in almost all instances and is summarized in
Figure 7. The corrosion potentials drop about 100 mV during the first week of immersion, after which the
values display a slight tendency to increase with immersion time. A similar trend was observed for the corrosion potential of SUS316L coatings in Seawater [
7]. The average corrosion current
of the coatings did not vary as strongly, ranging between 20–60 µA·cm
−2 throughout the 3-week testing.
3.4. Changes in Electrode Surface and Cross Section
The effects of corrosion on the HVOF coating surfaces are illustrated in
Figure 8. As compared with the pristine polished coatings, the corroded electrodes display an increase in porosity and in the quantity of corrosion products which seem to form preferentially in the re-solidified zone, while the unmolten particles remain apparently unaffected. As suggested in [
24,
25,
26], these results endorse the hypothesis of the formation of galvanic microcells between coating constituents. As revealed by EDX analysis (
Table 4), the heterogenous chromium distribution throughout the coating may be the driving force for the corrosion reactions. As the electrolyte penetrates through the coating thickness, more such heterogeneities are exposed to the solution, leading to a preferential corrosion path through boundaries between splats.
It has been proposed in [
26] that the coating corrosion resistance may be improved by reducing or eliminating the chemical heterogeneities between microconstituents. While coating HVAF #1 is mostly comprised of unmolten splats, its electrochemical behavior was not markedly different from that of the other materials. On the other hand, the microstructural features observed after the corrosion tests deserve mention. As illustrated in
Figure 9, several pits were formed on the coating splats. The formation of pits implies the breakdown of a passive film or the formation of a micro/nano galvanic couple. Evidence of such films was shown in
Figure 6, where the anodic current of coating HVAF#1 nearly stabilizes at 35 µA.cm
−2. Similar behavior was reported for SS444 HVOF coatings in [
18]. At the HVAF #1 coating cross-section, the formation of corrosion products rich in Cl highlights the ability of the electrolyte to penetrate through the voids between splats. The same was observed at the HVOF coatings though to a much lesser extent. The effect may be attributed to the relatively large gaps at the HVAF #1 splat boundaries (
Figure 9a).
3.5. XPS Analysis
The survey XPS analysis summarized in
Table 5 reveals that the Bulk SS444, arc melted feedstock and the HVAF #1 coating have similar oxygen concentrations, substantially lower than what was measured for the remaining coatings. When comparing polished coatings (not exposed to the tests) to those exposed to the test solution for 3-weeks, one notices an increase in the Cr/Fe ratio. This phenomenon may be attributed to a preferential dissolution of Fe at the electrode surface which was also observed in [
18] and on early studies regarding the passivity of Fe-Cr alloys [
27]. If one considers the standard electrode potentials of Cr(III) and Fe(II), respectively −0.74 V and −0.41 V versus a standard hydrogen electrode, one could say that the shift observed in the
of the coatings during the first week of immersion is caused by the surface Cr-enrichment.
The deconvolution of a sample’s high-resolution XPS spectra allows one to estimate the contribution of each element’s oxidation state to the overall signal. Following the rationale used in [
28], observation of
Figure 10 and
Table 6 implies that chromium surface atoms manifest in three main oxidation states, corresponding to metallic Cr(0), Cr(III)-(Cr
2O
3), and Cr(II)-Cr(OH)
3. One may easily notice that the contributions of the Cr oxidation states are very similar in the Bulk SS444, arc melted feedstock and in the HVAF #1 coating. However, during the 3-week tests, the surface of coating HVAF #1 is enriched in Cr-oxides and depleted in hydroxides, resembling the other sprayed coatings, which have a marked presence of Cr
2O
3 from the as-polished condition.
Deconvolution of the iron XPS peaks indicate that, when compared to a bulk SS444 plate, more Fe atoms achieve a higher oxidation state Fe(III) on the surface of the sprayed coatings and on the arc melted feedstock. This implies that the film formed on the surface of the latter materials has a limited ability in preventing Fe from oxidation in the test solution.
Concerning the oxidation state of oxygen, metallic oxides (O2−) and hydroxides (OH−) have a relatively stronger influence on the XPS spectra of the HVOF coatings, which may be attributed to the effects of high-temperature oxidation during spray as well as to the corrosion process itself, since the relative contribution of O2− and OH− to the XPS spectra is further increased at the corroded electrodes. Hydrocarbon contamination (C=O) possibly deriving from sample manipulation did not have a marked influence in the overall oxygen signal obtained from the investigated materials.