1. Introduction
Oil–water separation has become a pivotal issue globally due to the occurrence of the disastrous oil-spills [
1,
2]. This results in vital complications to marine aquatic systems. Nanomaterials play an important role in separating oil–water mixture due to their wettability properties [
3]. The hybrid materials show remarkable properties and act as a model system for the separation of oil–water mixture [
4,
5]. Carbon nanotube/polymer hybrid membranes for the separation of oil in water emulsion was synthesized via surface-grafting poly (N-diethylaminopropyl) methacrylate (PDEAEMA) on carbon nanotubes by Abraham et al [
6]. They utilized the switchable surface wettable properties such as hydrophobic to hydrophilic for the separation of oil-in-water emulsion [
6]. On the other hand, Zhao et al. [
7] have synthesized poly(dimethylsiloxane)/graphene oxide composite sponge for the separation of oil–water mixture. The nanocomposite exhibited excellent adsorption capacity, oil–water selectivity, and mechanical stability. The results also showed that the adsorbed weight of the oil is 724 times the weight of sponge [
7]. Similarly, Cao et al. [
8] have developed an eco-friendly approach for the preparation of reduced graphene oxide/polydopamine composite aerogel to separate oil–water mixture. The composite aerogel exhibited super-amphiphilicity in the nnair and underwater superoleophobicity. The composite showed more than 90% separation efficiency and recyclability [
8]. An oleophobic/superhydrophilic poly(diallyldimethylammonium-perfluorooctanoate) (PDDA-PFO) membrane was deposited on a carbon/silica nanoparticles coated stainless steel mesh by Liu et al. [
9]. The mesh demonstrated the selective separation of oil–water mixture and more than 97% separation efficiency was achieved [
9]. Likewise, a stable underwater superoleophobic poly (diallyldimethylammonium chloride) (PDDA) and halloysite nanotubes (HNTs) coated mesh was prepared using layer by layer assembly method [
10]. The mesh was coated with 10 deposition cycles and displayed outstanding performance in the separation of oil–water mixture with more than 97% separation efficiency [
10]. Zhang et al. [
11] have fabricated a self cleaning, durable, superhydrophobic stainless steel mesh via deposition of carbon soot and polydimethylsiloxane for the separation of oil–water mixture. The coating showed contact angle of 156° and roll off angle 3°. The separation efficiency reached up to 94% for the mesh [
11]. Recently, Li et al. [
12] have fabricated SiO
2 nanoparticles functionalized with octadecyltrimethoxysilane and coated those on a cotton fabric. The fabric showed the superhydrophobic nature with water contact angle 159° ± 1° and superholeophilic nature with oil contact angle 0°. The coating was tested up to 40 cycles and separation efficiency reached more than 94.6% [
12]. On the other hand, Wang et al. [
13] have synthesized the eco-friendly pure poly (lactic acid) foam for the separation of oil–water mixture. The foam exhibited the water contact angle 151°. For continuous water pumping, foam showed 98% efficiency for more than 15 h [
13].
Xu et al. [
14] have fabricated the superwettable twines via simple treatment of commercial twines by silica nanoparticles and plasma. They showed that the microgrooves were helping in the oil absorption and self-transportation [
14]. Similarly, Ge et al. [
15] synthesized 3D porous carbon-based hybrid by immobilizing the cobalt based metal–organic framework (Co-MOF) nanosheets on carbon foam (CF). The treatment is done by vapor phase hydrothermal process followed by carbonization under nitrogen atmosphere. Their technology exhibits remarkable oil collection and antifouling performance. They have demonstrated the recyclability up to 20 cycles and no obvious reduction in the capacity was observed [
15]. Li et al. [
16] have fabricated the compressible and conductive aerogels by oxidation-oven drying carbonization method. They prepared these aerogels from the waste papers. They are superhydrophobic and superoleophilic in nature and exhibits the removal of free oils from water [
16]. Hu et al. [
17] have fabricated the carbon nanotube-based membranes with stimuli switchable fluxes for the separation of oil–water mixture. Furthermore, the fluxes changed 832, 679, and 641 L·m
−2·h
−1·MPa
−1 for emulsions on the application of irradiation by UV-light, redoxed by FeCl
3, and acidified by HCl, respectively [
17]. Meanwhile, Saththasivam et al. [
18] have prepared the carbon nanotube-based membrane for oil water separation with separation efficiency > 99.9% and possess water flux as high as 5500 L/m
2·h·bar [
18]. Zhang et al. [
19] have synthesized multiwall carbon nanotube and polyurethane hybrid with superhydrophobic properties with contact angle ≥ 153°. They worked as efficient oil adsorbents for separation of oil–water mixture [
19]. Furthermore, polyvinylpyrrolidone coated magnetite nanoparticles were synthesized for the separation of oil–water mixture by Mirshahghassemi and Lead [
20]. With the help of fluorescence and nuclear magnetic resonance study, they showed 100% removal of hydrocarbons. On the basis of gas chromatography results, they showed the removal of 100% of lower alkanes (C9–C21) within 10 min and while increasing the time up to 40 min more than 67% of C22–C25 alkanes removed [
20].
