Artificial Patination of Copper and Copper Alloys in Wet Atmosphere with Increased Content of SO2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
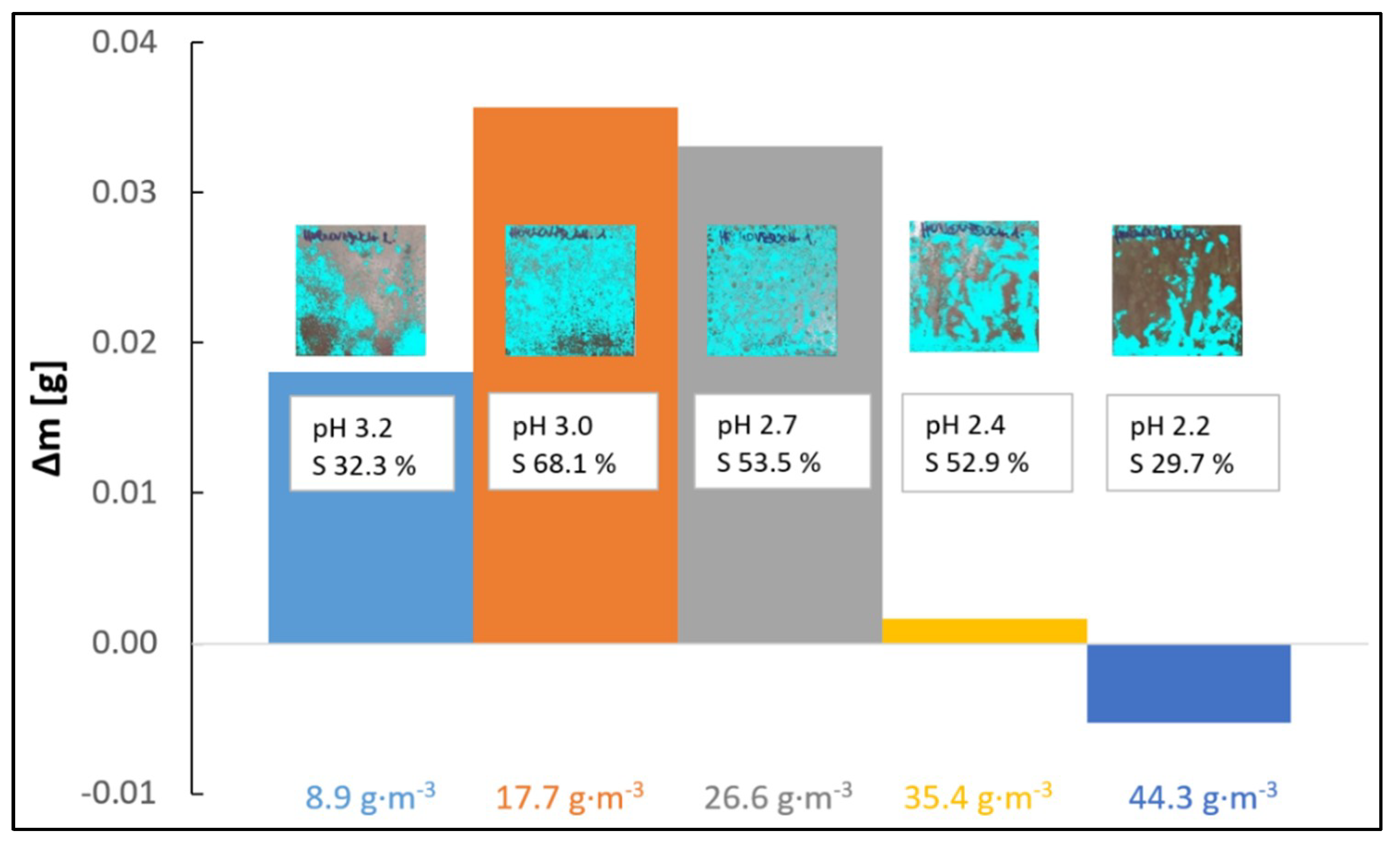
3.1. Influence of SO2 Inlet Dose and pH of Superficial Electrolyte
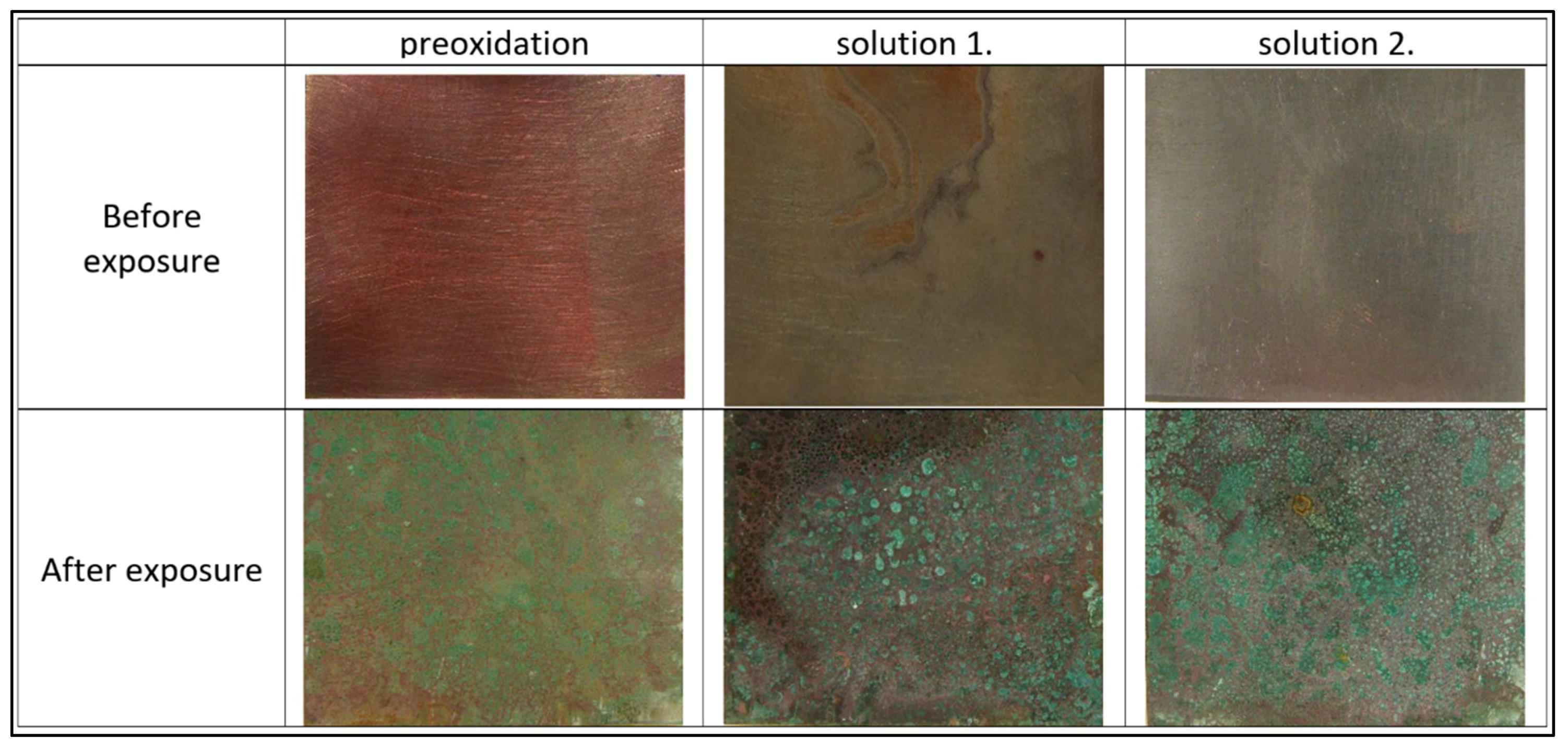
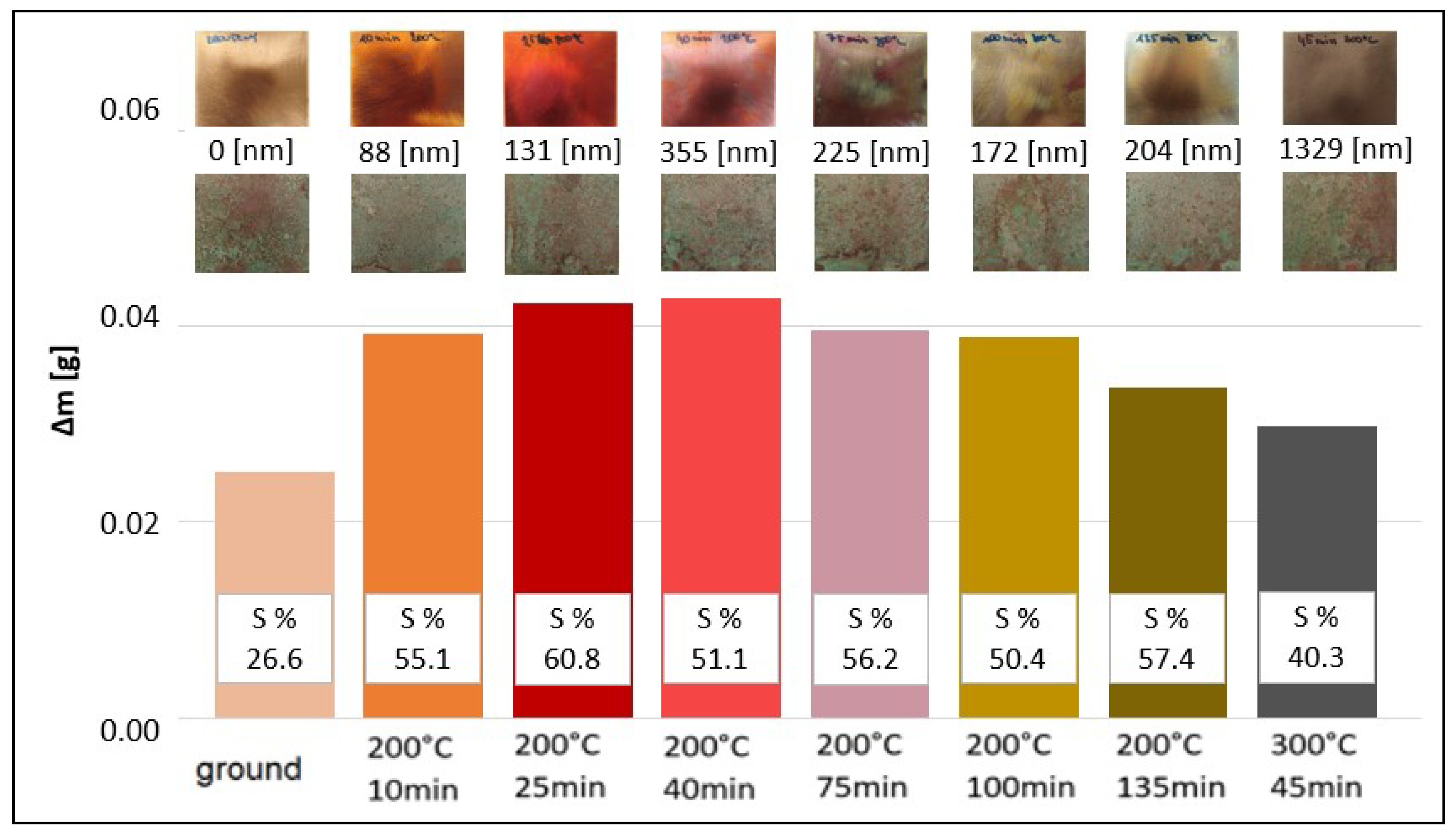
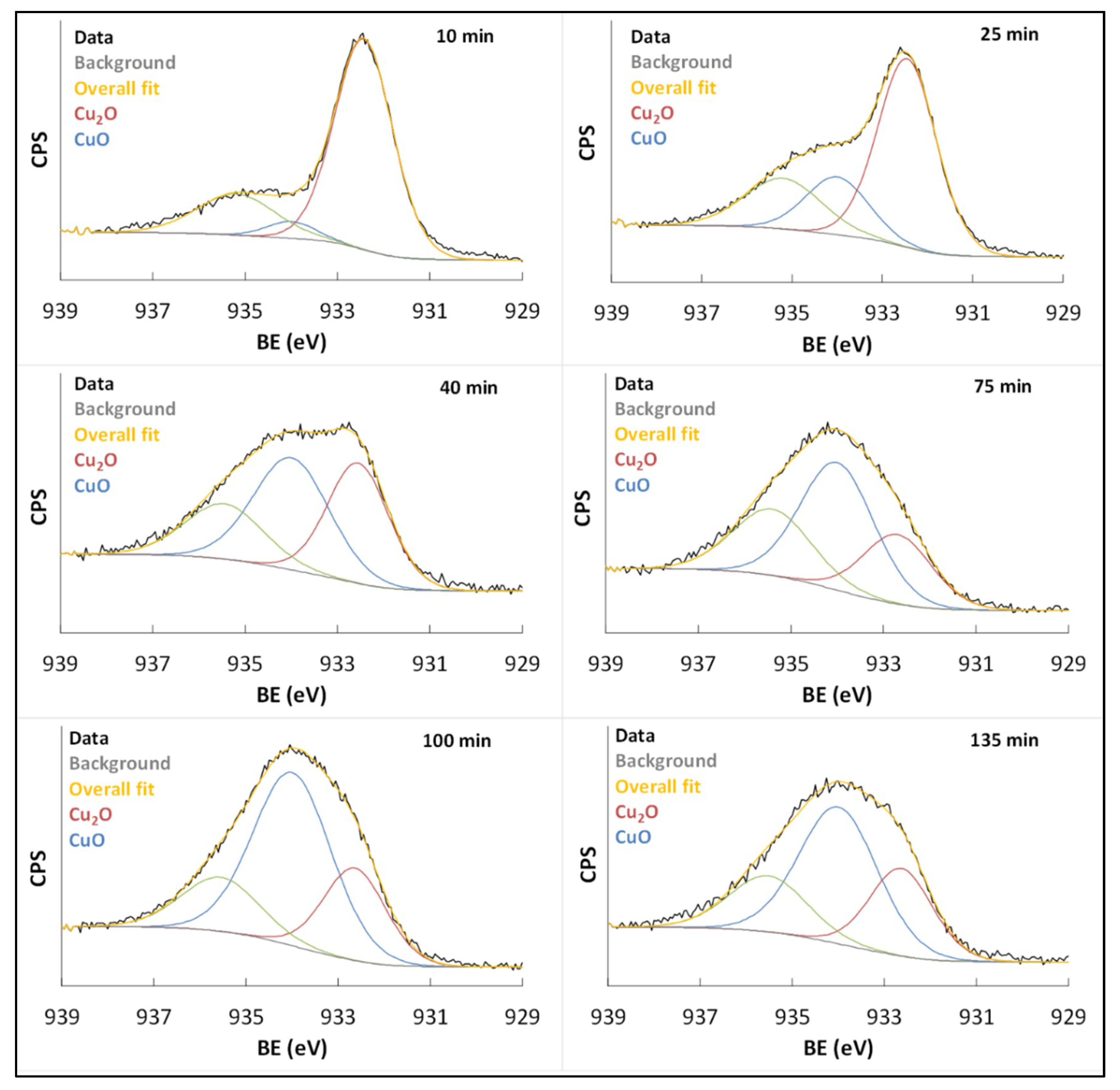
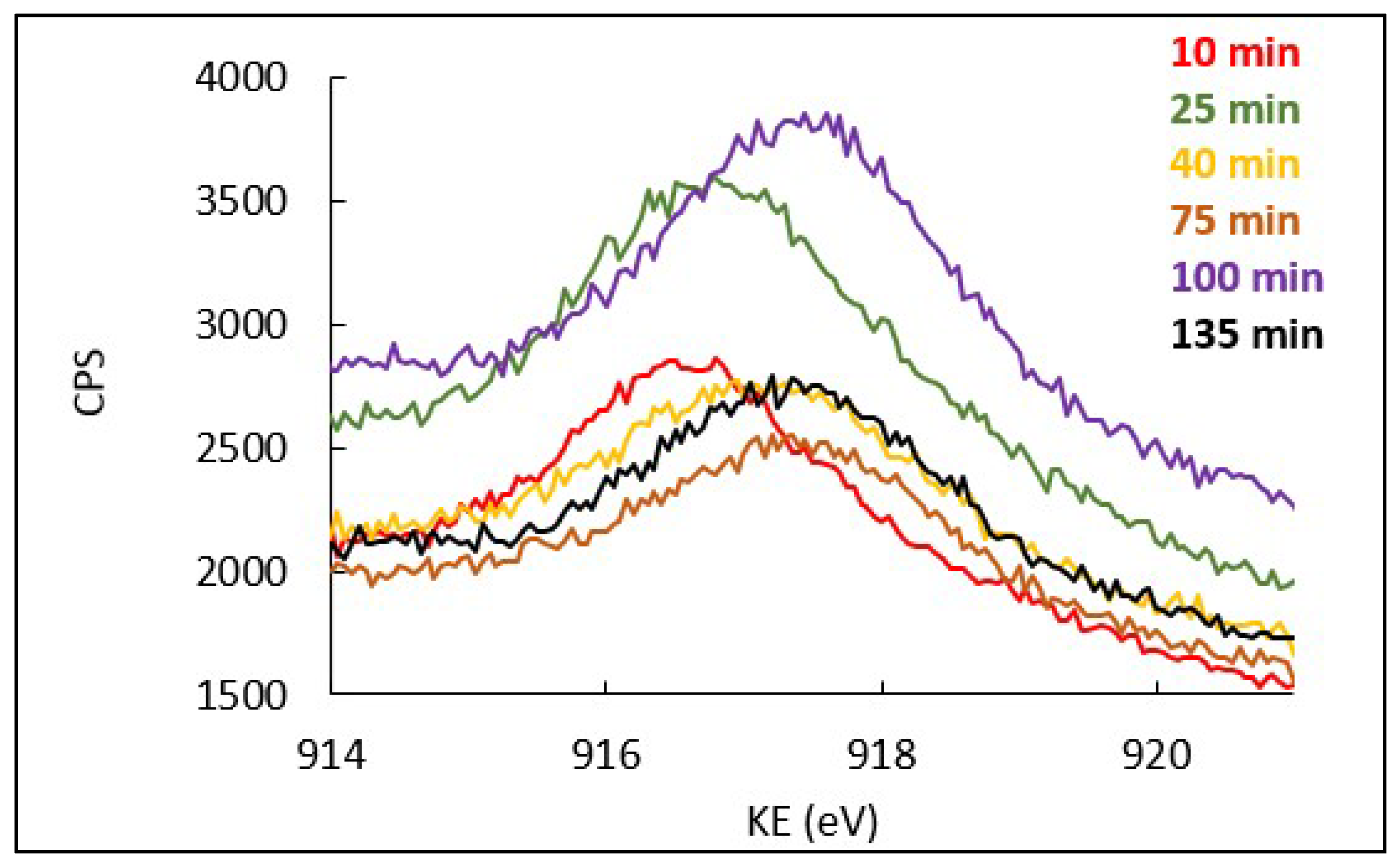
3.2. Effect of Copper Surface Pre-Treatment
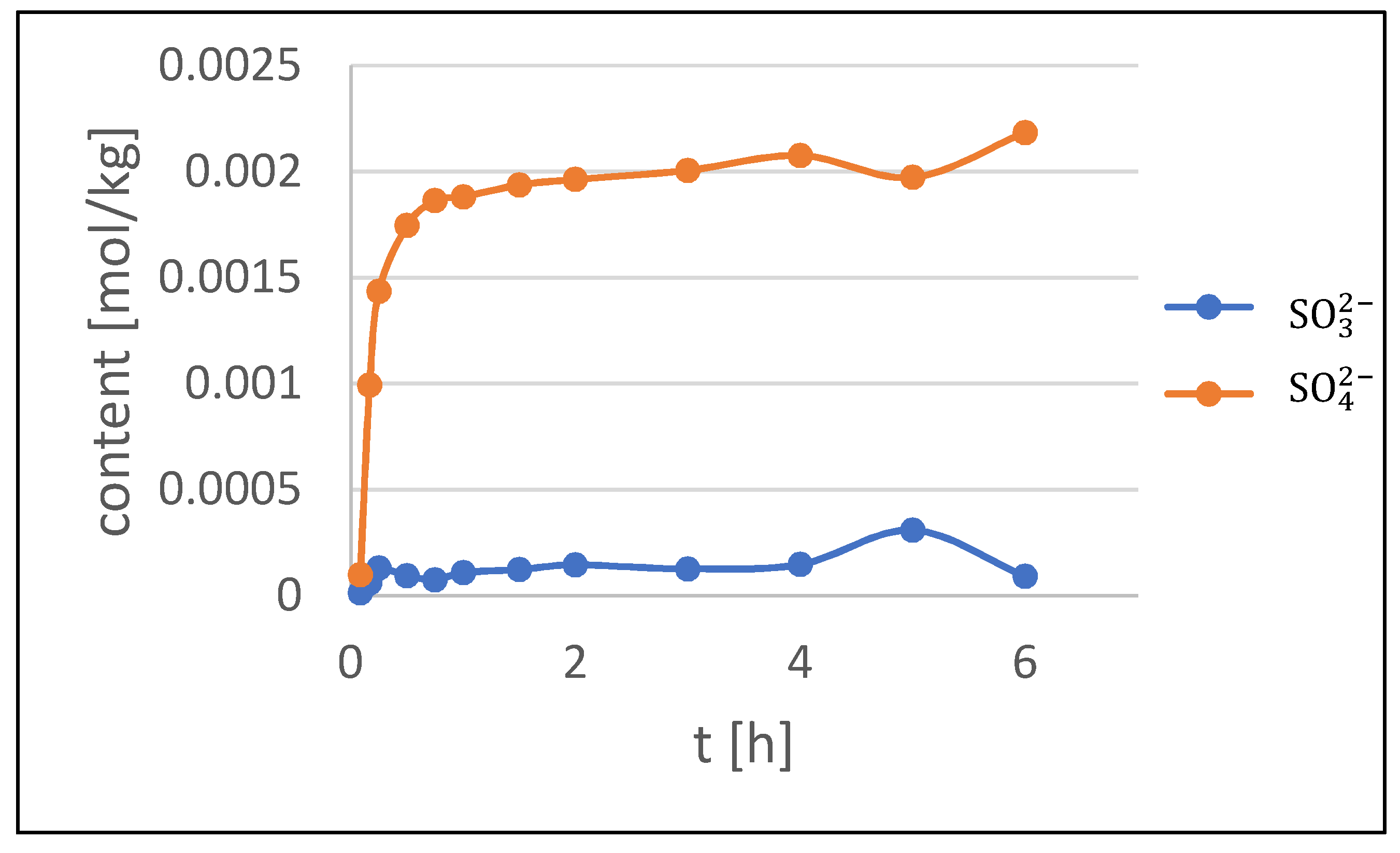
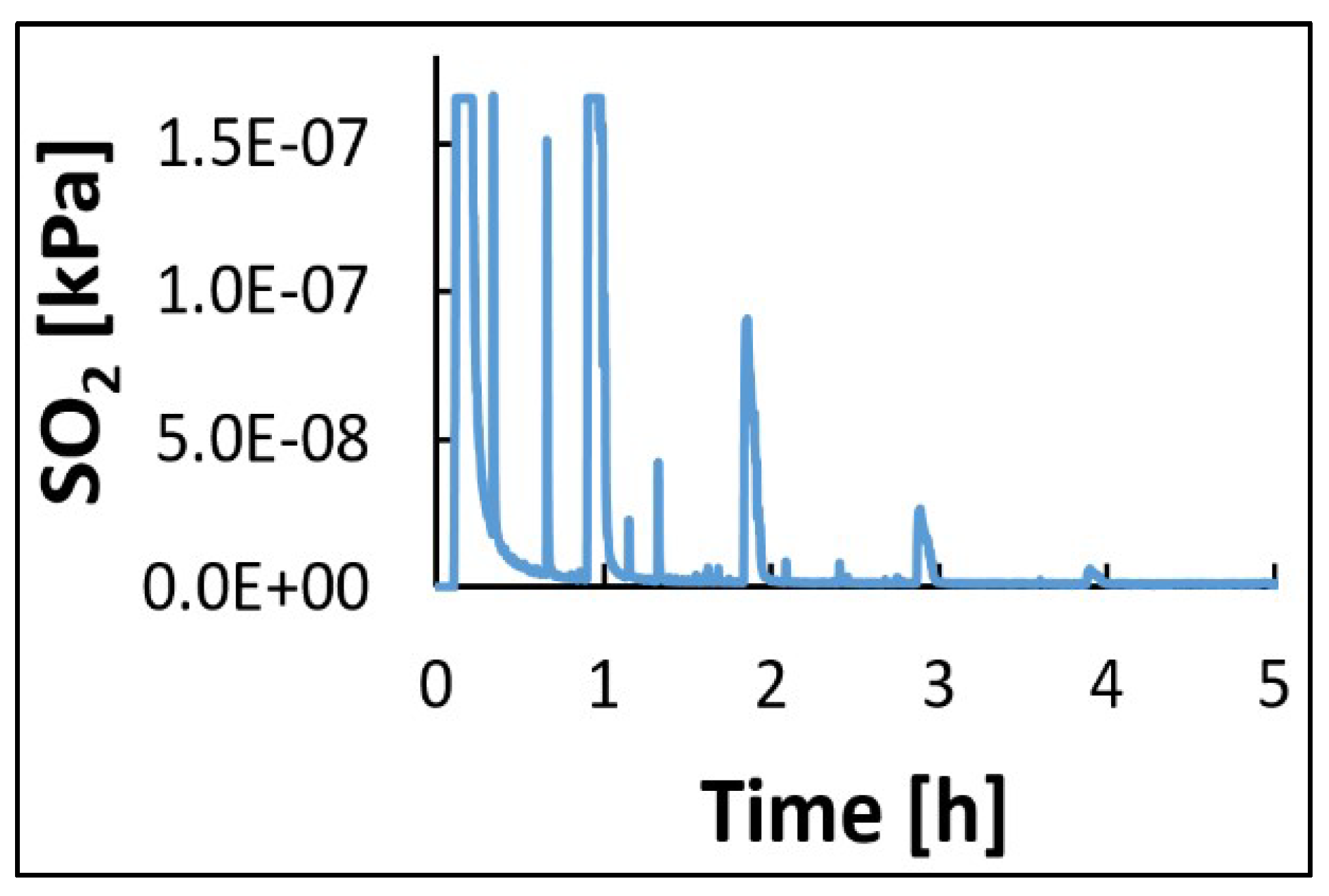
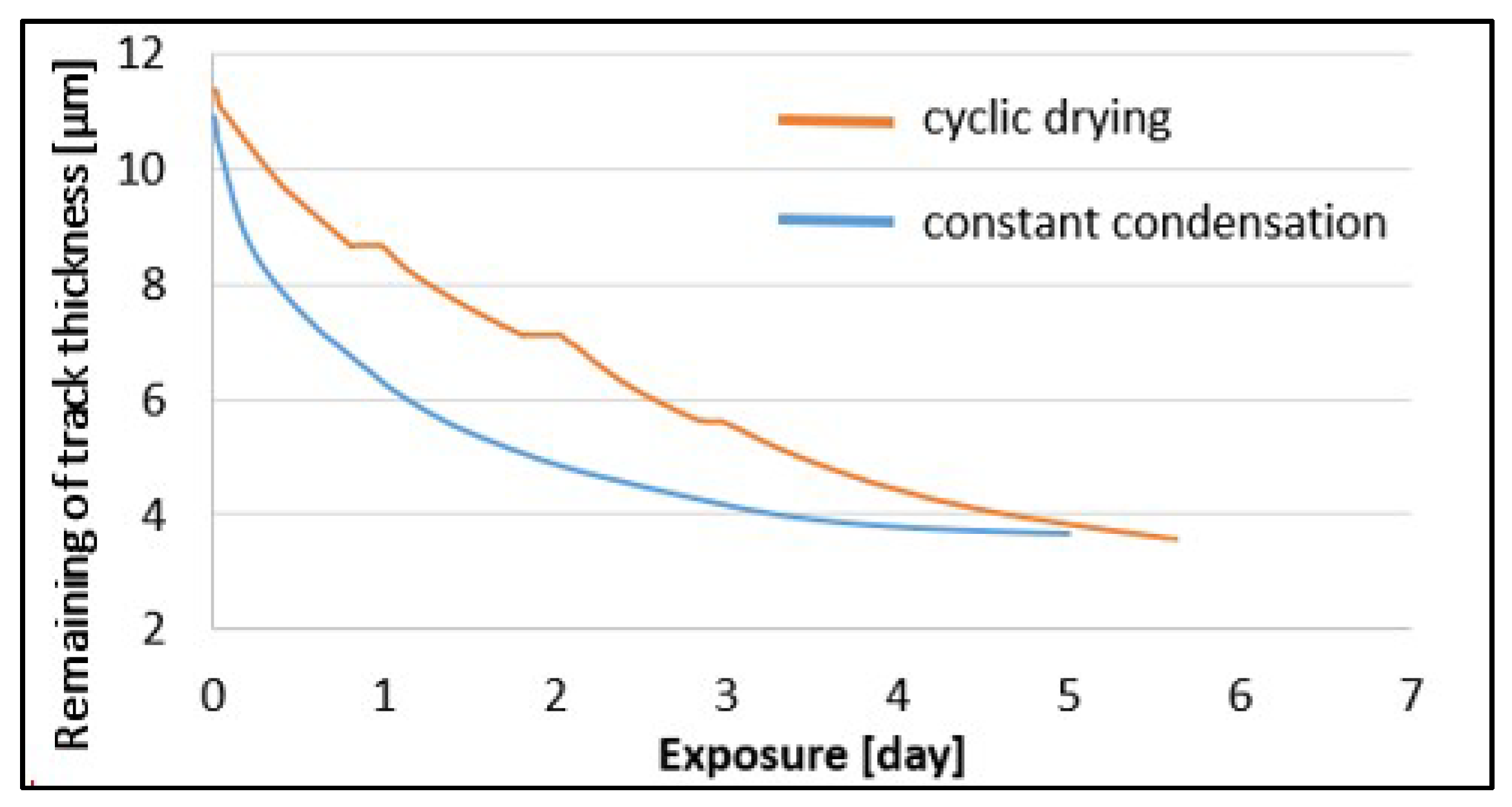
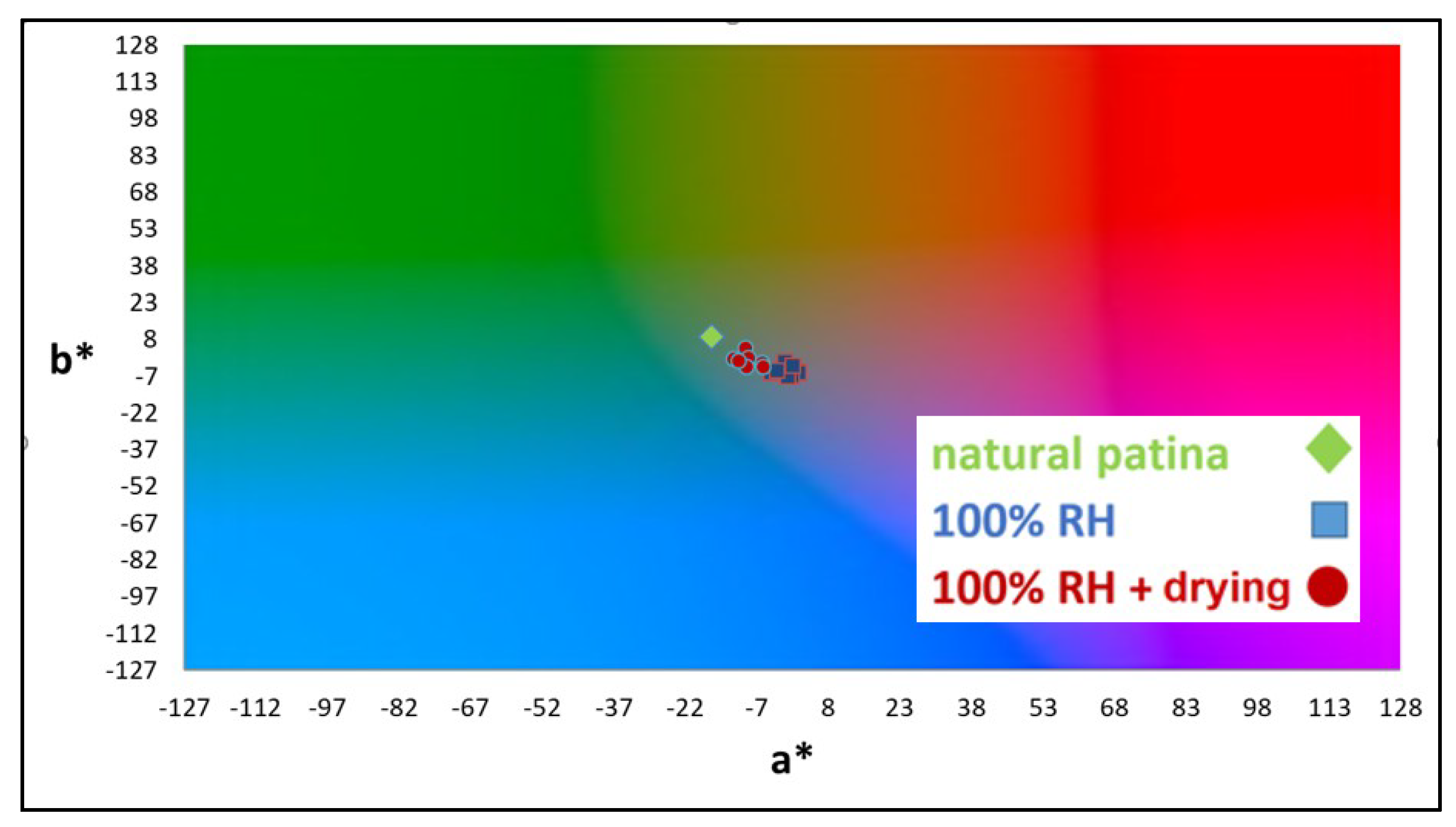
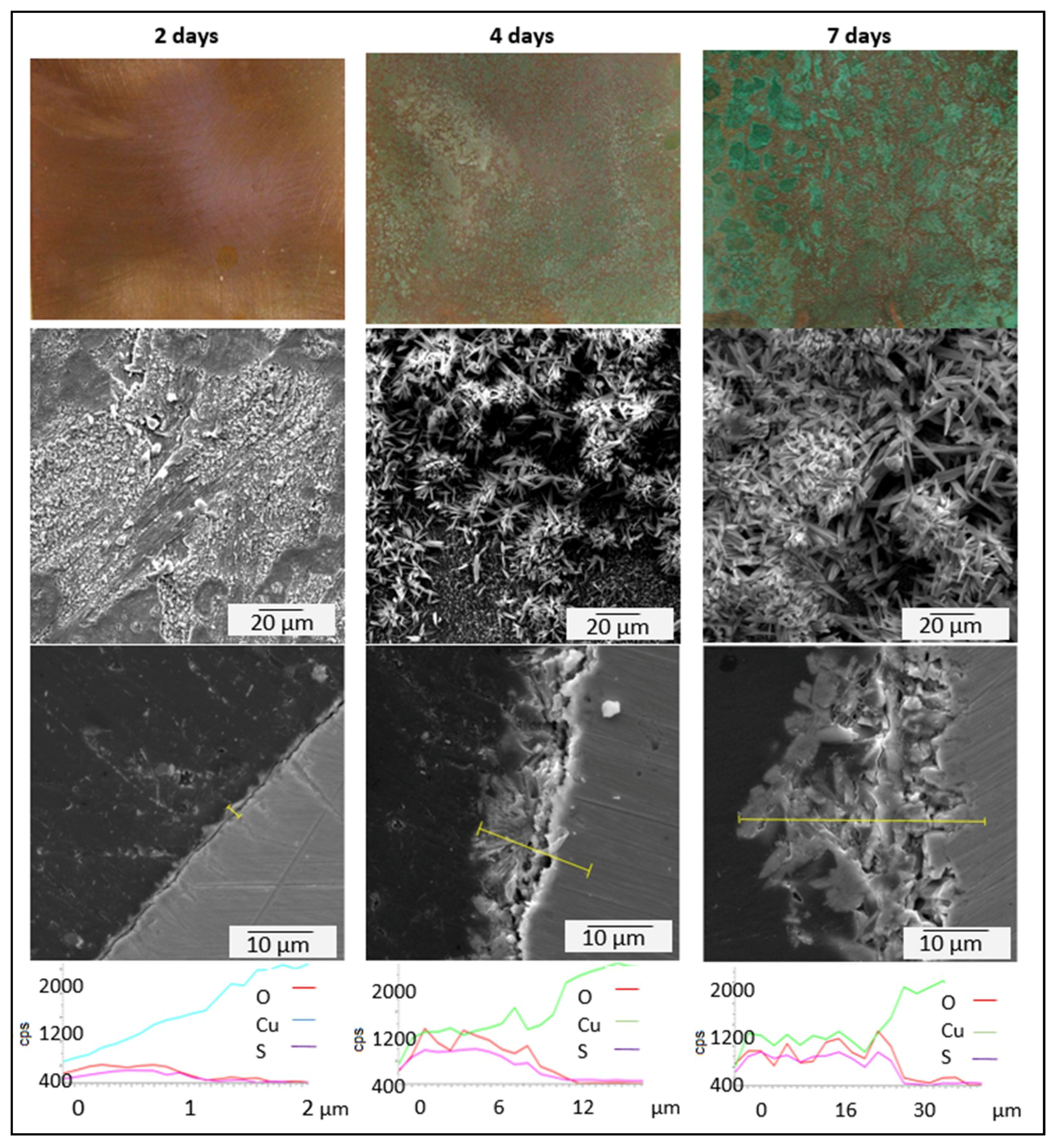
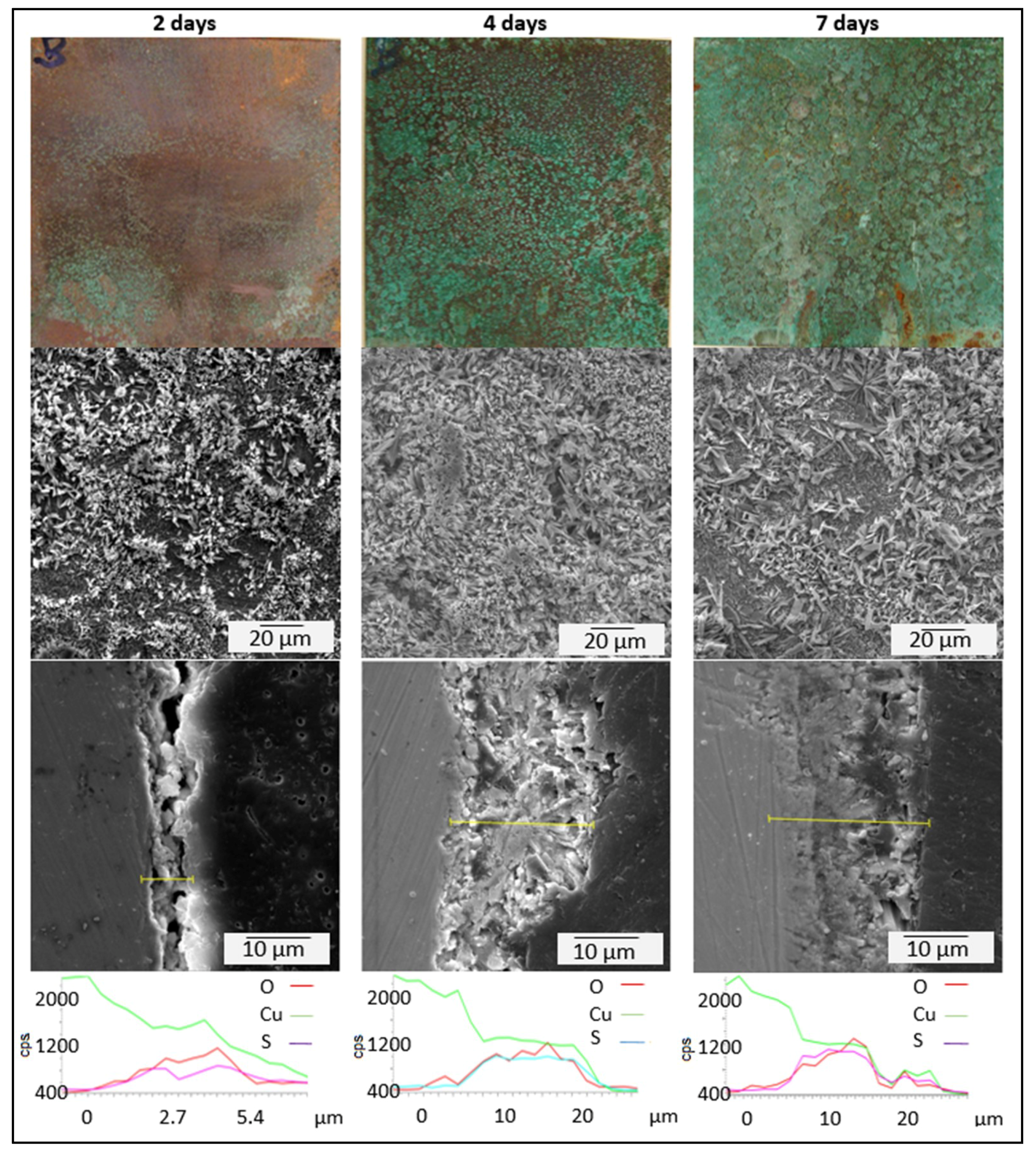
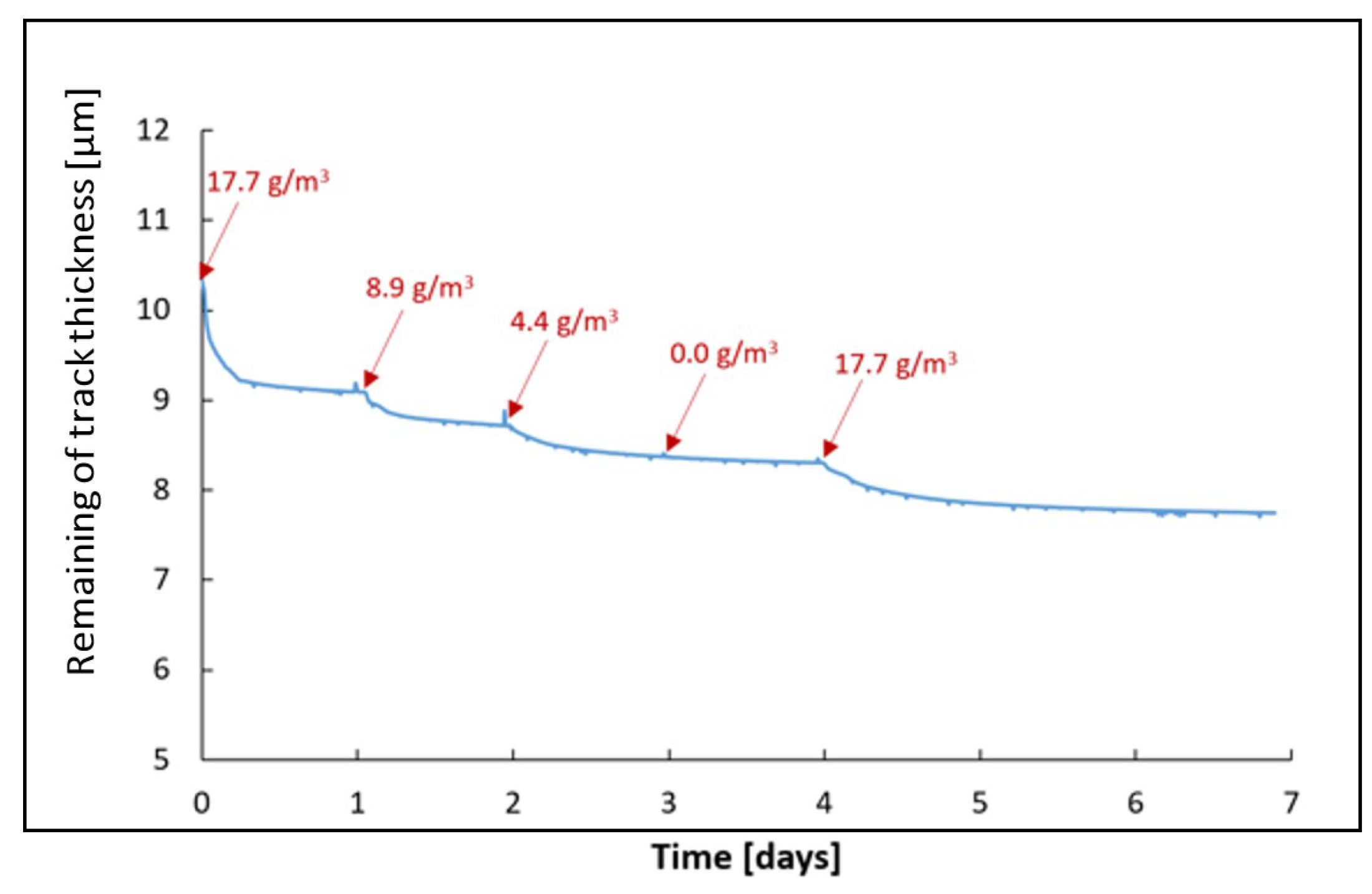
3.3. Kinetics of Patina Formation and Influence of Drying Phase
3.4. Optimisation of Patination Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, K.P.; Nairn, J.; Atrens, A. The chemistry of copper patination. Corros. Sci. 1998, 40, 2029–2050. [Google Scholar] [CrossRef]
- Bostan, R.; Varvara, S.; Gaina, L.; Petrisor, T.; Muresan, L.M. Protective effect of inhibitor-containing nitrocellulose lacquer on artificially patinated bronze. Prog. Org. Coat. 2017, 111, 416–427. [Google Scholar] [CrossRef]
- Di Carlo, G.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127. [Google Scholar] [CrossRef]
- Fonseca, I.T.E.; Picciochi, R.; Mendonca, M.H.; Ramos, A.C. The atmospheric corrosion of copper at two sites in Portugal: A comparative study. Corros. Sci. 2004, 46, 547–561. [Google Scholar] [CrossRef]
- Hernandez, R.D.B.; Aoki, I.V.; Tribollet, B.; de Melo, H.G. Electrochemical impedance spectroscopy investigation of the electrochemical behaviour of copper coated with artificial patina layers and submitted to wet and dry cycles. Electrochim. Acta 2011, 56, 2801–2814. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu-Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Sandberg, J.; Wallinder, I.O.; Leygraf, C.; le Bozec, N. Corrosion-induced copper runoff from naturally and pre-patinated copper in a marine environment. Corros. Sci. 2006, 48, 4316–4338. [Google Scholar] [CrossRef]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Kreislova, K.; Geiplova, H. Prediction of the long-term corrosion rate of copper alloy objects. Mater. Corros.