The current study aims to find a novel protocol for the separation of oil in water emulsion. The development of two-stage process anticipates in reducing the total petroleum hydrocarbons (TPHs). The current paper also focuses on the human health risks assessment from the polycyclic aromatic hydrocarbons (PAHs) dissolved in the water, separated from oil. The geometry of nanoscale hybrid (grapheme oxide (GO) and polydiallyl dimethyl ammonium chloride (PDDA)) system is expected to play an important role in the separation of oil water mixture and recyclability. The hybrid system is self-cleaning and withstands number of cycles without fouling effect.
2. Experimental Methods
10 g × 35.5″ Carbon Veil used as carbon substrate was purchased from Composite Canada (Mississauga, ON, Canada). Graphite flakes and sulfuric (95%–98%) acid were purchased by the Fisher Scientific. Phosphoric acid (85%), citric acid, ethylene glycol, polydiallyl dimethyl ammonium chloride (PDDA), hexane, dichloromethane (DCM), and hydrogen peroxide (30%) were purchased from Sigma Aldrich (Winston Park Dr. Oakville, Ontario, Canada). Stainless steel mesh of 60 µm average pore size was purchased from Alfa Aesar (Radcliff Rd, Tewksbury, MA, USA).
Graphene was prepared by the improved Hummers method [
21]. In 9:1 of H
2SO
4 and H
3PO
4 solution, 3 gm of graphite flakes were incorporated. The solution then transferred to ice bath to maintain temperature below 10 °C. Consecutively, 18 gm of potassium permanganate (KMnO
4) added slowly into the abovementioned solution. Furthermore, 200 mL water was added after 1 h and maintains the temperature 90 °C for 30 min. H
2O
2 was added slowly and kept it for stirring for 30 min. For the cleaning purpose, 150 mL HCl was added. After cleaning for various times disperse the end product in 1000 mL water.
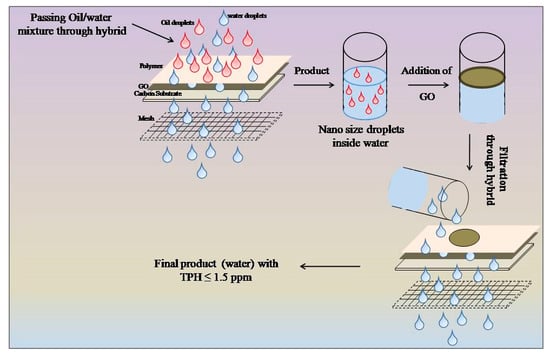
The self-cleaning nanoscale coating was performed via layer-by-layer deposition method (
Figure 1a,b). 1 mL of PDDA was deposited on carbon substrate. In continuation of this, 1 mL of GO was further incorporated above that layer. This assembly was covered by another layer of PDDA. This procedure was repeated for three times. Finally, substrate was placed in the oven at 60 °C. Consecutively, 1 mL of PDDA was deposited on top of the stainless-steel mesh. The carbon substrate along with the mesh is considered to be nanoscale hybrid system and used to separate oil–water mixture.
Figure 1c shows the schematic representation of the apparatus used to perform the experiment.
Laboratory based emulsion was created by mixing 7 gm of NaCl, 200 µL of crude oil, and 200 mL of water with the help of homogenizer at 11,000 rpm for 10 min.
3. Results and Discussions
The present work also focuses on the development of two stage process. These processes are classified as mesh and polish. Mesh is the processes where emulsion directly passes through nanohybrid and polish is the process where GO incorporated into the water which is received from the mesh process. Now, nanodroplate flocculate with GO on top of the water. This solution was further passed through the hybrid. This process is called polishing. Water received from each process was extracted through the dichloromethane (DCM) and hexane for GCMS and GC-FID to measure PAHs and TPHs. The detailed process of hexane extraction can be found in
Supplementary Information (Section 1.3).