-Werkstoffe Und Korrosion 2016, 67, 152–159. [Google Scholar] [CrossRef]
- Morcillo, M.; Chang, T.; Chico, B.; de la Fuente, D.; Wallinder, I.O.; Jimenez, J.A.; Leygraf, C. Characterisation of a centuries-old patinated copper roof tile from Queen Anne’s Summer Palace in Prague. Mater. Charact. 2017, 133, 146–155. [Google Scholar] [CrossRef]
- Chiavari, C.; Rahmouni, K.; Takenouti, H.; Joiret, S.; Vermaut, P.; Robbiola, L. Composition and electrochemical properties of natural patinas of outdoor bronze monuments. Electrochim. Acta 2007, 52, 7760–7769. [Google Scholar] [CrossRef]
- Saha, D.; Pandya, A.; Singh, J.K.; Paswan, S.; Singh, D.D.N. Role of environmental particulate matters on corrosion of copper. Atmos. Pollut. Res. 2016, 7, 1037–1042. [Google Scholar] [CrossRef]
- De la Fuente, D.; Simancas, J.; Morcillo, M. Morphological study of 16-year patinas formed on copper in a wide range of atmospheric exposures. Corros. Sci. 2008, 50, 268–285. [Google Scholar] [CrossRef]
- Kratschmer, A.; Wallinder, I.O.; Leygraf, C. The evolution of outdoor copper patina. Corros. Sci. 2002, 44, 425–450. [Google Scholar] [CrossRef]
- Veleva, L.; Farro, W. Influence of seawater and its aerosols on copper patina composition. Appl. Surf. Sci. 2012, 258, 10072–10076. [Google Scholar] [CrossRef]
- Graedel, T.E.; Nassau, K.; Franey, J.P. Copper Patinas Formed in the Atmosphere.1. Introduction. Corros. Sci. 1987, 27, 639–657. [Google Scholar] [CrossRef]
- Noli, F.; Misaelides, P.; Hatzidimitriou, A.; Pavlidou, E.; Kokkoris, M. Investigation of artificially produced and natural copper patina layers. J. Mater. Chem. 2003, 13, 114–120. [Google Scholar] [CrossRef]
- Nassau, K.; Miller, A.E.; Graedel, T.E. The Reaction of Simulated Rain with Copper, Copper Patina, and Some Copper-Compounds. Corros. Sci. 1987, 27, 703–719. [Google Scholar] [CrossRef]
- Watanabe, M.; Toyoda, E.; Handa, T.; Ichino, T.; Kuwaki, N.; Higashi, Y.; Tanaka, T. Evolution of patinas on copper exposed in a suburban area. Corros. Sci. 2007, 49, 766–780. [Google Scholar] [CrossRef]