The structural characterization of as prepared graphene oxide and hybrid was done by Raman spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). Wettable properties of coating is measured by contact angle measurement. For the surface energy measurement, a home made set up is used (See
Supplementary Information, Figure S1) Wetting phenomena on coating is elucidated through Young’s equation [
22]
where θ, γ
lv, γ
sv, and γ
sl are the contact angle, interfacial free energies of liquid–vapor, solid–vapor, and solid–liquid respectively. Surface energy of the nanoscale hybrid coating was determined by Owens, Wendt, Rabel, and Kaelble method (OWRK) method [
23]. This expresses the underlying interactions between the molecule in terms of polar and dispersive interactions. For OWRK method at least two liquids with polar and dispersive surface tensions are used to measure the surface energy. OWRK method is defined as
where γ
D and γ
P are dispersive and polar components of surface tensions. To measure the surface energy dispersive and polar components for three liquids—formamide, water, and ethylene glycol—are known and wettable properties of coating for these liquids are also calculated (see
Supplementary Information, Figures S2 and S3, Tables S1 and S2). The surface energy of the coating is calculated 29.43 mN/m. Surface energy depends upon the wettablity of the surface. Wettability reduces when overall surface energy of the solid surface is lowered [
24]. The detailed calculations can be found in the
Supplementary Information (Section 1.9).
Figure 2a shows the underwater contact angle of crude oil at the coating i.e., 135°. This shows the oleophobic nature of the nanoscale coating.
Figure 2b shows the scanning electron image of graphene oxide while
Figure 2c represents the secondary electron image of PDDA coated graphene oxide-based coating. This shows that coating is well uniformed. In
Figure 2d Raman spectrum of graphene oxide shows the presence of D and G bands at 1200–1300 cm
–1 [
25] and 1500–1600 cm
–1 [
26] respectively. D band occurs due to disordered structure of graphene while G band represents the graphitic nature. The comparison between GO and GO/PDDA coatings are shown in
Supplementary information Figure S4. This shows the red shift in D and G bands. More details can be found in
Supplementary Information (Section 1.10). Surface roughness of the coating is investigated by the 3D optical surface profiler (
Figure 2e). 3D topographical image shows the surface roughness of the sample. Surface roughness of the coating is 4.18 µm. This shows that surface roughness plays an important role in the wettable properties of the coating and contributes in the separation of oil–water mixture.
Figure 2f shows the Fourier-transform infrared spectroscopy (FTIR) of graphene oxide (GO). The characteristic peaks for graphene oxide were analyzed at 3320.88 for O–H stretching, 1714.07 for C=O (carboxyl/carbonyl) stretching vibrations, 1608.62 for C=C (aromatic), 1135.66 for C–O (epoxy), 1016.72 for C–O (alkoxy), 851.04 and 566.41 for C–H aromatic deformation.
Figure 3 shows the two-stage filtration process for the separation of oil–water mixture. Oil in water emulsion was subjected to the hybrid system and the filtered water contains nanosized oil droplets (mesh process). Now, GO was incorporated into the water received from the mesh process. Oil droplets flocculated on atop of the water along with GO, which is further filtered by the same hybrid system (polish process). The optical micrographs of emulsion, water obtained after mesh and polish processes can be found in
Supplementary Information Figure S5. In this coating, the carbon substrate has randomly oriented micro carbon fibers. This substrate has special properties such as high temperature resistance, abrasion resistance, corrosion resistance, and high specific strength. On the other hand, when PDDA is coated as a polymer to form coating, the main feature of PDDA is that it does not exfoliate from the coating and does not flow along with the water. It sticks on the surface very nicely and maintains the stability of the coating. Carbon substrate, PDDA, and GO form an oleophobic hydrophilic nanoscale hybrid coating. The self-cleaning behavior of the coating is shown in the
Supplementary Information (Figure S6).
This is expected to reduce the TPHs and PAHs significantly. The TPHs and separation efficiency are calculated by gas chromatography coupled to Flame ionization detector (GC-FID) and Total petroleum hydrocarbons (TPHs) analyzer.