- Stoulil, J.; Šedá, P.; Anisová, M.; Fencl, Z.; Novák, P.; Děd, J. Defects of copper patina. Koroze a Ochrana Materiálů 2015, 59, 87–90. [Google Scholar] [CrossRef]
- Bernardi, E.; Chiavari, C.; Lenza, B.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The leaching action of acid rain. Corros. Sci. 2009, 51, 159–170. [Google Scholar] [CrossRef]
- Strandberg, H. Reactions of copper patina compounds - I. Influence of some air pollutants. Atmos. Environ. 1998, 32, 3511–3520. [Google Scholar] [CrossRef]
- Zittlau, A.H.; Shi, Q.; Boerio-Goates, J.; Woodfield, B.F.; Majzlan, J. Thermodynamics of the basic copper sulfates antlerite, posnjakite, and brochantite. Chem. Der Erde-Geochem. 2013, 73, 39–50. [Google Scholar] [CrossRef]
- Guadagnini, L.; Chiavari, C.; Martini, C.; Bernardi, E.; Morselli, L.; Tonelli, D. The use of scanning electrochemical microscopy for the characterisation of patinas on copper alloys. Electrochim. Acta 2011, 56, 6598–6606. [Google Scholar] [CrossRef]
- Lv, G.C.; Nadykto, A.B.; Sun, X.M.; Zhang, C.X.; Xu, Y.S. Towards understanding the role of amines in the SO2 hydration and the contribution of the hydrated product to new particle formation in the Earth’s atmosphere. Chemosphere 2018, 205, 275–285. [Google Scholar] [CrossRef]
- Zhang, Q.; Tie, X.X.; Lin, W.L.; Cao, J.J.; Quan, J.N.; Ran, L.; Xu, W.Y. Variability of SO2 in an intensive fog in North China Plain: Evidence of high solubility of SO2. Particuology 2013, 11, 41–47. [Google Scholar] [CrossRef]
- Knotková, D.; Kreislová, K.; Had, J. Transformation of the Artificial Green Patina Layers; AKI: Pelhřimov, Czech Republic, 2001. [Google Scholar]
- Hernandez, R.P.B.; Goncalves, R.P.; Neiva, A.C.; de Melo, H.G. EIS and microstructural characterization of artificial nitrate patina layers produced at room temperature on copper and bronze. J. Braz. Chem. Soc. 2007, 18, 54–64. [Google Scholar]
- Mendoza, A.R.; Corvo, F.; Gomez, A.; Gomez, J. Influence of the corrosion products of copper on its atmospheric corrosion kinetics in tropical climate. Corros. Sci. 2004, 46, 1189–1200. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods: Reston, VA, USA, 2013; book 6, Chapter A43, 497; p. 2013.