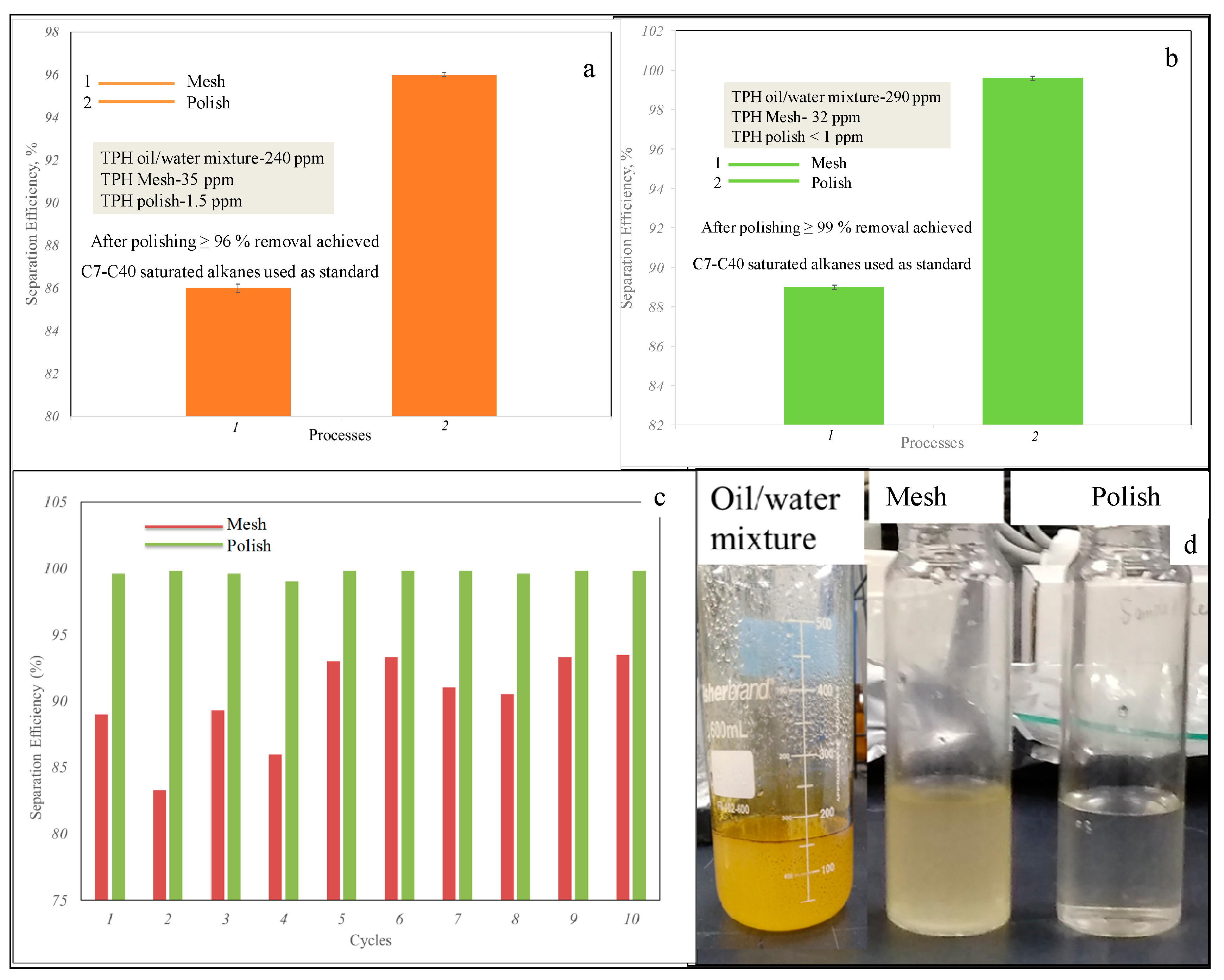
Figure 4a,b show the separation efficiency of the nanohybrid for two-stage process. The separation efficiency is calculated through the formula
where T
Mesh/Polish is the TPHs after mesh or polish process. T
Emulsion is the TPHs of the emulsion.
Initially the TPHs of the emulsion were 290 ppm determined by GC-FID. After passing through the mesh, it reduces to 32 ppm and finally it is further reduced to less than 1 ppm during polishing process.
The separation efficiency after the mesh is 89% while the polishing process it reaches above 99%. On the other hand, the TPHs results through TPH analyzer have approximately same value as GC-FID, i.e., separation efficiency reached to 86% for mesh and 96% for polish. The separation efficiency of nanohybrid has also been tested for ten cycles by GC-FID and apparently,
Figure 4c shows approximately the same separation efficiency and removal of hydrocarbons (C10–C40 alkanes). This implies that no fouling effect is taking place.
Figure 4d shows the different stages of water samples (oil–water mixture, water received from mesh and water received from polish). The separation efficiency was also tested for different content of GO for mesh process (see
Supplementary Information, Figure S7). This shows that there is a slight increase in the separation efficiency by increasing the GO content. Furthermore, cyclability results also show the stability of the nanohybrid material. Unequivocally, the nanohybrid also shows the removal of PAHs from the emulsion by two stage processes.
Table 1 shows the PAHs found in the sample after mesh process. The detection of PAHs in the water samples obtained from the two-stage process was carried out by the gas chromatography coupled to mass spectrometry (GC-MS).
It is to be noted that, after the mesh process, the concentration of the total PAHs in the sample was 0.5 ppm (naphthalene 0.2 ppm, anthracene 0.15 ppm, and phenanthrene 0.15 ppm). While no PAHs were detected in the final sample after polishing process.
The hybrid was also tested for 5 and 10 cycles to measure the PAHs concentration and found the concentration remains the same for each cycle (see
Supplementary Information, Table S3). In the current study, graphene is playing an important role in both processes. Graphene oxide possesses layered structure and high surface area, which make it most promising to adsorb oil droplets. Eventually, GO comprises oxygenated functional groups that attached with the dissolved part of hydrocarbons and finally assists the separation process [
27].