- Blanc, P.; Lassin, A.; Piantone, P.; Azaroual, M.; Jacquemet, N.; Fabbri, A.; Gaucher, E.C. Thermoddem: A Geochemical Database Focused on Low Temperature Water/Rock Interactions and Waste Materials. Appl. Geochem. 2012, 27, 2107–2116. [Google Scholar] [CrossRef]
- Young, C.L. Sulfur Dioxide, Chlorine, Fluorine and Chlorine Oxides: Solubility Data Series; Pergamon Press: Oxford, UK, 1983; Volume 12. [Google Scholar]
- Constable, F.H. The Cause of the Colours Shown during the Oxidation of Metallic Copper. Proc. R. Soc. Lond. Ser. A 1927, 115, 570–588. [Google Scholar] [CrossRef]
- Fredj, N.; Burleigh, T.D. Transpassive Dissolution of Copper and Rapid Formation of Brilliant Colored Copper Oxide Films. J. Electrochem. Soc. 2011, 158, C104–C110. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Han, S.; Sung, T.; No, K. Biaxial texturing of Cu sheets and fabrication of ZrO2 buffer layer for YBCO HTS films. Phys. C Supercond. 2000, 334, 243–248. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Kouril, M.; Jindrova, E.; Jamborova, T.; Stoulil, J.; Dernovskova, J. Contribution of protein and polysaccharide binders to corrosion of gold-imitating brass in illuminated manuscripts. Mater. Corros.-Werkstoffe Und Korrosion 2016, 67, 130–140. [Google Scholar] [CrossRef]
- Msallamova, S.; Kouril, M.; Strachotova, K.C.; Stoulil, J.; Popova, K.; Dvorakova, P. Historical lead seals and the influence of disinfectants on the lead corrosion rate. Herit. Sci. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Msallamova, S.; Kouril, M.; Strachotova, K.C.; Stoulil, J.; Popova, K.; Dvorakova, P.; Lhotka, M. Protection of lead in an environment containing acetic acid vapour by using adsorbents and their characterization. Herit. Sci. 2019, 7, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Stoulil, J.; Fialova, A.; Jamborova, T.; Kouril, M. Combined tannate/decanoate coatings. J. Cult. Herit. 2014, 15, 339–344. [Google Scholar] [CrossRef]
- Stoulil, J.; Kanok, J.; Kouril, M.; Parschova, H.; Novak, P. Influence of temperature on corrosion rate and porosity of corrosion products of carbon steel in anoxic bentonite environment. J. Nucl. Mater. 2013, 443, 20–25. [Google Scholar] [CrossRef]
- Švadlena, J.; Stoulil, J. Evaluation of protective properties of acrylate varnishes used for conservation of historical metal artefacts. Koroze a Ochrana Materialu 2017, 61, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Dubus, M.; Kouril, M.; Nguyen, T.-P.; Prosek, T.; Saheb, M.; Tate, J. Monitoring copper and silver corrosion in different museum enviroments by electrical resistance measurement. Stud. Conserv. 2010, 55, 121–133. [Google Scholar] [CrossRef]
- Dubus, M.; Prosek, T. Standardized assessment of cultural heritage enviroments by electrical scientific paper resistance measurements. e-Preserv. Sci. 2012, 9, 67–71. [Google Scholar]
- Kouril, M.; Prosek, T.; Scheffel, B.; Degres, Y. Corrosion monitoring in archives by the electrical resistance technique. J. Cult. Herit. 2014, 15, 99–103. [Google Scholar] [CrossRef]
- Kouril, M.; Prosek, T.; Scheffel, B.; Dubois, F. High sensitivity electrical resistance sensors for indoor corrosion monitoring. Corros. Eng. Sci. Technol. 2013, 48, 282–287. [Google Scholar] [CrossRef]
- Kouril, M.; Prosek, T.; Dubus, M.; Taube, M.; Hubert, V.; Scheffel, B.; Degres, Y.; Jouannic, M.; Thierry, D. Corrosion monitoring in the hands of restorers an conservators. Koroze a Ochrana Materiálů 2012, 56, 67–75. [Google Scholar]
- Prosek, T.; le Bozec, N.; Thierry, D. Application of automated corrosion sensors for monitoring the rate of corrosion during accelerated corrosion tests. Mater. Corros. 2014, 65, 448–458. [Google Scholar] [CrossRef]
- Prosek, T.; Kouril, M.; Hilbert, L.R.; Degres, Y.; Blazek, V.; Thierry, D.; Hansen, M.Ø. Real time corrosion monitoring in atmosphere using automated battery driven corrosion loggers. Corros. Eng. Sci. Technol. 2008, 43, 129–133. [Google Scholar] [CrossRef]


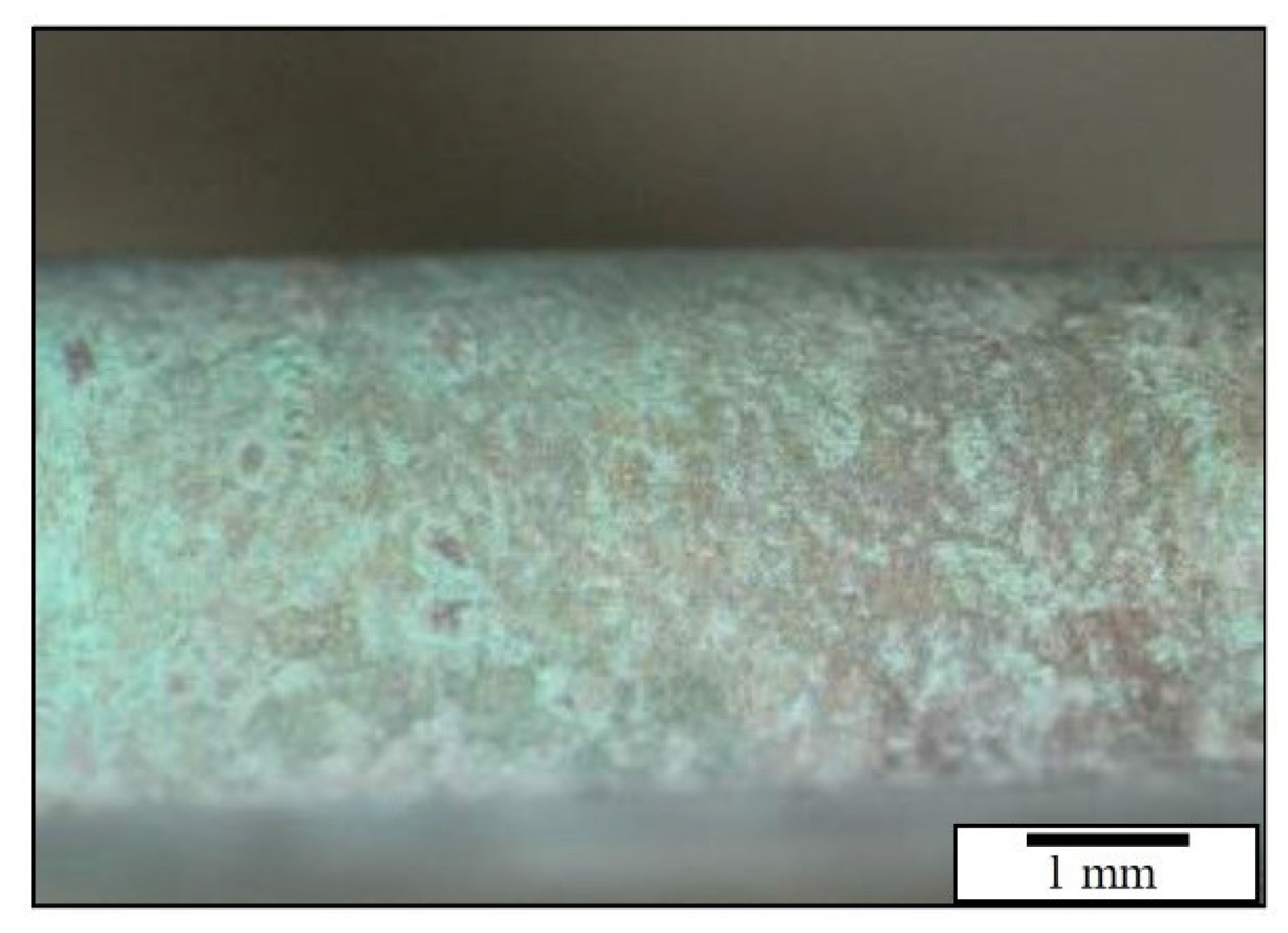

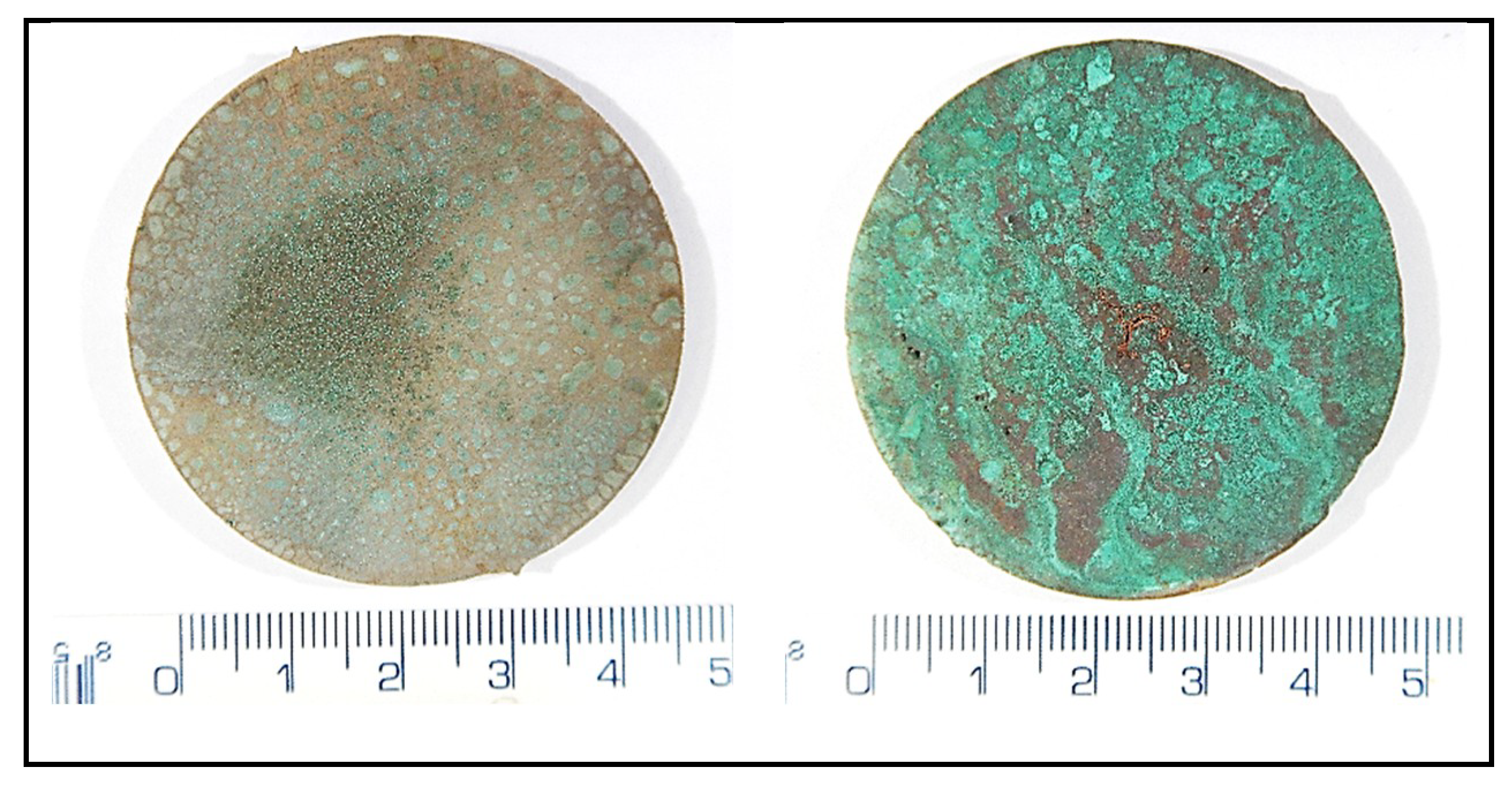
| Experiment | Duration | Temperature | SO2 Inlet Dose [g·m−3] | Cycle |
|---|---|---|---|---|
| 1 | 24 h | 40 °C | 8.9–44.3 | Condensation |
| 2 | 1 week | 40 °C | 8.9 | Condensation |
| 24 h | 40 °C | 17.7 | ||
| 3 | 1 week | 40 °C | 8.9 | Condensation |
| Condensation/drying phase | ||||
| 4 | 1 week | 40 °C | 17.7–0.0 | Condensation/drying phase |
| SO2 (g/m3) | Oxidation not Allowed | Oxidation Allowed | ||
|---|---|---|---|---|
| pH | PSO2 (kPa) | pH | PSO2 (kPa) | |
| 4.4 | 3.4 | 1.9 × 10−3 | 3.1 | 6.1 × 10−44 |
| 8.9 | 3.2 | 6.9 × 10−3 | 2.9 | 4.0 × 10−43 |
| 17.7 | 2.9 | 2.4 × 10−2 | 2.6 | 2.3 × 10−42 |
| 26.6 | 2.7 | 4.7 × 10−2 | 2.4 | 6.3 × 10−42 |
| 35.4 | 2.6 | 7.5 × 10−2 | 2.3 | 1.2 × 10−41 |
| 44.3 | 2.6 | 1.1 × 10−1 | 2.2 | 2.1 × 10−41 |
| T (°C) | Oxidation not Allowed | Oxidation Allowed | |||
|---|---|---|---|---|---|
| pH | PSO2 (kPa) | CSO2 (mol/kg) | pH | PSO2 (kPa) | |
| 40 | 3.2 | 6.9 × 10−3 | 7.77 × 10−4 | 2.9 | 4.0 × 10−43 |
| 50 | 3.2 | 1.1 × 10−2 | 7.76 × 10−4 | 2.9 | 7.2 × 10−43 |
| 60 | 3.2 | 1.8 × 10−2 | 7.74 × 10−4 | 2.9 | 1.3 × 10−42 |
| 70 | 3.2 | 2.7 × 10−2 | 7.71 × 10−4 | 2.9 | 2.1 × 10−42 |
| 80 | 3.2 | 4.0 × 10−2 | 7.65 × 10−4 | 2.9 | 3.4 × 10−42 |
| 90 | 3.3 | 5.5 × 10−2 | 7.58 × 10−4 | 2.9 | 5.2 × 10−42 |
| Features | Pre-Oxidation | Solution 1. | Solution 2. |
|---|---|---|---|
| Thickness [nm] | 129 | 737 | 939 |
| XRD–composition | Cuprite Cu2O | Cuprite Cu2O Djurleite Cu31S16 | Chalkocite Cu2S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bureš, R.; Klajmon, M.; Fojt, J.; Rak, P.; Jílková, K.; Stoulil, J. Artificial Patination of Copper and Copper Alloys in Wet Atmosphere with Increased Content of SO2. Coatings 2019, 9, 837. https://doi.org/10.3390/coatings9120837
Bureš R, Klajmon M, Fojt J, Rak P, Jílková K, Stoulil J. Artificial Patination of Copper and Copper Alloys in Wet Atmosphere with Increased Content of SO2. Coatings. 2019; 9(12):837. https://doi.org/10.3390/coatings9120837
Chicago/Turabian StyleBureš, Richard, Martin Klajmon, Jaroslav Fojt, Pavol Rak, Kristýna Jílková, and Jan Stoulil. 2019. "Artificial Patination of Copper and Copper Alloys in Wet Atmosphere with Increased Content of SO2" Coatings 9, no. 12: 837. https://doi.org/10.3390/coatings9120837
APA StyleBureš, R., Klajmon, M., Fojt, J., Rak, P., Jílková, K., & Stoulil, J. (2019). Artificial Patination of Copper and Copper Alloys in Wet Atmosphere with Increased Content of SO2. Coatings, 9(12), 837. https://doi.org/10.3390/coatings9120837




